Abstract
Background:
We conducted a survey of 111 medical oncologists across India to understand the current pattern of epidermal growth factor receptor (EGFR) mutation testing at their respective centers.
Methods:
Medical oncologists from 111 institutes across India were interviewed face to face using a structured questionnaire. They were divided into two groups – Group 1 with in-house EGFR testing and Group 2 who send samples to central/commercial laboratories outside their institutions. Answers of the two groups were analyzed to see the prevailing patterns of EGFR testing and differences between the two groups if any.
Results:
Ninety-five percent (105/111) of medical oncologists recommended testing for EGFR mutations in patients with adenocarcinoma histology and 40% (44/111) recommended EGFR testing in squamous cell histology. The average time duration to get EGFR test results was 10 days in Group 1 centers versus 18 days in Group 2 centers. Ninety-six percent (106/111) of the medical oncologists from Group 1 centers requested for factoring additional sample for biomarker testing compared to 69% (77/111) of the oncologists from Group 2 centers. Sixty-nine percent (77/111) of medical oncologists in Group 1 centers would prefer to wait for the test results before initiating treatment compared to 46% (51/111) in Group 2. EGFR tyrosine-kinase inhibitors were used in only approximately 60% of patients with diagnosed EGFR mutation in the first line. For patients in whom chemotherapy was initiated while waiting for test results, 50% (56/111) of medical oncologists would prefer to complete 4–6 cycles before switching to targeted therapy. At the time of progression, rebiopsy was possible in approximately 25% of the patients.
Conclusions:
Turnaround time for molecular testing should improve so that eligible patients can benefit from targeted therapies in the first line. There is a need to increase the awareness among pulmonologists, oncologists, and interventional radiologists regarding the importance of adequate samples required for molecular tests.
Keywords: Anaplastic lymphoma kinase, biopsy, epidermal growth factor receptor, fine-needle aspiration cytology, non-small cell lung cancer
Introduction
Lung cancer is the most common type of cancer in men in India. Although lung cancer constitutes 6.9% of all cancer cases for both sexes in India, it is responsible for 9.3% of all cancer-related deaths.[1] Non-small lung cancer constitutes 85% of the total lung cancer pools and has been classified into three major subtypes – adenocarcinoma, squamous cell carcinoma (SCC), and large cell carcinoma. Treatment of non-small cell lung cancer (NSCLC) was dependent on histology till recent past. Pemetrexed being active only in nonsquamous NSCLC, but not squamous cell or SCLC, emphasized the need for specific histologic diagnosis for therapy selection. The discovery of epidermal growth factor receptor (EGFR) mutations, rearrangements of anaplastic lymphoma kinase (ALK) and other receptor tyrosine kinases, and the development of targeted treatments have transformed the standard of care of patients with lung cancer. Molecular genotyping of lung cancer now involves routine testing of these mutations and rearrangements since these patients respond better to targeted treatments than to conventional chemotherapy.[2,3] Testing of EGFR mutations in patients with NSCLC has become the standard practice in India. Studies from different centers have reported the incidence of EGFR mutations in the range of 23%–44%.[4,5,6,7] Although some tertiary care centers in India have in-house facility to test the EGFR mutations, majority of the centers send the samples to central laboratories. Moreover, there are differences in the techniques used for EGFR testing at various centers.
Materials and Methods
To understand the pattern of EGFR testing across different oncology treating centers in India, we conducted a survey of medical oncologists during 2015–2016 using face-to-face questionnaire. We attempted to understand the flow of lung cancer patients with medical oncologists and criteria for selecting patients for molecular profiling, determine the number of hospitals conducting EGFR testing in-house or sending the samples to the centralized laboratories, determine any gaps in the molecular testing, and determine scope for improvement.
One hundred and eleven hospitals and institutes across India where EGFR testing for the management of NSCLC is routinely practiced were identified. The centers were classified into two groups. Group 1 centres (n=26) - where the EGFR testing was done in-house and Group 2 centres (n=85) - which sent the samples to the central/commercial laboratories outside their institutions. Medical oncologists at these institutes were interviewed using a structured questionnaire. The answers to the questionnaire were presented as absolute numbers and simple percentage and were analyzed for Group 1 and Group 2 centers separately using descriptive statistics to understand the prevailing patterns of EGFR testing and differences between the two groups if any. The questionnaire can be found in the supplementary appendix.
Results
The average number of cancer patients treated by a medical oncologist was 570/month. Lung cancer accounted for one-fourth of the patients (143 cases). Seventy-seven percent of these patients were either Stage III or Stage IV. More than half (53%) of the patients seen by medical oncologists in their clinical practice had adenocarcinoma histology. SCC formed 32% of total NSCLC cases.
In a medical oncologist's practice, 52% of the patients of lung cancer at the time of presentation had already undergone fine-needle aspiration cytology (FNAC) or primary biopsy. The percentage of patients with biopsy already done before consulting medical oncologist was higher in Group 1 centers (58% biopsy and 42% FNAC) compared to Group 2 centers (46% biopsy and 54% FNAC) [Figure 1]. Interventional radiologist was the primary specialty carrying out primary biopsy followed by pulmonologists and surgeons in centers from both groups [Figure 2].
Figure 1.
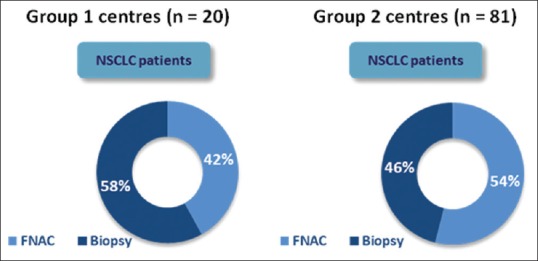
Percentage of non-small cell lung cancer patients who had already undergone primary biopsy/fine-needle aspiration cytology at the time of clinical presentation in Group 1 and Group 2 centers
Figure 2.
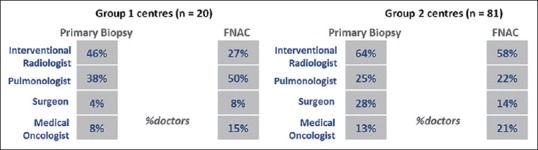
Specialty responsible for carrying out primary biopsy/fine-needle aspiration cytology among non-small cell lung cancer patients in Group 1 and Group 2 centers
Biopsy was ordered in 71% of patients who consulted medical oncologists with FNAC reports. For those who consulted without any biopsy/FNAC report, biopsy was ordered in 79%. In 29% of the patients, treatment was started based on FNAC report only [Figure 3] and majority of them (81%) received chemotherapy. These results were similar in Group 1 and Group 2 separately.
Figure 3.
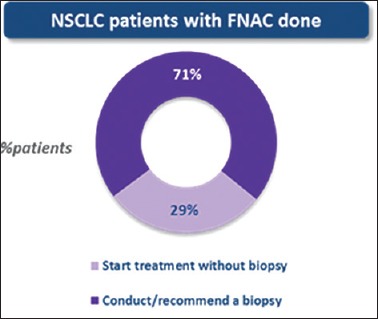
Percentage of non-small cell lung cancer patients in whom treatment was started based on fine-needle aspiration cytology reports
Rebiopsy was recommended in 16% of the patients who consulted medical oncologist for the first time [Figure 4]. The most common reason for rebiopsy was insufficiency of the sample to do biomarker testing.
Figure 4.
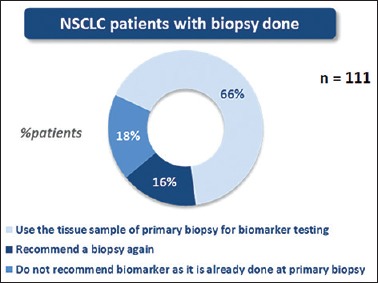
Percentage of non-small cell lung cancer patients in whom rebiopsy was recommended in the first line
Eighty percent of medical oncologists recommend biomarker testing (reflex testing) at the time of primary biopsy. This percentage was higher in Group 2 centers (82%) as compared to Group 1 centers (73%) [Figure 5a]. However, when it comes to recommending factoring sample for additional biomarker testing, 96% of medical oncologists from Group 1 centers recommended factoring in of additional samples as compared to only 69% of medical oncologists from Group 1 centers [Figure 5b].
Figure 5.
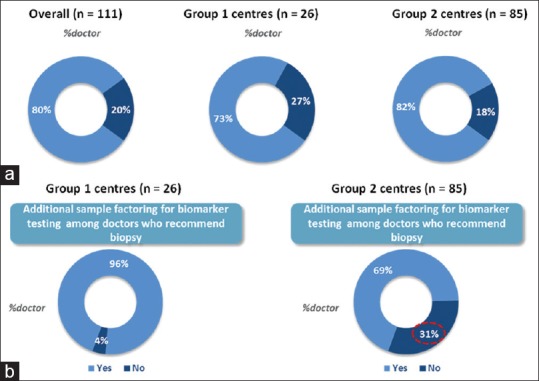
(a) Percentage of medical oncologists recommending reflex testing (Overall, Group 1 centers and Group 2 centers), (b) Percentage of medical oncologists recommending factoring sample for additional biomarker testing (Group 1 centers and Group 2 centers)
Ninety-five percent of medical oncologists recommend biomarker testing in NSCLC patients with adenocarcinoma histology. Five percent of respondents who did not test for biomarkers in adenocarcinoma did not use targeted therapies; therefore, biomarker testing was not required. Long waiting time to get the test results was another reason given for not doing biomarker testing. Forty percent of medical oncologists also did biomarker testing in patients with squamous cell histology. EGFR and ALK were the most common biomarkers tested by the medical oncologists. Simultaneous testing for EGFR and ALK was practiced by 53% of medical oncologists and 29% of medical oncologists tested for only EGFR. Thirty-eight percent of NSCLC patients of adenocarcinoma histology seen by respondents of this survey had EGFR mutations, most common mutation being del 19 (42%).
The average time required for EGFR testing was 10 days for Group 1 hospitals and 18 days for Group 2 centers. Polymerase chain reaction (PCR) and sequencing were the common methods used for EGFR mutation testing. Thirty-one percent of the medical oncologists from Group 1 hospitals and 41% of medical oncologists from Group 2 hospitals believed immunohistochemistry (IHC) to be a reliable method for EGFR testing.
Sixty-nine percent of the medical oncologists from Group 1 hospitals would wait for test results before initiating EGFR-directed therapies as compared to only 46% of medical oncologists from Group 2 hospitals [Figure 6]. Worsening general condition of the patients was the most common reason given for starting systemic treatment with chemotherapy.
Figure 6.
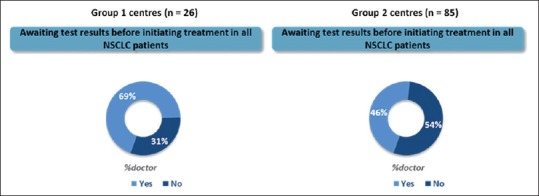
Percentage of medical oncologists waiting for test results before initiating epidermal growth factor receptor-directed therapies
If the systemic chemotherapy was started, 49% of medical oncologists will stop chemotherapy and immediately switch to EGFR tyrosine-kinase inhibitors (TKIs); in case, the patient is positive for EGFR mutation. Others will continue with 4–6 cycles of chemotherapy before shifting to maintenance TKI [Figure 7].
Figure 7.
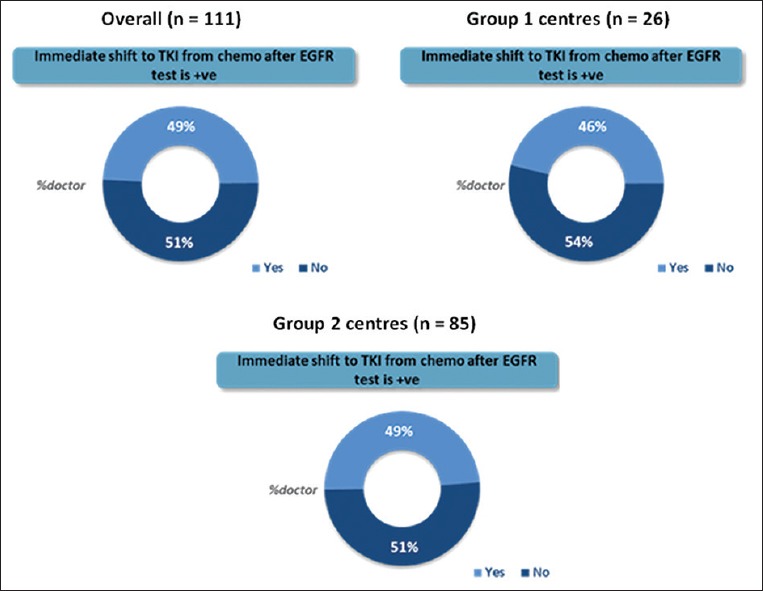
Percentage of medical oncologists immediately switching patients to epidermal growth factor receptor tyrosine-kinase inhibitors in case the patient is positive for epidermal growth factor receptor mutation (Overall, Group 1 and Group 2 centers)
Once patients progress on first-line therapy, medical oncologists were able to recommend rebiopsy in only 25% of the patients. This percentage was similar for Group 1 (21%) and Group 2 (26%) centers. Rapid disease progression and poor performance status were the two most common reasons given for the low rebiopsy rates. Increase in awareness among medical oncologists on the importance of EGFR testing and proper training of interventional radiologists on biopsy techniques followed by increase in EGFR testing facility were mentioned as the key factors that can increase the EGFR testing rates among NSCLC patients.
Discussion
The results of this survey reflect the flow of an NSCLC patient in a medical oncologist's clinic. The average number of patients seen by one medical oncologist in India was 570/month which is very high. This is because India has merely 2000 oncologist to take care of 10 million cancer patients.[8] More than 50% of the patients who present to medical oncologist with lung cancer symptoms have already undergone FNAC or biopsy. This may be due to that fact that majority of these patients initially see a general physician or a pulmonologist for their symptoms who would have ordered these investigations. For the diagnosis of lung cancer, biopsy is the gold standard diagnostic approach. Having an adequate biopsy sample is not only important for the histological diagnosis but also a very critical factor for the molecular diagnosis. Although FNAC sample can also be used for histological diagnosis, it has to be converted into cellblock for adequate molecular testing. In this survey, biopsy was recommended in 71% of the patients who presented to medical oncologist with an FNAC specimen. This could be because cellblock was not available for mutation analysis. There is a need to educate the general physician and pulmonologists regarding the importance of biopsy and cellblocks for molecular testing so that patients do not have to undergo these diagnostic procedures repeatedly. The rate of rebiopsy in the first-line setting was 16% as estimated by this survey, the major reason for rebiopsy being inadequate sample for molecular testing. Training of interventional radiologists on the biopsy taking techniques and adoption of rapid on-site evaluation protocols in the institutes can bring down the rates of rebiopsy in the first-line setting by allowing the adequate and good quality sample for molecular testing in the very first instance.
Rebiopsy may be challenging in certain situations. The results of this survey suggested that only 25% of the patients go for rebiopsy after progression. Poor performance status of the patients and rapid disease progression were the two most common reasons given for low rebiopsy rates. Cell-free circulating tumor DNA can be a potential surrogate for tissue biopsy in such patients. In a study which assessed EGFR mutation status in 803 plasma samples, the concordance between baseline tumor and plasma samples was 94.3%, with a sensitivity of 65.7% and specificity of 99.8%.[9] A liquid biopsy may also be useful in detecting ALK rearrangements.[10]
The rate of reflex testing was high in the participating institutes; however, factoring of samples for additional biomarker testing needs to improve, specifically in centers without in-house laboratories. This is important since the treatment of lung cancer is changing very fast with lot of data coming up on additional biomarkers. PD-L1 and BRAF are examples of such biomarkers which will need to be additionally tested. In a study of pembrolizumab done in patients with advanced NSCLC and PD-L1 expression on at least 50% of tumor cells, pembrolizumab was associated with significantly longer progression-free and overall survival. Therefore, adequacy of tumor specimen becomes of paramount importance since more and more tissue would be required for additional biomarker testing. The rate of EGFR testing was impressive with 95% of patients of adenocarcinoma histology tested for EGFR mutation. Moreover, 40% of oncologists tested EGFR mutation even in squamous cell histology. In a study done at Tata Memorial Hospital's treatment, it was shown that the incidence of EGFR mutation in SCC was 5.6%.[11] Subsequently, it was also shown that treatment with TKI in EGFR-mutated SCC of the lung is associated with improvement in survival.[12]
When this survey was conducted, the average time required for EGFR testing was 18 days in centers who sent the samples to the central laboratories. This could have been due to the logistical delays in sending the samples to the outside laboratories. However, there has been a constant effort by different laboratories in India to deal with the logistical delay time and the turnaround time could have changed now. Since the hospitals with in-house testing do not face the logistics issues, the turnaround time was less. Nevertheless, there is a need to decrease the turnaround time in all instances. This will also reduce the current scenario of commencing chemotherapy without waiting for molecular test results. The awareness of the techniques required to do EGFR testing was limited at the time of the conduct of this survey with approximately 40% believing that IHC was the appropriate method for molecular testing. However, the authors recognize that this percentage could have changed now since the knowledge of medical oncologists on molecular testing is increasing with various national bodies such as Lung Cancer Consortium Asia, Indian Cooperative Oncology Network, Indian Society of Medical and Pediatric Oncology, and Molecular Oncology Society working in this direction. With the lung cancer treatment becoming more complicated due to identification of various biomarkers, a constant effort by academia, national bodies regarding molecular diagnosis are the need of hour.
Based on the insights generated from this survey, Tata Memorial Hospital, Mumbai, and other oncology institutes such as Rajiv Gandhi Cancer Institute, Delhi; Christian Medical College, Vellore; and Indo American Institute have initiated 2–3 days’ preceptorship programs for pathologists/molecular scientists and interventional radiologists. In these programs, pathologists/molecular scientists are given hands-on training on EGFR testing techniques such as real-time PCR, sequencing and single nucleotide primer extension (SNaPshot) assay, and interventional radiologists are trained on techniques to obtain adequate tissue for biomarker testing. Till date, five such programs have been conducted in Tata Memorial Hospital and one program each at RGCI, CMC, and Indo American. The outcomes of such training will be evaluated taking feedbacks from the delegates and will be published.
Joint collaboration of academia and industry is the need of hour to improve the molecular diagnosis of lung cancer in India. This will go a long way in ensuring proper patient care in the era of targeted therapy for lung cancer in our country.
Conclusion
Importance of predictive and prognostic biomarkers for the management of lung cancer patients is increasing. There is an urgent need to increase the awareness among general physicians, pulmonologists, interventional radiologists and oncologists regarding the importance of adequate samples required for molecular testing. There is scope and need for reduced turnaround time for molecular testing. Increasing application of liquid biopsy will help in initial diagnosis as well as at relapse/follow up, especially for patients with poor performance status and/or difficult access to tumor site.
Financial support and sponsorship
This study was financially supported by unrestricted educational grant from Boehringer Ingelheim India Pvt. Ltd.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Malik PS, Raina V. Lung cancer: Prevalent trends & emerging concepts. Indian J Med Res. 2015;141:5–7. doi: 10.4103/0971-5916.154479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sebastian M, Schmittel A, Reck M. First-line treatment of EGFR-mutated nonsmall cell lung cancer: Critical review on study methodology. Eur Respir Rev. 2014;23:92–105. doi: 10.1183/09059180.00008413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–77. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 4.Noronha V, Prabhash K, Thavamani A, Chougule A, Purandare N, Joshi A, et al. EGFR mutations in indian lung cancer patients: Clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8:e61561. doi: 10.1371/journal.pone.0061561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veldore VH, Rao RM, Kakara S, Pattanayak S, Tejaswi R, Sahoo R, et al. Epidermal growth factor receptor mutation in non-small-cell lung carcinomas: A retrospective analysis of 1036 lung cancer specimens from a network of tertiary cancer care centers in india. Indian J Cancer. 2013;50:87–93. doi: 10.4103/0019-509X.117013. [DOI] [PubMed] [Google Scholar]
- 6.Chougule A, Prabhash K, Noronha V, Joshi A, Thavamani A, Chandrani P, et al. Frequency of EGFR mutations in 907 lung adenocarcioma patients of indian ethnicity. PLoS One. 2013;8:e76164. doi: 10.1371/journal.pone.0076164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahoo R, Harini VV, Babu VC, Patil Okaly GV, Rao S, Nargund A, et al. Screening for EGFR mutations in lung cancer, a report from india. Lung Cancer. 2011;73:316–9. doi: 10.1016/j.lungcan.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 8.India has just 2,000 oncologists for 10 million Patients. 2016. [Last accessed on 2017 Dec15]. Available from: https://timesofindia.indiatimes.com/india/India-hasjust-2000-oncologists-for-10-million-patients/articleshow/50842842.cms .
- 9.Douillard JY, Ostoros G, Cobo M, Ciuleanu T, Cole R, McWalter G, et al. Gefitinib treatment in EGFR mutated caucasian NSCLC: Circulating-free tumor DNA as a surrogate for determination of EGFR status. J Thorac Oncol. 2014;9:1345–53. doi: 10.1097/JTO.0000000000000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nilsson RJ, Karachaliou N, Berenguer J, Gimenez-Capitan A, Schellen P, Teixido C, et al. Rearranged EML4-ALK fusion transcripts sequester in circulating blood platelets and enable blood-based crizotinib response monitoring in non-small-cell lung cancer. Oncotarget. 2016;7:1066–75. doi: 10.18632/oncotarget.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choughule A, Noronha V, Joshi A, Desai S, Jambhekar N, Utture S, et al. Epidermal growth factor receptor mutation subtypes and geographical distribution among indian non-small cell lung cancer patients. Indian J Cancer. 2013;50:107–11. doi: 10.4103/0019-509X.117023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joshi A, Zanwar S, Noronha V, Patil VM, Chougule A, Kumar R, et al. EGFR mutation in squamous cell carcinoma of the lung: Does it carry the same connotation as in adenocarcinomas? Onco Targets Ther. 2017;10:1859–63. doi: 10.2147/OTT.S125397. [DOI] [PMC free article] [PubMed] [Google Scholar]


