Abstract
Background
Accurate prediction of mastectomy skin flap viability is vital as necrosis causes significant morbidity, potentially compromising results and delaying oncological management. Traditionally assessed by clinical judgement, a more objective evaluation can be provided using intraoperative imaging modalities. This systematic review aimed to compare all intraoperative techniques for assessment of mastectomy flap viability.
Methods
A systematic literature review was performed using MEDLINE and Embase databases. Primary outcomes reported included specificity, sensitivity and predictive values of each test, and mean rates of mastectomy flap necrosis and reoperation. Secondary outcomes included cost analysis.
Results
Some 18 studies were included. Designs were prospective cohort study (8), retrospective case series (4), prospective case series (3), retrospective case–control study (1), prospective pilot trial (1) and cost analysis study (1). The studies compared indocyanine green angiography (ICGA) (16 studies) and fluorescein dye angiography (FA) (3 studies) with clinical judgement. Sensitivity and specificity were highest for ICGA (5 studies) ranging from 38 to 100 and 68 to 91 per cent respectively. Both methods overpredicted necrosis. Mean rates of flap necrosis and reoperation decreased with ICGA (7·9 and 5·5 per cent respectively) and FA (3 and 0 per cent) compared with clinical judgement (19·4 and 12·9 per cent). Two studies were designed to define numerical parameters corresponding to perfusion using intraoperative techniques. Two studies performed a cost analysis for ICGA; one claimed a cost benefit and the other advocated its use in high‐risk patients only.
Conclusion
ICGA and FA are potentially useful tools for mastectomy flap assessment. However, the predictive accuracy is subject to the specific settings and model of equipment used. Current recommendations support their use in high‐risk patients.
Introduction
Since its introduction by Toth and Lappert1, rates of skin‐sparing mastectomy (SSM) and immediate breast reconstruction have been increasing2. Both SSM and nipple‐sparing mastectomy preserve the native skin envelope and inframammary fold of the breast, which can be used to facilitate immediate reconstructive procedures. Surgeons are able to achieve superior aesthetic results compared with those of conventional mastectomy or delayed reconstruction, as well as offer both therapeutic and reconstructive procedures in one sitting. These advantages lead to higher levels of patient satisfaction, psychological outcomes and cost‐effectiveness3.
Native mastectomy skin flap necrosis is a common complication when using skin‐sparing techniques. Reported rates are as high as 30 per cent4. The challenge lies in complete removal of all breast tissue to ensure oncological safety, while leaving sufficient skin flap thickness to maintain skin viability5. The superficial plane of dissection between the subcutaneous fat and breast tissue has been found to be indistinct under microscopic examination in almost half of patients6, making it technically difficult, especially with the more limited field of view compared with that of conventional mastectomy. Immediate breast reconstruction is also associated with a significantly higher rate of complications (up to 50 per cent) compared with delayed procedures (up to 36 per cent)7.
Other factors identified to pose a greater risk of mastectomy flap necrosis include patient‐related factors such as smoking8 9, diabetes mellitus10 11, high BMI10, 11, 12, high mastectomy specimen weight10 11, 13, and previous exposure to radiotherapy12 14. Intraoperative factors such as tumescent mastectomy technique9 15 and Wise‐pattern mastectomy incision13 16 have also been associated with a higher rate of mastectomy flap necrosis17.
Traditionally, mastectomy flap viability has been assessed clinically in the intraoperative setting18. Subjective parameters such as skin colour, capillary refill time and dermal bleeding from skin edges are used to guide resection of non‐viable tissue. Over‐resection of potentially unviable tissue may be the safest option, but this may limit reconstructive options and compromise aesthetic outcomes12. Rates of necrosis reported using this approach are approximately 10–15 per cent19, with a specificity of 10–30 per cent20.
Depending on the severity of skin flap ischaemia, the patient may develop superficial epidermolysis to full‐thickness necrosis21. Management can vary between dressing care in an outpatient setting, or more significant consequences such as surgical debridement, additional reconstruction and exposure of the breast prosthesis requiring removal. Planned oncological therapies may be delayed and the patient's quality of life affected10. Inadequate perfusion can also contribute to more generic complications such as delayed wound healing and infection22. Finding a means of objectively assessing mastectomy flap perfusion accurately and cost‐effectively is desirable.
Fluorescence angiography techniques have been used to great effect by disciplines such as ophthalmology and other surgical specialties23. They involve the injection of intravenous dye such as fluorescein or indocyanine green (ICG), which emits infrared energy in conjunction with use of a light source. By enabling real‐time assessment of tissue perfusion, a potentially more objective measure of viability can aid more accurate prediction of skin flap survival and help reduce rates of necrosis. Current applications of fluorescence angiography techniques in plastic surgery include sentinel lymph node biopsy, pedicled and free flap reconstructions as well as mastectomy skin flaps24.
Previous reviews24, 25, 26, 27 have considered use of fluorescence angiography techniques in a variety of clinical applications without focusing on mastectomy flap viability. The aim of this systematic review was to identify all intraoperative techniques for assessment of mastectomy flap viability in patients undergoing SSM or nipple‐sparing mastectomy and immediate reconstruction with prosthesis or autologous tissue, and to compare outcomes with clinical judgement.
Methods
Search strategy
A literature review of MEDLINE (1946–2017) and Embase (1947–2017) databases was conducted using keywords in the English language combined with Boolean logical operators. Search strategies are available in full in Tables S1 and S2 (supporting information). No further supplementary searches were undertaken. The primary outcomes were accuracy of each modality in predicting necrosis, and rates of mastectomy flap necrosis and reoperation. Secondary outcomes included cost analysis and complications directly associated with assessment modality.
Study selection
Duplicate articles were removed and all abstracts written in the English language were screened for full‐text review by two authors independently. This included articles published in languages other than English. Inclusion criteria were all original comparative studies using intraoperative techniques to determine mastectomy flap perfusion in patients undergoing immediate breast reconstruction after SSM or nipple‐sparing mastectomy. Exclusion criteria were studies with alternative populations, interventions or outcomes of interest, such as use of intraoperative modalities to assess sentinel lymph node biopsies or autologous perforator flap perfusion.
Data extraction
Data were extracted by two independent reviewers, including: authors; year of publication; study design; number of patients; number of breasts; type of mastectomy; type of reconstruction; duration of follow‐up; technique for mastectomy flap assessment; rate of necrosis; rate of reoperation; sensitivity, specificity and predictive values of each technique; cost analysis; and complications relating to technique. Sensitivity, specificity and predictive values were calculated from the data presented when not specified. Discrepancies were checked and resolved by consensus.
Assessment of study quality
All studies were assigned a level of evidence (LOE) adapted from the Oxford Centre for Evidence‐Based Medicine (http://www.cebm.net/index.aspx?o=1025) based on methodology and study design. The assigned levels were: LOE 1, RCT; LOE 2, cohort study; LOE 3, case–control study; LOE 4, case series; and LOE 5, expert opinion or case report.
Results
Study identification
A total of 1886 abstracts were retrieved from searches (Fig. 1). After detailed examination of 30 full‐text articles, 18 studies7 19, 20 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, including 2077 patients, were included in this review.
Figure 1.
PRISMA diagram showing selection of articles for review
Study characteristics
Designs were prospective cohort study (8), retrospective case series (4), prospective case series (3), retrospective case–control study (1), prospective pilot trial (1) and cost analysis study (1). Three intraoperative modalities for determining mastectomy flap perfusion were identified: indocyanine green angiography (ICGA) (16 studies), fluorescein dye angiography (FA) (3) and optical diffusion imaging spectroscopy (ODIS) (1).
Study design and level of evidence
Two different approaches were taken in the evaluation of mastectomy flap viability assessment using intraoperative techniques. One was to compare rates of mastectomy necrosis and other complications, such as reoperation rates, and clinical outcomes between the techniques used (Table 1). Seven case series7 28, 29, 30, 31, 32 34 and one cohort study33 used this approach. The alternative approach in seven prospective cohort studies19 20, 35, 36, 37, 38, 39 was to evaluate flap perfusion using the intraoperative modalities and to record the predicted areas of necrosis using photographic documentation or video recording. Tissue resection, however, was done according to the surgeon's clinical judgement. The clinical outcome of the mastectomy flap was then compared with previously predicted outcomes of the assessment technique to calculate sensitivity, specificity and predictive values (Table 2). One retrospective case–control study40 and one case series41 were designed to define numerical parameters corresponding to perfusion when using intraoperative techniques. Two studies30 42 performed retrospective cost analysis for the use of ICGA (including a secondary cost analysis in a case series30).
Table 1.
Summary of all studies reporting rates of mastectomy flap necrosis and reoperation using intraoperative techniques
| Reference | Study design | LOE | No. of breasts | Type of reconstruction | Follow‐up | Technique | Overall complications (%) | Mastectomy flap necrosis (%) | Reoperation (%)* | |
|---|---|---|---|---|---|---|---|---|---|---|
| Autologous | Prosthetic | |||||||||
| Rao et al. 34 | Prospective pilot study | 4 | 5 | 0 | 5 | 4 weeks | ODIS | – | 20 | 20 (M) |
| Jones et al. 28 | Prospective case series | 4 | 57 | 31 | 26 | – | ICGA | – | 6 | 2 (M) |
| Komorowska‐Timek and Gurtner7 | Prospective case series | 4 | 24 | 8 | 16 | – | ICGA | 4 | 4 | 4 (M) |
| 206 | – | – | – | CJ | 15·1 | – | – | |||
| Sood and Glat29 | Retrospective case series | 4 | 62 | 0 | 62 | – | ICGA | 18 | 10 | 7 (M) |
| 80 | – | – | – | CJ | 37 | 17 | 13 (M) | |||
| Duggal et al. 30 | Retrospective case series | 4 | 184† | 129 (91‡ ) | 149 (91‡ ) | 8·7 months | ICGA | 42·7 | 13·0 | 5·9 (O) |
| 184† | 24·7 months | CJ | 46·7 | 23·4 | 14·1 (O) | |||||
| Harless and Jacobson31 | Retrospective case series | 4 | 213 | 0 | 213 | 4·6 months | ICGA | 6·6 | 0·9 | 0·9 (M) |
| 254 | 0 | 254 | 16·9 months | CJ | 13·8 | 6·7 | 4·7 (M) | |||
| Diep et al. 32 | Retrospective case series | 4 | 77 | 0 | 77 | 90 days | ICGA | 51 | 7 (moderate–severe) | 5 (M) |
| 68 | 0 | 68 | CJ | 43 | 19 (severe) | 19 (M) | ||||
| Rinker33 | Prospective cohort study | 2 | 35 | 14 | 21 | 10 months | ICGA | – | 14 | 15 (O) |
| 34 | 10 | 24 | FA | – | 3 | 0 (O) | ||||
| 30 | 11 | 19 | CJ | – | 27 | 20 (O) | ||||
Reoperation rates are shown for overall complications (O) and mastectomy flap necrosis (M).
Number of patients.
Combined autologous/prosthetic reconstructions in a total of 91 breasts. LOE, level of evidence; ODIS, optical diffusion infrared spectroscopy; ICGA, indocyanine green angiography; CJ, clinical judgement; FA, fluorescein dye angiography.
Table 2.
Summary of all studies reporting sensitivity, specificity, positive and negative predictive values using intraoperative techniques
| Reference | No. of breasts | Type of reconstruction | Follow‐up (months) | Technique | Mastectomy flap necrosis (%) | Reoperation (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Autologous | Prosthetic | ||||||||||
| Losken et al. 39 | 50 | 31 (19*) | 0 (19*) | – | FA | 75 | 71 | 96 | 25 | ||
| Newman et al. 35 | 20 | 1 | 19 | – | ICGA | 100 | 91 | 90 | 10 | ||
| CJ | 45 | 35 | |||||||||
| Murray et al. 36 | 227 | – | – | – | ICGA | – | – | 100 | – | ||
| CJ | 4·4 | 1·8 | |||||||||
| Moyer and Losken20 | 15 | 2† (6‡) | 6† (6‡) | – | ICGA | 85 | 88 | 88 | 16 | ||
| 118† | – | – | CJ | 14 | – | ||||||
| Phillips et al. 37 | 51 | 0 | 51 | 13·3 | ICGA | 100 | 70–90¶ | ||||
| FA | 90 | 30 | 48 | 82 | |||||||
| CJ | 41 | 10 | |||||||||
| Munabi et al. 19 | 62 | 12 | 50 | 8·8 | ICGA | 38–100 | 72–83¶ | 44 | 98 | ||
| CJ | 13 | 0 | |||||||||
| Mattison et al. 38 | 55 | 0 | 55 | – | ICGA | 100 | 68 | 35 | 100 | ||
| CJ | 15 | 15 | |||||||||
All are prospective comparative cohort studies, with level of evidence 2.
Combined autologous/prosthetic reconstructions in a total of 19 breasts.
Number of patients.
Combined autologous/prosthetic reconstructions in a total of six patients.
Range of values for different absolute perfusion cut‐off values for mastectomy flap necrosis. PPV, positive predictive value; NPV, negative predictive value; FA, fluorescein dye angiography; ICGA, indocyanine green angiography; CJ, clinical judgement.
Mastectomy flap necrosis and reoperation
Mastectomy flap necrosis rates were compared with clinical judgement in seven7 28, 29, 30, 31, 32, 33 studies using ICGA, one study using FA33 and one pilot study using ODIS34. Use of ICGA in 652 and FA in 34 breasts resulted in a decrease in mean mastectomy flap necrosis compared with clinical judgement in 1964 breasts (7·9 and 3 compared with 19·4 per cent) (Table 3). Use of ODIS in five patients resulted in a mastectomy flap necrosis rate of 20 per cent34. Studies varied in their classification of skin necrosis; some did not offer a definition, whereas others30 32, 35 used a scoring system such as mild, moderate or severe, with a description of what was meant by these terms.
Table 3.
Summary of all studies reporting mastectomy flap necrosis and reoperation rates using clinical judgement compared with indocyanine green angiography and fluorescein dye angiography
| Reference | Clinical judgement | ICGA | FA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of breasts | Mastectomy flap necrosis (%) | Reoperation (%) | No. of breasts | Mastectomy flap necrosis (%) | Reoperation (%) | No. of breasts | Mastectomy flap necrosis (%) | Reoperation (%) | |
| Rinker33 | 30 | 27 | 20 | 35 | 14 | 15 | 34 | 3 | 0 |
| Diep et al. 32 | 68 | 19 | 19 | 77 | 7 | 5 | |||
| Mattison et al. 38 | 55 | 15 | 15 | ||||||
| Harless and Jacobson31 | 254 | 6·7 | 4·7 | 213 | 0·9 | 0·9 | |||
| Duggal et al. 30 | 186 | 23·4 | 14·1 | 184 | 13·0 | 5·9 | |||
| Kanuri et al. 42 | 710 | 11·1 | 11·1 | ||||||
| Munabi et al. 19 | 62 | 13 | 0 | ||||||
| Sood and Glat29 | 80 | 17 | 13 | 62 | 10 | 7 | |||
| Murray et al. 36 | 227 | 4·4 | 1·8 | ||||||
| Phillips et al. 37 | 51 | 41 | 10 | ||||||
| Moyer and Losken20 | 15 | 14 | – | ||||||
| Komorowska‐Timek and Gurtner7 | 206 | 15 | – | 24 | 4 | 4 | |||
| Newman et al. 35 | 20 | 45 | 35 | ||||||
| Jones et al. 28 | 57 | 6 | 2 | ||||||
| Total no. of breasts | 1964 | 652 | 34 | ||||||
| Mean % rate* | 19·4 | 12·9 | 7·9 | 5·5 | 3 | 0 | |||
Mean % rate was calculated by dividing the sum of % rates (to one decimal place) by the total number of studies.
ICGA, indocyanine green angiography; FA, fluorescein dye angiography.
Use of ICGA and FA led to a decrease in mean reoperation rate compared with clinical judgement (5·5 and 0 compared with 12·9 per cent) (Table 3). The use of ODIS was associated with a reoperation rate of 20 per cent34. Rate of reoperation owing to direct causes of mastectomy flap necrosis was extracted when possible, but in cases of ambiguity reoperation for overall complications was used to calculate the mean rate (Table 2).
Predictive accuracy
The sensitivity and specificity were the highest for ICGA in five studies19 20, 35 37, 38, ranging from 38 to 100 per cent and 68 to 91 per cent respectively. Two studies37 39 using FA reported a sensitivity of 75–90 per cent and specificity of 30–71 per cent. Both ICGA and FA overpredicted necrosis; one study37 reported that the overpredicted surface area was 6·57 cm2 (72 per cent) for ICGA and 18·86 cm2 (88 per cent) for FA.
It is notable that in both the studies using FA, any ambiguity in fluorescence was classified as non‐viable, and no effort was made to delineate between intermediate mottled appearances, which may explain the high rate of overprediction. This may also account for the findings of Rinker33, who compared rates of mastectomy flap necrosis using all three modalities (clinical judgement, ICGA and FA) and reported a necrosis rate of 3 per cent and reoperation rate of 0 per cent with use of FA, which was the lowest of all techniques (Table 1). If all tissues with a mottled appearance were excised as described in the former two studies37 39, a low necrosis rate is to be expected owing to over‐resection of potentially viable tissue. Indeed, the mean area of resection was larger when FA was used than with use of ICGA (6·2 versus 5·4 cm2 respectively), although the difference was not statistically significant33. In a series of 50 consecutive SSMs with autologous reconstruction39, a recommendation was therefore made to consider additional variables related to flap survival, such as surface area, previous irradiation and location of non‐fluorescence, when deciding resection margins.
Studies involving ICGA varied with respect to the device and software analysis used. Previously, only qualitative information was available through the SPY™ System software (Novadaq, Toronto, Ontario, Canada), which rendered grey‐scale images that required subjective interpretation by the surgeon similar to FA. This was used in two studies. Newman and colleagues35 reported a 95 per cent correlation between intraoperative imaging and clinical outcome, with a sensitivity of 100 per cent and a specificity of 91 per cent (Table 2). Phillips et al. 37 reported a sensitivity of 100 per cent and a specificity of 70–90 per cent, compared with 90 and 30 per cent respectively for FA, and concluded that both modalities overpredicted areas of necrosis but that ICGA had higher predictive accuracy.
With the introduction of new software (SPY‐Q™ Analysis Toolkit; Novadaq, Toronto, Ontario, Canada) that enables more objective measurement of perfusion by assigning perfusion units to grey‐scale and colour images40, studies have emerged that aimed to determine numerical threshold values that can be used to guide surgical resection. Perfusion values can be absolute or relative; absolute perfusion units (APUs) are based on the intensity of fluorescence at a given point compared with a standardized grey‐scale pixel brightness, and relative perfusion units (RPUs) are reported as a percentage of perfusion compared with a reference point that is selected by the user as an area of 100 per cent perfusion on a colourized image38 (Fig. 2). APU values also vary according to different generations of laser‐assisted ICGA equipment; Phillips and co‐workers41 found that an APU value of 3·7 on SPY® 2001 (Novadaq, Bonita Springs, Florida, USA) equated to 23·8 on SPY® Elite (Lifecell, Branchburg, New Jersey, USA) devices.
Figure 2.
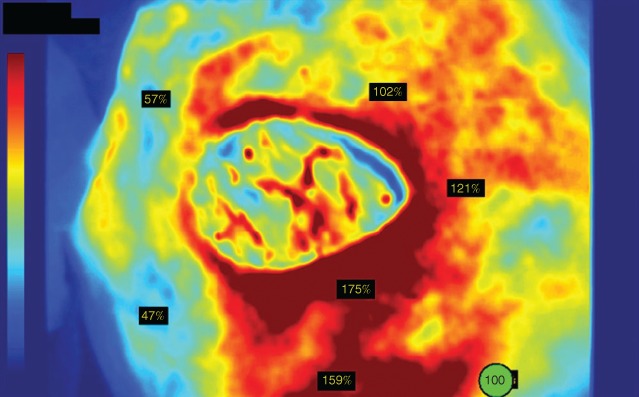
Relative perfusion units on a colourized image generated by SPY‐Q™ software (courtesy of Novadaq Technologies; http://novadaq.com/products/spy-elite/)
Five studies19 20, 37 38, 40 used SPY‐Q™ software to determine perfusion values to guide resection. There were divided opinions on whether absolute or relative perfusion units were more reliable. Phillips and colleagues37 advocated the use of APU as there was less variability between patients and the ability to extrapolate across patients. They reported that a perfusion unit threshold less than 3·7 was predictive of mastectomy flap necrosis, with 90 per cent sensitivity and 100 per cent specificity using the SPY® 2001 device. Munabi et al.19 also used APU values to determine a threshold of less than 7 to predict necrosis (sensitivity 88 per cent, specificity 83 per cent) using the SPY® Elite device. Interestingly, when they excluded patients who received adrenaline (epinephrine)‐containing tumescent solution or those with a history of smoking, a greater specificity was obtained at that perfusion level (96 per cent). Mattison and colleagues38 used contour levels to demarcate areas under chosen absolute values of 10, 15 and 20, and advised that the contour of 10 would provide the most acceptable results (sensitivity 100 per cent, specificity 68 per cent). All reported APU levels and associated predictive accuracy values are shown in Table 4, with recommended cut‐off values highlighted.
Table 4.
Absolute perfusion unit cut‐off value and associated predictive accuracy
| APU cut‐off value | Sensitivity (%) | Specificity (%) | Reference |
|---|---|---|---|
| SPY® Elite | |||
| 20 | 100 | 28 | Mattison et al.38 |
| 15 | 100 | 51 | Mattison et al.38 |
| 13 | 100 | 72 | Munabi et al.19 |
| 10* | 100 | 68 | Mattison et al.38 |
| 7* | 88 | 83 | Munabi et al.19 |
| 6 | 75 | 83 | Munabi et al.19 |
| 3 | 38 | 83 | Munabi et al 19 |
| SPY® 2001 | |||
| 8 | 100 | 70 | Phillips et al.37 |
| 3·7* | 100 | 90 | Phillips et al.37 |
APU, absolute perfusion unit.
Recommended values.
Two studies recommended use of relative perfusion values. Moyer and Losken20 found a RPU value of 25 per cent or less predicted non‐viable tissue 90 per cent of the time, whereas a value of 45 per cent or more predicted viable tissue 98 per cent of the time. Having a cut‐off value at 33 per cent gave a positive predictive value of 88 per cent and a negative predictive value of 16 per cent. Newman et al.40 reviewed 20 SPY® images, ten of which had mastectomy flap necrosis and ten with adequate healing, to correlate absolute and relative values. They found that a mean APU value of 18·5 was not statistically significant in predicting necrosis (P = 0·068), but a mean RPU of 25·2 per cent was statistically significant (P < 0·001). The authors concluded that the relative values gave more benefit, acting as an internal control for variances in patient factors. They recommended a relative value of 30 per cent or lower to be indicative of tissue ischaemia.
Secondary outcomes
Two studies carried out a cost analysis for ICGA. Duggal and co‐workers30 performed a retrospective comparison of a cohort of patients undergoing SSM and immediate reconstruction assessed using ICGA with a demographically matched historical cohort evaluated using clinical judgement. When clinical judgement was used, the total cost of complications resulting in unexpected reoperation was calculated at US $417 576·27 (€336 950·64; exchange rate 6 March 2018). This was compared with the total cost of complications and cost of equipment for ICGA, which was calculated to be $304 562·62 (€245 757·67), resulting in a total cost‐saving of $113 013·65 (€91 192·97), or $614 (€495·45) per patient. In contrast, Kanuri et al.42 claimed that an extra cost of US $1537·30 (€1240·48) would be incurred for every case of necrosis prevented if ICGA were used in all immediate reconstruction procedures. They went on to suggest that selective use in patients at high risk of skin flap necrosis would be an alternative and more cost‐effective approach. No cost analysis was performed for FA; however, a hand‐held Wood's lamp is reported to cost approximately US $300 (€242), with each vial of 10 per cent fluorescein costing less than $15 (€12)33.
Two studies recorded adverse reactions to ICGA or FA7 33. One severe allergic reaction to FA was reported33.
Discussion
Mastectomy flap necrosis is a common complication of SSM and immediate reconstruction that can result in significant morbidity, which may be reduced by intraoperative imaging. However, the value of intraoperative technology does not lie in its ability to confirm a clinically obvious outcome, but in its ability to predict a course that is not suspected, uncertain or contrary to clinical judgement20. Not only should the technique correctly predict areas of poor perfusion, but it should be able to distinguish between poorly perfused tissue that will survive and tissue that is destined for necrosis in a quantitative manner, so that the user should not need to rely on subjective parameters. Other factors to consider include ease of administration, operator dependency, side‐effect profile and cost when considering adoption of intraoperative techniques in the clinical setting.
Two main modalities were identified in this study: FA and ICGA. Fluorescein dye was first used by Ehrlich43 to assess fluid in the anterior chamber of the eye. It has since been applied in various medical situations to determine vascular perfusion of tissue44. Its value in assessing skin flap viability was first described by Myers45, who used it to predict and prevent skin slough after mastectomy. Singer et al.46 then used it to assess mastectomy skin flap viability in immediate breast reconstructions. When injected intravenously it accumulates in the extracellular compartment and emits a yellow–green fluorescence on exposure to ultraviolet light, indicating adequate (yellow) or poor (blue) perfusion37 47. Advantages of this technique are that it is readily accessible and affordable; however, the slow rate of action and long half‐life of fluorescein make it impractical for repeated imaging in the intraoperative setting7, and the qualitative nature of the data is subject to interobserver variability. Although its use has led to a decrease in mean mastectomy flap necrosis compared with clinical judgement33, the ambiguity in intermediate areas of fluorescence has the tendency to lead the operator to underpredict mastectomy flap survival, resulting in the resection of viable tissue37 39. Quantification of tissue fluorescein content by means of fibre‐optic dermofluorometry has been attempted in order to define numerical thresholds for skin flap viability48, 49, 50, 51; however, it has not been implemented further in the monitoring of mastectomy skin flaps. There is also a steep learning curve associated with use of a Wood's lamp24, and side‐effects ranging from urticarial to anaphylactic reactions have been reported33 52.
The lack of sufficient objectivity of data that FA offers for intermediately perfused skin flaps renders it useful solely for identifying clearly viable tissue, which may also be adequately assessed by clinical judgement. This and the physiological limitations of fluorescein dye, which make it inferior to ICG, a second‐generation dye, may explain why it has not been adopted widely for use in the plastic and reconstructive setting for assessment of mastectomy skin flaps53.
ICGA can be used for real‐time imaging of tissue perfusion. First used by Kogure and colleagues54 to visualize choroidal veins, it is now used to evaluate blood flow in a range of specialties including cardiology, hepatology and ophthalmology24. When ICG is injected, it binds to the plasma proteins and remains in the intravascular compartment. It emits fluorescence when excited by infrared light, to demonstrate perfusion in the dermis and superficial soft tissues to a depth of 3·6 mm, which is deeper than the superficial plexus shown by FA55. Advantages of ICG compared with fluorescein include the faster rate of action that allows quicker imaging, and the added benefit of a shorter half‐life (2·5 min), which allows multiple images to be captured in a short space of time. It also has a superior side‐effect profile24 56.
The use of ICGA to evaluate mastectomy flap viability has also resulted in a decrease in mean mastectomy flap necrosis and reoperation rates (Table 3). With the introduction of quantitative software there is the potential for more accurate designation of numerical thresholds to guide tissue resection, but at present there is still a tendency for overprediction of necrosis37. Intravascular imaging can also be affected by any source of regional vasoconstriction secondary to operative technique (such as use of adrenaline‐containing tumescent solution) or patient factors (for example smoking)26. This was reflected in the higher incidence of false‐positives found in such populations by Munabi and colleagues19. Moyer and Losken20 noted that smokers tended to require a greater percentage of perfusion (5 per cent higher) to sustain viable tissue compared with non‐smokers (P = 0·243). On the contrary, among patients with a history of hypertension and those with African American skin types flap tissue remained viable at a lower mean perfusion score (P = 0·117 and P = 0·015 respectively). These factors will require consideration when attributing perfusion thresholds as well as accounting for the variability resulting from the concentration of ICG dye, operating room temperature, mean arterial BP, systemic vascular resistance, ambient room light and interdevice variability40.
Alternative methods that have been suggested for use in predicting skin flap viability include heat‐measuring devices such as a thermometer, hydrogen electrodes, laser and colour Doppler instruments, and probes measuring tissue oxygen saturation, carbon dioxide and pH39. None of these has translated into the intraoperative setting, although ODIS (tissue oximetry) was used in a small pilot study34 to measure the ratio of oxyhaemoglobin to deoxyhaemoglobin to evaluate perfusion in five patients. One of these patients developed mastectomy flap necrosis that required reoperation. Although the authors concluded that this was a potential method for skin flap evaluation, no further study of this technique has been undertaken. Laser Doppler flowmetry has been used to evaluate the nipple–areola complex after subcutaneous reduction mammaplasties and subcutaneous mastectomies, but it did not accurately predict necrosis in all patients who experienced skin slough57. These techniques are used more commonly in the postoperative setting to monitor the anastomotic patency of free flaps, either by external and implantable Doppler monitoring or laser Doppler flowmetry58.
There was a lack of homogeneity in the reporting of mastectomy necrosis. This was echoed by Lemaine et al.59, who highlighted the lack of a standardized definition of mastectomy flap necrosis or consistency between studies, ranging from binary classifications to subjective grading systems. They suggested a scoring system (skin ischaemia necrosis (SKIN) score) which takes into account both the depth and surface area involved, and correlates the score with the need for reoperation. This may be a potentially useful tool in reporting necrosis rates, providing a means of better communication and understanding of outcomes.
Similarly, there was an inconsistency in reporting complications, and not all studies were clear in defining the reason for reoperation. As inadequate skin flap perfusion may contribute to other seemingly unrelated complications, such as delayed wound healing and infection22, consistent and systematic reporting of infection, seroma and delayed wound healing should be carried out, along with the severity of reoperation undertaken as a result of skin flap necrosis (such as simple debridement, readjustment of reconstruction or implant removal). To facilitate cost analysis, there should be reporting of prolonged hospital stay, duration of prolonged wound care owing to complications, and prolonged time to completion of breast reconstruction resulting from mastectomy flap necrosis.
This review is limited in that references of included papers were not reviewed and grey literature searches were not undertaken. The included studies were assigned a LOE adapted from the Oxford Centre for Evidence‐Based Medicine, but no other assessment of scientific quality was undertaken.
Currently, intraoperative evaluation of mastectomy flaps using ICGA provides better predictive accuracy than FA and clinical judgement, leading to reduced rates of mastectomy flap necrosis. However, owing to its tendency to overpredict areas of necrosis, leading to potential loss of viable tissue and a negative impact on reconstructive options, there is a need to establish reliable numerical thresholds to guide surgical resection. There is heterogeneity in current studies regarding the device and software being used, and a lack of consensus on whether absolute or relative perfusion should be used as the reference unit. Appropriately powered prospective studies should be set up to evaluate predictive accuracies using standardized operating protocols and patient inclusion and exclusion criteria.
It has been suggested that intraoperative modalities should be used selectively for patients at high risk of mastectomy flap necrosis to facilitate cost‐effectiveness42. Patients with pigmentation or skin discolouration that poses a challenge for clinical judgement may also benefit from use of intraoperative imaging modalities26.
Supporting information
Table S1 Boolean Literature Search Strategy [MEDLINE]
Table S2 Boolean Literature Search Strategy [EMBASE]
Acknowledgements
D.G. and A.M. are joint senior authors of this article.
Disclosure: The authors declare no conflict of interest.
Funding information No funding
References
- 1. Toth B, Lappert P. Modified skin incisions for mastectomy: the need for plastic surgical input in preoperative planning. Plast Reconstr Surg 1991; 87: 1048–1053. [PubMed] [Google Scholar]
- 2. Agrawal A, Sibbering D, Courtney C. Skin sparing mastectomy and immediate breast reconstruction: a review. Eur J Surg Oncol 2013; 39: 320–328. [DOI] [PubMed] [Google Scholar]
- 3. Patani N, Mokbel K. Oncological and aesthetic considerations of skin‐sparing mastectomy. Breast Cancer Res Treat 2008; 111: 391–403. [DOI] [PubMed] [Google Scholar]
- 4. Garwood ER, Moore D, Ewing C, Hwang ES, Alvarado M, Foster R et al Total skin‐sparing mastectomy: complications and local recurrence rates in 2 cohorts of patients. Ann Surg 2009; 249: 26–32. [DOI] [PubMed] [Google Scholar]
- 5. Robertson SA, Rusby JE, Cutress RI. Determinants of optimal mastectomy skin flap thickness. Br J Surg 2014; 101: 899–911. [DOI] [PubMed] [Google Scholar]
- 6. Beer G, Varga Z, Budi S, Seifert B, Meyer V. Incidence of the superficial fascia and its relevance in skin‐sparing mastectomy. Cancer 2002; 94: 1619–1625. [DOI] [PubMed] [Google Scholar]
- 7. Komorowska‐Timek E, Gurtner G. Intraoperative perfusion mapping with laser‐assisted indocyanine green imaging can predict and prevent complications in immediate breast reconstruction. Plast Reconstr Surg 2010; 125: 1065–1073. [DOI] [PubMed] [Google Scholar]
- 8. Padubidri AN, Yetman R, Browne E, Lucas A, Papay F, Larive B et al Complications of postmastectomy breast reconstructions in smokers, ex‐smokers, and nonsmokers. Plast Reconstr Surg 2001; 107: 342–349. [DOI] [PubMed] [Google Scholar]
- 9. Mlodinow AS, Fine NA, Khavanin N, Kim JY. Risk factors for mastectomy flap necrosis following immediate tissue expander breast reconstruction. J Plast Surg Hand Surg 2014; 48: 322–326. [DOI] [PubMed] [Google Scholar]
- 10. Vargas CR, Koolen PG, Anderson KE, Paul MA, Tobias AM, Lin SJ et al Mastectomy skin necrosis after microsurgical breast reconstruction. J Surg Res 2015; 198: 530–534. [DOI] [PubMed] [Google Scholar]
- 11. Matsen CB, Mehrara B, Eaton A, Capko D, Berg A, Stempel M et al Skin flap necrosis after mastectomy with reconstruction: a prospective study. Ann Surg Oncol 2015; 23: 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abedi N, Ho AL, Knox A, Tashakkor Y, Omeis T, Van Laeken N et al Predictors of mastectomy flap necrosis in patients undergoing immediate breast reconstruction: a review of 718 patients. Ann Plast Surg 2016; 76: 629–634. [DOI] [PubMed] [Google Scholar]
- 13. Di Candia M , Lie KH, Forouhi P, Malata CM. Experience with the wise mammaplasty skin resection pattern in skin‐sparing mastectomy and immediate breast reconstruction for large breast volumes. Int J Surg 2011; 9: 41–45. [DOI] [PubMed] [Google Scholar]
- 14. Khansa I, Colakoglu S, Curtis MS, Yueh JH, Ogunleye A, Tobias AM et al Postmastectomy breast reconstruction after previous lumpectomy and radiation therapy: analysis of complications and satisfaction. Ann Plast Surg 2011; 66: 444–451. [DOI] [PubMed] [Google Scholar]
- 15. Chun YS, Verma K, Rosen H, Lipsitz SR, Breuing K, Guo L et al Use of tumescent mastectomy technique as a risk factor for native breast skin flap necrosis following immediate breast reconstruction. Am J Surg 2011; 201: 160–165. [DOI] [PubMed] [Google Scholar]
- 16. Davies K, Allan L, Roblin P, Ross D, Farhadi J. Factors affecting post‐operative complications following skin sparing mastectomy with immediate breast reconstruction. Breast 2011; 20: 21–25. [DOI] [PubMed] [Google Scholar]
- 17. Robertson SA, Jeevaratnam JA, Agrawal A, Cutress RI. Mastectomy skin flap necrosis: challenges and solutions. Breast Cancer (Dove Med Press) 2017; 9: 141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jallali N, Ridha H, Butler PE. Postoperative monitoring of free flaps in UK plastic surgery units. Microsurgery 2005; 25: 469–472. [DOI] [PubMed] [Google Scholar]
- 19. Munabi NC, Olorunnipa OB, Goltsman D, Rohde CH, Ascherman JA. The ability of intra‐operative perfusion mapping with laser‐assisted indocyanine green angiography to predict mastectomy flap necrosis in breast reconstruction: a prospective trial. J Plast Reconstr Aesthet Surg 2014; 67: 449–455. [DOI] [PubMed] [Google Scholar]
- 20. Moyer H, Losken A. Predicting mastectomy skin flap necrosis with indocyanine green angiography: the gray area defined. Plast Reconstr Surg 2012; 129: 1043–1048. [DOI] [PubMed] [Google Scholar]
- 21. Carlson GW. Technical advances in skin sparing mastectomy. Int J Surg Oncol 2011; 2011: 396901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khammash MR, Obeidat KA. Prevalence of ischemia in diabetic foot infection. World J Surg 2003; 27: 797–799. [DOI] [PubMed] [Google Scholar]
- 23. Wu C, Kim S, Halvorson EG. Laser‐assisted indocyanine green angiography: a critical appraisal. Ann Plast Surg 2013; 70: 613–619. [DOI] [PubMed] [Google Scholar]
- 24. Griffiths M, Chae MP, Rozen WM. Indocyanine green‐based fluorescent angiography in breast reconstruction. Gland Surg 2016; 5: 133–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chae MP, Hunter‐Smith DJ, Rozen WM. Comparative analysis of fluorescent angiography, computed tomographic angiography and magnetic resonance angiography for planning autologous breast reconstruction. Gland Surg 2015; 4: 164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gurtner GC, Jones GE, Neligan PC, Newman MI, Phillips BT, Sacks JM et al Intraoperative laser angiography using the SPY system: review of the literature and recommendations for use. Ann Surg Innov Res 2013; 7: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chatterjee A, Krishnan NM, Van Vliet MM, Powell SG, Rosen JM, Ridgway EB. A comparison of free autologous breast reconstruction with and without the use of laser‐assisted indocyanine green angiography: a cost‐effectiveness analysis. Plast Reconstr Surg 2013; 131: 693e–701e. [DOI] [PubMed] [Google Scholar]
- 28. Jones GE, Garcia CA, Murray J, Elwood ET, Whitty LA. Fluorescent intraoperative tissue angiography for the evaluation of the viability of pedicled TRAM flaps. Plast Reconstr Surg 2009; 124: 53. [DOI] [PubMed] [Google Scholar]
- 29. Sood M, Glat P. Potential of the SPY intraoperative perfusion assessment system to reduce ischemic complications in immediate postmastectomy breast reconstruction. Ann Surg Innov Res 2013; 7: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Duggal CS, Madni T, Losken A. An outcome analysis of intraoperative angiography for postmastectomy breast reconstruction. Aesthet Surg J 2014; 34: 61–65. [DOI] [PubMed] [Google Scholar]
- 31. Harless CA, Jacobson SR. Tailoring through technology: a retrospective review of a single surgeon's experience with implant‐based breast reconstruction before and after implementation of laser‐assisted indocyanine green angiography. Breast J 2016; 22: 274–281. [DOI] [PubMed] [Google Scholar]
- 32. Diep GK, Hui JY, Marmor S, Cunningham BL, Choudry U, Portschy PR et al Postmastectomy reconstruction outcomes after intraoperative evaluation with indocyanine green angiography versus clinical assessment. Ann Surg Oncol 2016; 23: 4080–4085. [DOI] [PubMed] [Google Scholar]
- 33. Rinker B. A comparison of methods to assess mastectomy flap viability in skin‐sparing mastectomy and immediate reconstruction: a prospective cohort study. Plast Reconstr Surg 2016; 137: 395–401. [DOI] [PubMed] [Google Scholar]
- 34. Rao R, Saint‐Cyr M, Ma AM, Bowling M, Hatef DA, Andrews V et al Prediction of post‐operative necrosis after mastectomy: a pilot study utilizing optical diffusion imaging spectroscopy. World J Surg Oncol 2009; 7: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Newman MI, Samson MC, Tamburrino JF, Swartz KA. Intraoperative laser‐assisted indocyanine green angiography for the evaluation of mastectomy flaps in immediate breast reconstruction. J Reconstr Microsurg 2010; 26: 487–492. [DOI] [PubMed] [Google Scholar]
- 36. Murray JD, Jones GE, Elwood ET, Whitty LA, Garcia C. Laser angiography as a predictor of mastectomy flap necrosis after breast reconstruction. Plast Reconstr Surg 2012; 129: 1017e–1018e. [DOI] [PubMed] [Google Scholar]
- 37. Phillips BT, Lanier ST, Conkling N, Wang ED, Dagum AB, Ganz JC et al Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: results of a prospective trial. Plast Reconstr Surg 2012; 129: 778e–788e. [DOI] [PubMed] [Google Scholar]
- 38. Mattison GL, Lewis PG, Gupta SC, Kim HY. SPY imaging use in postmastectomy breast reconstruction patients: preventative or overly conservative. Plast Reconstr Surg 2016; 138: 15e–21e. [DOI] [PubMed] [Google Scholar]
- 39. Losken A, Styblo T, Schaefer T, Carlson G. The use of fluorescein dye as a predictor of mastectomy skin flap viability following autologous tissue reconstruction. Ann Plast Surg 2008; 61: 24–29. [DOI] [PubMed] [Google Scholar]
- 40. Newman MI, Jack MC, Samson MC. SPY‐Q analysis toolkit values potentially predict mastectomy flap necrosis. Ann Plast Surg 2013; 70: 595–598. [DOI] [PubMed] [Google Scholar]
- 41. Phillips BT, Fourman MS, Rivara A, Dagum AB, Huston TL, Ganz JC et al Comparing quantitative values of two generations of laser‐assisted indocyanine green dye angiography systems: can we predict necrosis? Eplasty 2014; 14: e44. [PMC free article] [PubMed] [Google Scholar]
- 42. Kanuri A, Liu AS, Guo L. Whom should we SPY? A cost analysis of laser‐assisted indocyanine green angiography in prevention of mastectomy skin flap necrosis during prosthesis‐based breast reconstruction. Plast Reconstr Surg 2014; 133: 448e–454e. [DOI] [PubMed] [Google Scholar]
- 43. Ehrlich P. Uber provozierte Fluorescenz‐Erscheinungen am Auge. Deutsch Med Wchschr 1882; 8: 35–37. [Google Scholar]
- 44. Lange K, Boyd L. The use of fluorescein to determine the adequacy of the circulation. Am Heart J 1942; 24: 866. [Google Scholar]
- 45. Myers MB. Prediction of skin sloughs at the time of operation with the use of fluorescein dye. Surgery 1962; 51: 158–162. [PubMed] [Google Scholar]
- 46. Singer R, Lewis CM, Franklin JD, Lynch JB. Fluorescein test for prediction of flap viability during breast reconstruction. Plast Reconstr Surg 1978; 61: 371–375. [DOI] [PubMed] [Google Scholar]
- 47. O'goshi K, Serup J. Safety of sodium fluorescein for in vivo study of skin. Skin Res Technol 2006; 12: 155–161. [DOI] [PubMed] [Google Scholar]
- 48. Silverman DG, Norton KJ, Brousseau DA. Serial fluorometric documentation of fluorescein dye delivery. Surgery 1985; 97: 185–193. [PubMed] [Google Scholar]
- 49. Kreidstein ML, Levine RH, Knowlton RJ, Pang CY. Serial fluorometric assessments of skin perfusion in isolated perfused human skin flaps. Br J Plast Surg 1995; 48: 288–293. [DOI] [PubMed] [Google Scholar]
- 50. Graham BH, Walton RL, Elings VB, Lewis FR. Surface quantification of injected fluorescein as a predictor of flap viability. Plast Reconstr Surg 1983; 71: 829–831. [DOI] [PubMed] [Google Scholar]
- 51. Thomson JG, Kerrigan CL. Dermofluorometry: thresholds for predicting flap survival. Plast Reconstr Surg 1989; 83: 859–865. [PubMed] [Google Scholar]
- 52. Johnson RN, McDonald HR, Schatz H. Rash, fever, and chills after intravenous fluorescein angiography. Am J Ophthalmol 1998; 126: 837–838. [DOI] [PubMed] [Google Scholar]
- 53. Holm C, Mayr M, Höfter E, Becker A, Pfeiffer UJ, Mühlbauer W. Intraoperative evaluation of skin‐flap viability using laser‐induced fluorescence of indocyanine green. Br J Plast Surg 2002; 55: 635–644. [DOI] [PubMed] [Google Scholar]
- 54. Kogure K, David NJ, Yamanouchi U, Choromokos E. Infrared absorption angiography of the fundus circulation. Arch Ophthalmol 1970; 83: 209–214. [DOI] [PubMed] [Google Scholar]
- 55. Rübben A, Eren S, Krein R, Younossi H, Böhler U, Wienert V. Infrared videoangiofluorography of the skin with indocyanine green – rat random cutaneous flap model and results in man. Microvasc Res 1994; 47: 240–251. [DOI] [PubMed] [Google Scholar]
- 56. Meijer DK, Weert B, Vermeer GA. Pharmacokinetics of biliary excretion in man. VI. Indocyanine green. Eur J Clin Pharmacol 1988; 35: 295–303. [DOI] [PubMed] [Google Scholar]
- 57. Perbeck L, Proano E, Westerberg L. The circulation in the nipple–areola complex following subcutaneous mastectomy in breast cancer. Scand J Plast Reconstr Surg Hand Surg 1992; 26: 217–221. [DOI] [PubMed] [Google Scholar]
- 58. Keller A. Noninvasive tissue oximetry for flap monitoring: an initial study. J Reconstr Microsurg 2007; 23: 189–197. [DOI] [PubMed] [Google Scholar]
- 59. Lemaine V, Hoskin TL, Farley DR, Grant CS, Boughey JC, Torstenson TA et al Introducing the SKIN score: a validated scoring system to assess severity of mastectomy skin flap necrosis. Ann Surg Oncol 2015; 22: 2925–2932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Boolean Literature Search Strategy [MEDLINE]
Table S2 Boolean Literature Search Strategy [EMBASE]


