Abstract
Background
Previous analyses of the oesophageal circumferential resection margin (CRM) have focused on the prognostic validity of two different definitions of a positive CRM, that of the College of American Pathologists (tumour at margin) and that of the Royal College of Pathologists (tumour within 1 mm). This study aimed to analyse the validity of these definitions and explore the risk of recurrence and survival with incremental tumour distances from the CRM.
Methods
This cohort study included patients who underwent resection for adenocarcinoma of the oesophagus between 2000 and 2014. Kaplan–Meier and Cox regression analyses were performed to determine the hazard ratio (HR) with 95 per cent confidence intervals for recurrence and mortality in CRM increments: tumour at the cut margin, extending to within 0·1–0·9, 1·0–1·9, 2·0–4·9 mm, and 5·0 mm or more from the margin.
Results
A total of 444 patients were included in the study. Kaplan–Meier and unadjusted analyses showed a significant incremental improvement in overall survival (P < 0·001) and recurrence (P for trend < 0·001) rates with increasing distance from the CRM. Tumour distance of 2·0 mm or more remained a significant predictor of survival on multivariable analysis (HR for risk of death 0·66, 95 per cent c.i. 0·44 to 1·00). Multivariable analysis of overall survival demonstrated a significant difference between a positive and negative CRM with the Royal College of Pathologists' definition (HR 1·37, 1·01 to 1·85), but not with the College of American Pathologists' definition (HR 1·22, 0·90 to 1·65).
Conclusion
This study demonstrated an incremental improvement in survival and recurrence rates with increasing tumour distance from the CRM.
Introduction
The introduction of neoadjuvant treatment has increased the survival of patients undergoing surgery for oesophageal cancer1, 2, 3. Despite this, 5‐year survival rates following resection rarely exceed 50 per cent, and recurrence rates are still disappointingly high. Understanding how various clinicopathological factors influence survival and patterns of recurrence may be important in guiding future tailored treatment strategies.
Many studies have examined the prognostic significance of the two most commonly used definitions of tumour‐involved circumferential resection margin (CRM). The College of American Pathologists (CAP) defines a positive CRM as tumour at the cut margin (TAM), which has been advocated in some studies4, 5, 6, 7, 8, 9, whereas the Royal College of Pathologists (RCP) defines a positive CRM as tumour within 1 mm, preferred in other studies10, 11, 12, 13. Some studies have found the CRM to be independently prognostically significant4 7, 9 10, 13, 14, 15, 16, and others have not5 6, 11 12, 17, 18, 19. A positive margin may increase the likelihood of locoregional and systemic tumour recurrence9 20, although it is unclear whether the latter is simply a reflection of a larger, more advanced tumour.
The relationship between margin status and nodal status is already recognized as important. A study in 200615 found that a positive CRM had greater prognostic significance in the presence of fewer nodal metastases. The presence of positive lymph nodes is known to confer a significantly worse prognosis21, so it may be that any independent prognostic significance of a positive CRM would be overshadowed by the presence of nodal disease.
This study aimed to evaluate the prognostic role of the two existing definitions of a positive CRM on overall survival and tumour recurrence in patients with oesophageal adenocarcinoma, and examine the influence of incremental increases in margin clearance on these outcomes, considering confounding factors such as nodal status.
Methods
This was a cohort study using the database of consecutive resections performed at Guy's and St Thomas' Oesophago‐Gastric Centre, London, UK. The initial study cohort involved all patients who underwent oesophagectomy between 2000 and 2014. Only patients with adenocarcinoma who had undergone potentially curative oesophagectomy were included in the overall analysis. Patients with all other pathologies were excluded. The study exposure was CRM distance. For the analysis of incremental CRM distance, patients with a reported negative margin (no tumour within 1 mm) but with no documented CRM distance in millimetres, and patients who had a complete pathological response to chemotherapy, were excluded.
The primary outcome measures were overall all‐cause and disease‐specific mortality. Secondary outcomes were any recurrence, locoregional recurrence and systemic recurrence. Locoregional recurrence was defined as recurrent disease seen within the primary surgical or radiotherapy fields.
Patients underwent a standard protocol of investigation including oesophagogastroduodenoscopy, CT, endoscopic ultrasonography and fluorodeoxyglucose (FDG)‐PET. Neoadjuvant chemotherapy practice evolved during the study period, and followed standard indications and regimens as supported by RCT evidence3. The small number of patients who received neoadjuvant chemoradiotherapy were excluded. Patients judged to have an imaging status of T2 or above and/or lymph node positivity were considered for neoadjuvant chemotherapy if fit.
Transthoracic oesophagectomy included both Ivor Lewis and left thoracoabdominal approaches with two‐field lymphadenectomy22, 23, 24. Transhiatal oesophagectomy was performed in patients with lower oesophageal tumours in whom dissection of the primary could be achieved under direct vision from the abdomen along with an abdominal and lower mediastinal lymphadenectomy. Histological staging was standardized to meet the AJCC seventh edition TNM criteria. Pathological specimens were processed and reported using the RCP guidelines. The use of adjuvant chemotherapy or chemoradiotherapy was determined by multidisciplinary team consensus based on the positivity of resection margins, pathological nodal status and postoperative performance status of the patient.
Statistical analysis
For overall all‐cause mortality, duration of follow‐up was defined as the time from surgery to the date of death or last follow‐up. For disease‐specific mortality, length of follow‐up was defined as the time from surgery to the date of recurrence or last date of follow‐up if disease‐free.
Kaplan–Meier curves were used to investigate crude differences in survival across different categories of CRM (TAM, 0·1–0·9, 1·0–1·9, 2·0–4·9 and 5·0 mm or more), which were tested formally using the log rank test. Analyses were stratified by: T category (T1–2 versus T3–4), neoadjuvant chemotherapy versus surgery alone, pathological lymph node involvement versus no such involvement, and presence of lymphovascular invasion versus no lymphovascular invasion. Cox regression was employed to obtain hazard ratios (HRs) and 95 per cent confidence intervals for time to death or recurrence based on categories of CRM. TAM was used as the reference category. Additional multivariable analysis was performed looking at TAM (reference category), 0·1–0·9, 1·0–1·9 and 2·0 mm or more to examine 1·0–1·9 mm as a possible ‘at risk’ group.
Further analysis compared the CAP and RCP definitions of CRM positivity with their corresponding negative groups used as the reference category to determine significance as an independent prognostic variable. In addition, a test for trend used CRM categories as a continuous variable. Findings were considered significant at P < 0·050 for both univariable and multivariable analyses.
The following clinicopathological parameters were adjusted for in the multivariable analyses: sex (male or female), age (continuous), preoperative stenting (yes or no), neoadjuvant chemotherapy (yes or no), type of surgery (transhiatal or transthoracic), lymphovascular invasion (yes or no), pT category (pT1–2 N0, pT1–2 N1, pT1–2 N2–3, pT3–4 N0, pT3–4 N1 or pT3–4 N2–3), pathological grade (poorly, moderately or well differentiated), Mandard tumour regression score (2–3, 4–5 or not applicable) and adjuvant treatment (none, chemotherapy or chemoradiotherapy). All analyses were conducted in R statistical software version 3·2.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The original cohort included 578 patients with oesophageal adenocarcinoma. The CRM positivity rate was 41·3 per cent (239 of 578) using RCP criteria, compared with 18·0 per cent (104 of 578) with CAP criteria. Rates of margin positivity reduced over time, and were 33·0 per cent (RCP) and 11·3 per cent (CAP) in the latter half of the study.
For the univariable and multivariable analyses of CRM distance, 22 patients with a complete pathological response to chemotherapy and 112 patients with reported negative margins (no tumour within 1 mm) but no CRM distance documented in millimetres were excluded. Histology specimens for this latter group were not available for retrospective histological analysis. Some 444 patients remained for final analysis, resulting in 104 patients in the TAM group, 135 in the 0·1–0·9‐mm group, 46 in the 1–1·9‐mm group, 64 in the 2·0–4·9‐mm group and 95 in the 5·0 mm or above group.
Patient characteristics are shown in Table 1. CRM‐positive patients (CAP and RCP) had a higher rate of other adverse prognostic factors, such as T3–4 status, poor differentiation, Mandard score 4 and 5, nodal disease and lymphovascular invasion (Table 1).
Table 1.
Clinicopathological characteristics of patients with positive and negative resection margins, and according to resection margin increments
| Established margin definition | Specific margin difference (mm) | ||||||
|---|---|---|---|---|---|---|---|
| CAP CRM‐positive (TAM) (n = 104) | RCP CRM‐positive (< 1·0 mm) (n = 239) | CRM‐negative (≥ 1·0 mm) (n = 205) | 0·1–0·99 (n = 135) | 1–2 (n = 46) | 2–5 (n = 64) | > 5 (n = 95) | |
| Mean(s.d.) age at operation (years) | 63·06(10·57) | 62·17(9·58) | 61·49(10·57) | 61·49(8·72) | 62·60(9·12) | 63·64(10·49) | 62·58(10·49) |
| Sex ratio (M : F) | 84 : 20 | 196 : 43 | 185 : 20 | 113 : 22 | 40 : 6 | 56 : 8 | 89 : 6 |
| Tumour location | |||||||
| Siewert type 1 | 53 (51·0) | 111 (46·4) | 119 (58·0) | 58 (43·0) | 29 (63) | 31 (48) | 59 (62) |
| Siewert type 2 | 49 (47·1) | 121 (50·6) | 69 (33·7) | 72 (53·3) | 14 (30) | 27 (42) | 28 (29) |
| Lower oesophagus | 2 (1·9) | 7 (2·9) | 17 (8·3) | 5 (3·7) | 3 (7) | 6 (9) | 8 (8) |
| Neoadjuvant chemotherapy | |||||||
| Yes | 76 (73·1) | 191 (79·9) | 150 (73·2) | 115 (85·2) | 41 (89) | 52 (81) | 57 (60) |
| No | 28 (26·9) | 48 (20·1) | 55 (26·8) | 20 (14·8) | 5 (11) | 12 (19) | 38 (40) |
| Type of surgery | |||||||
| TTO | 51 (49·0) | 135 (56·5) | 90 (43·9) | 82 (60·7) | 23 (50) | 24 (38) | 43 (45) |
| THO | 53 (51·0) | 104 (43·5) | 115 (56·1) | 53 (39·3) | 23 (50) | 40 (63) | 52 (55) |
| Pathological stage | |||||||
| pT1–2 N− | 3 (2·9) | 11 (4·6) | 82 (40·0) | 8 (5·9) | 7 (15) | 19 (30) | 56 (59) |
| pT1–2 N+ | 6 (5·8) | 39 (16·3) | 47 (22·9) | 33 (24·4) | 6 (13) | 16 (25) | 25 (26) |
| pT3–4 N− | 18 (17·3) | 32 (13·4) | 33 (16·1) | 14 (10·4) | 10 (22) | 14 (22) | 9 (9) |
| pT3–4 N+ | 77 (74·0) | 157 (65·7) | 43 (21·0) | 80 (59·3) | 23 (50) | 15 (23) | 5 (5) |
| Pathological grade | |||||||
| Poorly differentiated | 58 (55·8) | 124 (51·9) | 64 (31·2) | 66 (48·9) | 12 (26) | 23 (36) | 29 (31) |
| Moderately differentiated | 43 (41·3) | 109 (45·6) | 134 (65·4) | 66 (48·9) | 34 (74) | 40 (63) | 60 (63) |
| Well differentiated | 4 (3·8) | 6 (2·5) | 7 (3·4) | 3 (2·2) | 0 (0) | 1 (1) | 6 (6) |
| Lymphovascular invasion | |||||||
| Yes | 75 (72·1) | 172 (72·0) | 80 (39·0) | 97 (71·9) | 26 (57) | 33 (52) | 21 (22) |
| No | 29 (27·9) | 67 (28·0) | 125 (61·0) | 38 (28·1) | 20 (43) | 31 (48) | 74 (78) |
| Mandard score | |||||||
| 2–3 (good or partial response) | 10 (9·6) | 43 (18·0) | 77 (37·6) | 33 (24·4) | 16 (35) | 23 (36) | 37 (39) |
| 4–5 (poor or no response) | 58 (55·8) | 138 (57·7) | 71 (34·6) | 80 (59·3) | 22 (48) | 29 (45) | 20 (21) |
| No chemotherapy | 28 (26·9) | 48 (20·1) | 55 (26·8) | 20 (14·8) | 5 (11) | 12 (19) | 38 (40) |
| Not recorded | 8 (7·7) | 10 (4·2) | 2 (1·0) | 2 (1·5) | 3 (7) | 0 (0) | 0 (0) |
| Adjuvant treatment | |||||||
| None/not tolerated | 35 (33·7) | 82 (34·3) | 117 (57·1) | 47 (34·8) | 23 (50) | 33 (52) | 61 (64) |
| Adjuvant chemotherapy | 18 (17·3) | 69 (28·9) | 78 (38·0) | 51 (37·8) | 20 (43) | 27 (42) | 31 (33) |
| Adjuvant CRT | 51 (49·0) | 88 (36·8) | 10 (4·9) | 37 (27·4) | 3 (7) | 4 (6) | 3 (3) |
| Recurrence | |||||||
| None | 35 (33·7) | 92 (38·5) | 125 (61·0) | 57 (42·2) | 21 (46) | 41 (64) | 63 (66) |
| Any | 69 (66·3) | 147 (61·5) | 80 (39·0) | 78 (57·8) | 25 (54) | 23 (36) | 32 (34) |
| Local | 32 (30·8) | 75 (31·4) | 47 (22·9) | 43 (31·9) | 15 (33) | 11 (17) | 21 (22) |
| Systemic | 55 (52·9) | 117 (49·0) | 59 (28·8) | 62 (45·9) | 18 (39) | 17 (27) | 24 (25) |
Values in parentheses are percentages unless indicated otherwise. CAP, College of American Pathologists; CRM, circumferential resection margin; TAM, tumour at the cut margin; RCP, Royal College of Pathologists; TTO, transthoracic oesophagectomy; THO, transhiatal oesophagectomy; CRT, chemoradiotherapy.
Mortality
Kaplan–Meier analysis of overall survival in all patients demonstrated a survival advantage as tumour distance increased from the margin (P < 0·001) (Fig. 1). In unadjusted analysis, the overall all‐cause mortality decreased in increments away from the margin, with a significant improvement in survival between TAM and 0·1–0·9 mm (HR 0·71, 95 per cent c.i. 0·53 to 0·95), TAM and 1·0–1·9 mm (HR 0·47, 0·30 to 0·72), TAM and 2·0–4·9 mm (HR 0·34, 0·22 to 0·52) and TAM and 5·0 mm or more (HR 0·24, 0·17 to 0·36). This trend remained significant in multivariable analysis (P for trend < 0·050), although each category in isolation did not (Table 2).
Figure 1.

Kaplan–Meier curves of overall survival in patients who underwent resection of oesophageal adenocarcinoma, according to distance from the circumferential resection margin: tumour at the cut margin (TAM), 0·1–0·9‐mm, 1·0–1·9‐mm, 2·0–4·9‐mm and 5·0 mm and above groups. P < 0·001 (log rank test)
Table 2.
Unadjusted and multivariable Cox regression survival and recurrence analyses for the five groups with increasing distance from the resection margin
| Overall survival | Time to local recurrence | Time to systemic recurrence | ||||
|---|---|---|---|---|---|---|
| Unadjusted | Multivariable | Unadjusted | Multivariable | Unadjusted | Multivariable | |
| CRM distance (mm) | ||||||
| TAM | 1·00 (reference) | 1·00 (reference) | 1·00 (reference) | 1·00 (reference) | 1·00 (reference) | 1·00 (reference) |
| 0·1–0·9 | 0·71 (0·53, 0·95) | 0·89 (0·65, 1·23) | 0·95 (0·60, 1·50) | 1·03 (0·62, 1·71) | 0·80 (0·56, 1·15) | 1·05 (0·71, 1·56) |
| 1·0–1·9 | 0·47 (0·30, 0·72) | 0·66 (0·44, 1·16) | 0·80 (0·44, 1·49) | 1·08 (0·54, 2·15) | 0·56 (0·33, 0·96) | 0·87 (0·49, 1·56) |
| 2·0–4·9 | 0·34 (0·22, 0·52) | 0·66 (0·41, 1·05) | 0·37 (0·18, 0·73) | 0·71 (0·33, 1·53) | 0·34 (0·20, 0·59) | 0·70 (0·38, 1·29) |
| ≥ 5·0 | 0·24 (0·17, 0·36) | 0·67 (0·41, 1·09) | 0·35 (0·20, 0·61) | 1·20 (0·58, 2·47) | 0·25 (0·15, 0·41) | 0·77 (0·41, 1·44) |
| P for trend | < 0·001 | 0·048 | < 0·001 | 0·985 | < 0·001 | 0·204 |
Values in parentheses are 95 per cent confidence intervals. CRM, circumferential resection margin; TAM, tumour at the cut edge.
Recurrence
The local recurrence rate was 27·5 per cent (122 of 444): 30·8 per cent (32 of 104) in the TAM group, 31·9 per cent (43 of 135) in the 0·1–0·9‐mm group, 33 per cent (15 of 46) in the 1·0–1·9‐mm group, 17 per cent (11 of 64) in the 2·0–4·9‐mm group and 22 per cent (21 of 95) in the 5·0 mm or above group (Table 1). In unadjusted analysis, time to locoregional recurrence improved significantly only in the 2·0–4·9‐mm (HR 0·37, 95 per cent c.i. 0·18 to 0·73) and 5·0 mm or above (HR 0·35, 0·20 to 0·61) groups (Table 2). This difference was not significant in multivariable analysis.
The systemic recurrence rate was 39·6 per cent (176 of 444): 52·9 per cent (55 of 104) in the TAM group, 45·9 per cent (62 of 135) in the 0·1–0·9‐mm group, 39 per cent (18 of 46) in the 1·0–1·9‐mm group, 27 per cent (17 of 64) in the 2·0–4·9‐mm group and 25 per cent (24 of 95) in the 5·0 mm or above group. In unadjusted analysis, time to systemic recurrence improved significantly in the 1·0–1·9‐mm (HR 0·56, 95 per cent c.i. 0·33 to 0·96), 2·0–4·9‐mm (HR 0·34, 0·20 to 0·59) and the 5·0 mm or above (HR 0·25, 0·15 to 0·41) groups (Table 2). This difference was not significant in multivariable analysis.
Margin definition: RCP versus CAP
Multivariable analysis of overall survival demonstrated a significant difference between a positive CRM and a negative CRM only with the RCP definition (RCP: HR 1·37, 95 per cent c.i. 1·01 to 1·85; CAP: HR 1·22, 0·90 to 1·65).
Additional high‐risk group (1·0–1·9 mm)
Further analysis was performed to determine whether a 1·0–1·9‐mm CRM indicated a further ‘at risk group’ in addition to current definitions of a positive CRM. Regression analysis combining the 2·0–4·9 and 5·0 mm or above groups (2·0 mm or above group) indicated a significant difference in both overall and disease‐free survival when TAM, 0·1–0·9‐mm, 1·0–1·9‐mm and 2·0 mm or above groups were compared (Table 3). In multivariable analysis, the risk of death was significantly reduced when tumour was detected 2·0 mm or more from the cut margin versus TAM (HR 0·66, 95 per cent c.i. 0·44 to 1·00) (Table 3).
Table 3.
Unadjusted and multivariable Cox regression survival and recurrence analysis for the four groups with increasing distance from the resection margin
| Overall survival | ||
|---|---|---|
| Unadjusted | Multivariable | |
| CRM distance (mm) | ||
| TAM | 1·00 (reference) | 1·00 (reference) |
| 0·1–0·9 | 0·71 (0·53, 0·95) | 0·89 (0·65, 1·23) |
| 1·0–1·9 | 0·47 (0·30, 0·72) | 0·66 (0·44, 1·16) |
| ≥ 2·0 | 0·28 (0·20, 0·38) | 0·66 (0·44, 1·00) |
| P for trend | < 0·001 | 0·045 |
Values in parentheses are 95 per cent confidence intervals. CRM, circumferential resection margin; TAM, tumour at the cut edge.
Kaplan–Meier analysis of locoregional and systemic recurrence demonstrated a significant disease‐free survival benefit for a CRM of 2·0 mm or more when TAM, 0·1–0·9‐mm, 1·0–1·9‐mm and 2·0 mm or above groups were compared (Figs 2 and 3). The locoregional recurrence rate was lower in the 2·0 mm or above group at 20·8 per cent, compared with 31·6 per cent in the TAM group).
Figure 2.
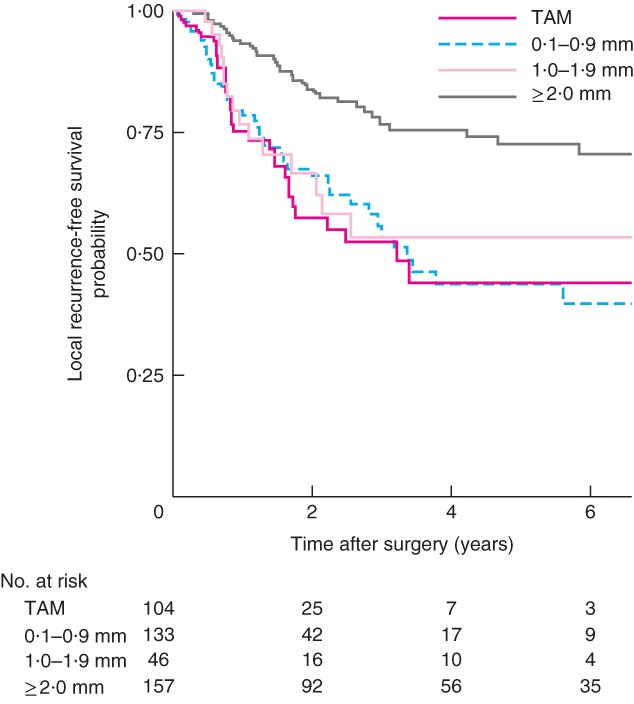
Kaplan–Meier curves of local recurrence‐free survival in patients who underwent resection of oesophageal adenocarcinoma, according to distance from the circumferential resection margin: tumour at the cut margin (TAM), 0·1–0·9‐mm, 1·0–1·9‐mm and 2·0 mm and above groups. P = 0·013 (2·0 mm and above versus TAM, log rank test)
Figure 3.

Kaplan–Meier curves of systemic recurrence‐free survival in patients who underwent resection of oesophageal adenocarcinoma, according to distance from the circumferential resection margin: tumour at the cut margin (TAM), 0·1–0·9‐mm, 1·0–1·9‐mm and 2·0 mm and above groups. P = 0·024 (2·0 mm and above versus TAM, log rank test)
Circumferential resection margin positivity in node‐negative patients
Kaplan–Meier analysis of TAM, 0·1–0·9‐mm, 1·0–1·9‐mm and 2·0 mm or above groups, stratified according to pathological node negativity, demonstrated a significant difference only between TAM and the 0·1–0·9‐mm cohort (P = 0·041) (Fig. 4). A similar pattern was observed in patients with no lymphovascular invasion, although the difference was not statistically significant (Fig. 5).
Figure 4.

Kaplan–Meier curves of overall survival in node‐negative patients who underwent resection of oesophageal adenocarcinoma, according to distance from the circumferential resection margin: tumour at the cut margin (TAM), 0·1–0·9‐mm, 1·0–1·9‐mm and 2·0 mm and above groups. P = 0·041 (0·1–0·9 mm versus TAM, log rank test)
Figure 5.

Kaplan–Meier curves of overall survival in lymphovascular‐negative patients who underwent resection of oesophageal adenocarcinoma, according to distance from the circumferential resection margin: tumour at the cut margin (TAM), 0·1–0·9‐mm, 1·0–1·9 mm and 2·0 mm and above groups. P = 0·074 (0·1–0·9 mm and 2·0 mm and above versus TAM, log rank test)
Discussion
This study has demonstrated that overall survival improved with incremental increases in CRM distance following oesophagectomy for adenocarcinoma. Patients with tumours 1·0–1·9 mm from the resection margin had a significantly poorer prognosis than those with tumours with greater margins of clearance. This may represent an additional, as yet undescribed, ‘at risk’ group. This study found the RCP definition of CRM positivity to be independently prognostic.
Some methodological issues deserve attention. Analysing survival and recurrence of adenocarcinoma independent of squamous cell carcinoma reduced the heterogeneity of the cohort. Although comprehensive data collection allowed adjustment for confounders that affect recurrence and survival, the retrospective nature of the study and the evolution of practice throughout the study interval may have been additional sources of bias.
The prognostic value of the oesophageal CRM has been demonstrated in many studies4 8, 10 11, 14, 15, 16 25, 26, 27, 28. A large multicentre study in 20169 demonstrated that the CAP definition of CRM positivity was independently predictive of poor survival, whereas a meta‐analysis29 suggested that, although the CAP definition identified the highest‐risk group, the RCP definition encompassed patients still at risk of poor survival.
Some studies6 9, 16 20, 30 have examined the effect of CAP and RCP on rates and patterns of recurrence. A multicentre study9 (using the CAP definition of CRM positivity) found a significant increase in local recurrence rates between CRM‐positive and ‐negative patients (41·2 per cent versus 26·2 per cent respectively; P < 0·001) after propensity score matching, but no difference in systemic recurrence rates (28·3 versus 28·9 per cent; P = 0·664). Another study20 (using the RCP definition) found no increase in locoregional recurrence in CRM‐positive patients (HR 0·7, 95 per cent c.i. 0·3 to 2·1) but an increased risk of systemic recurrence (HR 3·0, 1·5 to 5·9).
Locoregional recurrence rates were similar in the TAM, 0·1–0·9‐mm and 1·0–1·9‐mm groups in the present study. In unadjusted analysis there was a significant reduction in the risk of local recurrence between the 1·0–1·9‐mm group and 2·0 mm or above group, with the overall trend reaching significance. The lack of significance in multivariable analysis may be because patients with positive margins were offered adjuvant chemoradiotherapy or as a result of unmeasured confounders. As the study showed similar locoregional recurrence rates in TAM, 0·1–0·9‐mm and 1·0–1·9‐mm groups, the mechanism by which CRM distance impacts on survival may be more complicated than inadequate local tumour clearance.
Survival analysis of patients with node‐negative disease found the CAP definition (tumour at margin) to confer a significantly worse outcome in comparison with patients with no tumour at the margin, a relationship that was not found in node‐positive patients. It has long been known that pathological nodal status is one of the most important prognostic markers of adenocarcinoma of the oesophagus21. It would follow that the independence of the CRM as a prognostic marker might diminish with lymph node metastases, given the relationship between lymph node metastasis and poor outcome. A number of studies15 16, 31 have stratified node‐positive patients when analysing the impact of CRM positivity on survival, but with mixed results. One study15 showed that the RCP definition of CRM was adversely prognostic only when less than 25 per cent of the lymph node yield was positive. A meta‐analysis29 concluded that the presence of lymph node metastasis appeared to negate the importance of CRM involvement. This suggests that the focus of treatment of patients with nodal disease should be effective systemic control.
In the present study, patients with tumour within 1·0–1·9 mm of the CRM had an independent significant risk of worse prognosis. This was reflected in the disease‐free survival curves, where the only significant difference between curves was seen between the 1·0–1·9‐mm group and the 2·0 mm or above group. Tumour within 1·0–1·9 mm of the CRM seems to represent a prognostic ‘middle ground’ in terms of both survival and recurrence. This may be important when considering adjuvant treatment strategies.
Given the stepwise improvement in survival as tumour is found further away from the margin, it may be more helpful to consider CRM distance as a continuous parameter rather than a positive/negative phenomenon.
Collaborators
Other members of the Guy's and St Thomas' Oesophago‐Gastric Research Group are: M. Kelly, O. Hynes, G. Tham, C. Iezzi and A. Cowie (Department of Surgery, Guy's and St Thomas' Oesophago‐Gastric Centre, London, UK); S. Ngan and A. Qureshi (Department of Oncology, Guy's and St Thomas Hospital, London, UK); M. Green (Department of Pathology, Guy's and St Thomas' Hospital, London, UK); N. Griffin and A. Jacques (Department of Radiology, Guy's and St Thomas' Hospital, London, UK); V. Goh (Cancer Imaging, School of Biomedical Engineering and Imaging Sciences, King's College London, London, UK); H. Deere, F. Chang, U. Mahadeva, B. Gill‐Barman and S. George (Department of Cellular Pathology, Guy's and St Thomas' Hospital, London, UK); J. Dunn, S. Zeki and J. Meenan (Department of Gastroenterology, Guy's and St Thomas' Hospital, London, UK).
Acknowledgements
As a research fellow, W.R.C.K. was funded by Guy's and St Thomas' Cancer Charity (grant number C151002).
Disclosure: The authors declare no conflict of interest.
Contributor Information
W. R. C. Knight, Email: william.r.knight@gmail.com.
the Guy's and St Thomas' Oesophago‐Gastric Research Group:
M. Kelly, O. Hynes, G. Tham, C. Iezzi, A. Cowie, S. Ngan, A. Qureshi, M. Green, N. Griffin, A. Jacques, V. Goh, H. Deere, F. Chang, U. Mahadeva, B. Gill‐Barman, S. George, J. Dunn, S. Zeki, and J. Meenan
References
- 1. Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long‐term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 2009; 27: 5062–5067. [DOI] [PubMed] [Google Scholar]
- 2. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP et al; CROSS group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366: 2074–2084. [DOI] [PubMed] [Google Scholar]
- 3. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M et al Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006: 355: 11–20. [DOI] [PubMed] [Google Scholar]
- 4. Deeter M, Dorer R, Kuppusamy MK, Koehler RP, Low DE. Assessment of criteria and clinical significance of circumferential resection margins in esophageal cancer. Arch Surg 2009; 144: 618–624. [DOI] [PubMed] [Google Scholar]
- 5. Harvin JA, Lahat G, Correa AM, Lee J, Maru D, Ajani J et al Neoadjuvant chemoradiotherapy followed by surgery for esophageal adenocarcinoma: significance of microscopically positive circumferential radial margins. J Thorac Cardiovasc Surg 2012; 143: 412–420. [DOI] [PubMed] [Google Scholar]
- 6. Chao YK, Yeh CJ, Chang HK, Tseng CK, Chu YY, Hsieh MJ et al Impact of circumferential resection margin distance on locoregional recurrence and survival after chemoradiotherapy in esophageal squamous cell carcinoma. Ann Surg Oncol 2011; 18: 529–534. [DOI] [PubMed] [Google Scholar]
- 7. Verhage RJ, Zandvoort HJ, ten Kate FJ, van Hillegersberg R. How to define a positive circumferential resection margin in T3 adenocarcinoma of the esophagus. Am J Surg Pathol 2011; 35: 919–926. [DOI] [PubMed] [Google Scholar]
- 8. Hulshoff JB, Faiz Z, Karrenbeld A, Kats‐Ugurlu G, Burgerhof JG, Smit JK et al Prognostic value of the circumferential resection margin in esophageal cancer patients after neoadjuvant chemoradiotherapy. Ann Surg Oncol 2015; 22(Suppl 3): S1301–S1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Markar SR, Gronnier C, Duhamel A, Pasquer A, Théreaux J, Chalret du Rieu M et al; FREGAT Working Group‐FRENCH‐AFC . Significance of microscopically incomplete resection margin after esophagectomy for esophageal cancer. Ann Surg 2016; 263: 712–718. [DOI] [PubMed] [Google Scholar]
- 10. Pultrum BB, Honing J, Smit JK, van Dullemen HM, van Dam GM, Groen H et al A critical appraisal of circumferential resection margins in esophageal carcinoma. Ann Surg Oncol 2010; 17: 812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rao VS, Yeung MM, Cooke J, Salim E, Jain PK. Comparison of circumferential resection margin clearance criteria with survival after surgery for cancer of esophagus. J Surg Oncol 2012; 105: 745–749. [DOI] [PubMed] [Google Scholar]
- 12. Salih T, Jose P, Mehta SP, Mirza A, Udall G, Pritchard SA et al Prognostic significance of cancer within 1 mm of the circumferential resection margin in oesophageal cancer patients following neo‐adjuvant chemotherapy. Eur J Cardiothorac Surg 2013; 43: 562–567. [DOI] [PubMed] [Google Scholar]
- 13. Scheepers JJ, Van Der Peet DL, Veenhof AA, Cuesta MA. Influence of circumferential resection margin on prognosis in distal esophageal and gastroesophageal cancer approached through the transhiatal route. Dis Esophagus 2009; 22: 42–48. [DOI] [PubMed] [Google Scholar]
- 14. Dexter SP, Sue‐Ling H, McMahon MJ, Quirke P, Mapstone N, Martin IG. Circumferential resection margin involvement: an independent predictor of survival following surgery for oesophageal cancer. Gut 2001; 48: 667–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Griffiths EA, Brummell Z, Gorthi G, Pritchard SA, Welch IM. The prognostic value of circumferential resection margin involvement in oesophageal malignancy. Eur J Surg Oncol 2006; 32: 413–419. [DOI] [PubMed] [Google Scholar]
- 16. Sujendran V, Wheeler J, Baron R, Warren BF, Maynard N. Effect of neoadjuvant chemotherapy on circumferential margin positivity and its impact on prognosis in patients with resectable oesophageal cancer. Br J Surg 2008; 95: 191–194. [DOI] [PubMed] [Google Scholar]
- 17. Khan OA, Fitzgerald JJ, Soomro I, Beggs FD, Morgan WE, Duffy JP. Prognostic significance of circumferential resection margin involvement following oesophagectomy for cancer. Br J Cancer 2003; 88: 1549–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ghadban T, Reeh M, Koenig AM, Nentwich MF, Bellon E, Izbicki JR et al Prognostic significant or not? The positive circumferential resection margin in esophageal cancer: impact on local recurrence and overall survival in patients without neoadjuvant treatment. Ann Surg 2017; 266: 988–994. [DOI] [PubMed] [Google Scholar]
- 19. Thompson SK, Ruszkiewicz AR, Jamieson GG, Esterman A, Watson DI, Wijnhoven BP et al Improving the accuracy of TNM staging in esophageal cancer: a pathological review of resected specimens. Ann Surg Oncol 2008; 15: 3447–3458. [DOI] [PubMed] [Google Scholar]
- 20. Gilbert S, Martel AB, Seely AJ, Maziak DE, Shamji FM, Sundaresan SR et al Prognostic significance of a positive radial margin after esophageal cancer resection. J Thorac Cardiovasc Surg 2015; 149: 548–555. [DOI] [PubMed] [Google Scholar]
- 21. Peyre CG, Hagen JA, DeMeester SR, Altorki NK, Ancona E, Griffin SM et al The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg 2008; 248: 549–556. [DOI] [PubMed] [Google Scholar]
- 22. Gillies RS, Simpkin A, Sgromo B, Marshall RE, Maynard ND. Left thoracoabdominal esophagectomy: results from a single specialist center. Dis Esophagus 2011; 24: 138–144. [DOI] [PubMed] [Google Scholar]
- 23. Visbal AL, Allen MS, Miller DL, Deschamps C, Trastek VF, Pairolero PC. Ivor Lewis esophagogastrectomy for esophageal cancer. Ann Thorac Surg 2001; 71:1803–1808. [DOI] [PubMed] [Google Scholar]
- 24. Davies AR, Zylstra J, Baker CR, Gossage JA, Dellaportas D, Lagergren J et al A comparison of the left thoracoabdominal and Ivor‐Lewis esophagectomy. Dis Esophagus 2018; 31. [DOI] [PubMed] [Google Scholar]
- 25. Sagar PM, Johnston D, McMahon MJ, Dixon MF, Quirke P. Significance of circumferential resection margin involvement after oesophagectomy for cancer. Br J Surg 1993; 80: 1386–1388. [DOI] [PubMed] [Google Scholar]
- 26. Saha AK, Sutton C, Rotimi O, Dexter S, Sue‐Ling H, Sarela AI. Neoadjuvant chemotherapy and surgery for esophageal adenocarcinoma: prognostic value of circumferential resection margin and stratification of N1 category. Ann Surg Oncol 2009; 16: 1364–1370. [DOI] [PubMed] [Google Scholar]
- 27. Reid TD, Davies IL, Mason J, Roberts SA, Crosby TD, Lewis WG. Stage for stage comparison of recurrence patterns after definitive chemoradiotherapy or surgery for oesophageal carcinoma. Clin Oncol (R Coll Radiol) 2012; 24: 617–624. [DOI] [PubMed] [Google Scholar]
- 28. O'Neill JR, Stephens NA, Save V, Kamel HM, Phillips HA, Driscoll PJ et al Defining a positive circumferential resection margin in oesophageal cancer and its implications for adjuvant treatment. Br J Surg 2013; 100: 1055–1063. [DOI] [PubMed] [Google Scholar]
- 29. Chan DS, Reid TD, Howell I, Lewis WG. Systematic review and meta‐analysis of the influence of circumferential resection margin involvement on survival in patients with operable oesophageal cancer. Br J Surg 2013; 100: 456–464. [DOI] [PubMed] [Google Scholar]
- 30. Reid TD, Chan DS, Roberts SA, Crosby TD, Williams GT, Lewis WG. Prognostic significance of circumferential resection margin involvement following oesophagectomy for cancer and the predictive role of endoluminal ultrasonography. Br J Cancer 2012; 107: 1925–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scheepers JJ, Van Der Peet DL, Veenhof AA, Cuesta MA. Influence of circumferential resection margin on prognosis in distal esophageal and gastroesophageal cancer approached through the transhiatal route. Dis Esophagus 2009; 22: 42–48. [DOI] [PubMed] [Google Scholar]


