Abstract
Background
The clinical effectiveness of treating ipsilateral multifocal (MF) and multicentric (MC) breast cancers using breast‐conserving surgery (BCS) compared with the standard of mastectomy is uncertain. Inconsistencies relate to definitions, incidence, staging and intertumoral heterogeneity. The primary aim of this systematic review was to compare clinical outcomes after BCS versus mastectomy for MF and MC cancers, collectively defined as multiple ipsilateral breast cancers (MIBC).
Methods
Comprehensive electronic searches were undertaken to identify complete papers published in English between May 1988 and July 2015, primarily comparing clinical outcomes of BCS and mastectomy for MIBC. All study designs were included, and studies were appraised critically using the Newcastle–Ottawa Scale. The characteristics and results of identified studies were summarized.
Results
Twenty‐four retrospective studies were included in the review: 17 comparative studies and seven case series. They included 3537 women with MIBC undergoing BCS; breast cancers were defined as MF in 2677 women, MC in 292, and reported as MIBC in 568. Six studies evaluated MIBC treated by BCS or mastectomy, with locoregional recurrence (LRR) rates of 2–23 per cent after BCS at median follow‐up of 59·5 (i.q.r. 56–81) months. BCS and mastectomy showed apparently equivalent rates of LRR (risk ratio 0·94, 95 per cent c.i. 0·65 to 1·36). Thirteen studies compared BCS in women with MIBC versus those with unifocal cancers, reporting LRR rates of 2–40 per cent after BCS at a median follow‐up of 64 (i.q.r. 57–73) months. One high‐quality study reported 10‐year actuarial LRR rates of 5·5 per cent for BCS in 300 women versus 6·5 per cent for mastectomy among 887 women.
Conclusion
The available studies were mainly of moderate quality, historical and underpowered, with limited follow‐up and biased case selection favouring BCS rather than mastectomy for low‐risk patients. The evidence was inconclusive, weakening support for the St Gallen consensus and supporting a future randomized trial.
Introduction
Breast cancer affects 1·7 million women annually worldwide, the majority of whom are treated surgically1. Clinical evidence is well established for the treatment of unifocal cancers by breast‐conserving surgery (BCS) and whole‐breast radiotherapy (RT) in preference to mastectomy2 3. In contrast, there are no a priori randomized trials evaluating the clinical safety of BCS for treating multiple ipsilateral breast cancers (MIBC). MIBC are collectively defined as more than one synchronous ipsilateral cancer at diagnosis. In a national Association of Breast Surgery survey of UK breast surgeons in 2015, 91 per cent of surgeons thought that a randomized trial evaluating the safety and quality‐of‐life implications after BCS for MIBC was clinically important (Z. E. Winters, unpublished data)
Observational studies evaluating treatments for MIBC have shown wide variation in clinical outcomes. There have also been wide ranging expert opinions on optimal surgical treatments3 4. Inherent clinical inconsistencies include variable definitions, large variation in incidences depending on the sensitivity of preoperative imaging (for example mammography versus MRI), underestimating the tumour load using the current TNM staging classification and unknown clinical implications of MIBC, where multifocal (MF) cancers may be clinically and genetically distinguishable from multicentric (MC) ones5. These issues have challenged interstudy comparisons and clinical evidence regarding treatments for MIBC.
Historically, MF cancers have been defined as more than one cancer within the same breast quadrant, whereas MC cancers are widely spaced in different quadrants. MIBC may also include ductal carcinoma in situ (DCIS) and invasive breast cancer5. However, these definitions are problematic, with no breast anatomical boundaries and variably defined distances of clinically apparently normal tissue between cancers; MF cancers are foci separated by 40 mm or les s (or no more than 20 mm), and MC cancers are foci separated by more than 40 mm (or over 20 mm) or tumours in different quadrants6, 7, 8. According to the College of American Pathologists guidelines9, characterization of only the largest lesion in MIBC is sufficient, provided that all cancers have the same tumour grade. Despite this, several authors10 11 have suggested revising the current TNM staging system12, potentially avoiding underestimation of the overall disease burden for MIBC. Recently, Desmedt and colleagues8 suggested that genomically heterogeneous cancers tended to be situated further apart (MC cancers), whereas those closer together (MF cancers) showed intertumoral homogeneity. Molecular characterization of each cancer focus to distinguish between MC and MF cancers may be important in future classifications8. Intertumoral heterogeneity in MIBC has been reported in 11–27 per cent of patients8 13, 14. Generally, evaluation of the largest cancer using standard immunohistochemical (IHC) biomarkers (oestrogen and progesterone receptors, human epidermal growth factor receptor 2 (HER2) and Ki‐67) is performed3 9, 15. However in future, the potential to use extended IHC biomarkers and whole‐genome sequencing may increase the recognition of intertumoral heterogeneity14 16, 17, 18, with prognostic implications19, 20, 21, 22.
Clinically occult cancers may remain dormant, or may be treated adequately by adjuvant whole‐breast RT after BCS8. The incidence of clinically and radiologically detected MIBC ranges from 10 to 24 per cent of all breast cancers20, 21, 22, 23, increasing with time from earlier to later studies. This apparent doubling in incidence of MIBC over the 10 years between 1990 and 2000 may be due in part to improved breast imaging (digital mammography, ultrasonography and MRI) and increased screening24. Standard imaging of MIBC may include digital mammography, ultrasound examination and MRI, with biopsy confirmation of any additional suspected cancers on MRI to minimize their misdiagnosis, which occurs in 30 per cent of ‘lesions’24 25. Studies6 10, 11 19, 20, 21 23, 26 are conflicting on the prognostic implications of MIBC, with suggestions of increased axillary lymph node involvement latterly using sentinel lymph node biopsy (SLNB), and with worse overall outcomes than those for unifocal cancers19 22, 23. Increased rates of locoregional recurrence (LRR)5 and breast cancer events22 23, 27 28 secondary to BCS for MIBC have been reported, but vary widely across studies. Recently, a clear majority of the St Gallen expert consensus panel3 expressed the opinion that it was possible to treat MF and MC cancers with BCS, where there was margin clearance and whole‐breast RT was planned. However, this opinion was not contextualized to clinical outcomes specifically differentiating MF and MC cancers, or comparable outcomes following mastectomy as the standard of care. Current guidelines are concordant regarding adjuvant treatments (chemotherapy, endocrine therapy, targeted therapy and RT) informed by tumour subtypes3 5.
This systematic review critically appraises the levels of clinical evidence, and whether these support or weaken the case on which expert opinion is based in support of BCS. The primary aim was to compare disease‐specific outcomes following BCS versus mastectomy for treating MIBC (including MF and MC cancers). Studies comparing BCS for treating MIBC and unifocal disease were also evaluated.
Methods
A protocol was developed that followed the PRISMA statement for review methods and reporting29. A PICOS (participants, interventions, comparators, outcomes and study designs) sequence was used to describe the included studies. In this systematic review, the term MIBC refers to both MF and MC cancers. Where individual studies referred specifically to MF or MC cancers, these definitions were retained.
Literature search strategy
Web‐based search engines MEDLINE, Embase, PsycINFO, ISI Web of Knowledge and Cochrane databases were interrogated using keywords (Appendix S1, supporting information). The search was limited to human studies published in English from May 1988 to July 2015. Abstracts and conference reports were excluded owing to difficulties in evaluating incomplete information. Duplicate records were excluded. Two independent reviewers screened titles and abstracts for eligibility using predetermined criteria. Reference lists of screened articles and reviews were searched manually to identify further relevant studies. The intention was to focus on RCTs and non‐randomized longitudinal cohort studies, and this was extended to include all study designs.
Data extraction
A standardized data pro forma was created including study design, nature of data accrual, number of centres, years of data collection, study inclusion and exclusion criteria, diagnostic methods, clinical outcomes (LRR, disease‐free survival (DFS) and overall survival), duration of follow‐up, pathological details, adjuvant treatment and non‐clinical outcomes (Appendix S2, supporting information). All data were evaluated independently by two authors, and discrepancies resolved by the senior author.
Inclusion and exclusion criteria and outcomes
Included studies were those evaluating women aged at least 18 years with invasive cancer and/or DCIS diagnosed as MIBC before operation or after surgery (histological diagnosis). Eligible interventions comprised BCS, either primary or secondary to neoadjuvant treatment. To be included, studies required a direct comparison of BCS with mastectomy for MIBC (primary aim), or a comparison of BCS for MIBC versus BCS for unifocal cancers (secondary aim). There were no exclusion criteria relating to minimum numbers of participants or duration of follow‐up.
Other inclusion criteria required the study to be primary research as opposed to audit, and to include a minimum of one relevant clinical outcome such as: LRR (local relapse‐free, clinical local recurrence), distant metastasis (distant disease), DFS (disease‐free survival) or overall survival (breast cancer‐specific disease, overall metastases, breast cancer‐specific survival).
Study quality
Risk of study bias was assessed using the Newcastle–Ottawa scale (NOS)30 31. The NOS comprises a semiquantitative assessment of study quality, using eight separate measures. These measures score the study based on selection of patients, comparability of groups and reporting of outcomes. The score ranges from 0 to 9. Studies scoring 7 or more are considered high quality, those scoring 4–6 are of moderate quality, and those with a score of less than 4 are of poor quality. Two reviewers scored each study independently, with discussion and re‐evaluation of scoring discrepancies resulting in consensus.
Data analysis
Data on recurrence rates were analysed using a fixed‐effects model. Risk ratios and 95 per cent confidence intervals were calculated with Revman 5.3 software (Nordic Cochrane Centre, Copenhagen, Denmark) and presented as a forest plot. Heterogeneity among studies was assessed by means of the I 2 statistic.
Results
Study selection
Titles and abstracts of 471 citations were identified electronically (Fig. 1) After review of 37 full‐text articles, 24 primary papers were retained. Of the 13 articles excluded, six did not report primary research (review articles), three did not report on surgical interventions, two did not report on BCS for MIBC and two were not focused on MIBC.
Figure 1.

Selection of articles for review. MIBC, multiple ipsilateral breast cancers; BCS, breast‐conserving surgery
Study design
There were no RCTs and there was a paucity of published data addressing the primary aim. The 24 studies had retrospective observational designs, comprising 17 comparative studies20, 21, 22, 23 27, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 and seven case series28 44, 45, 46, 47, 48, 49 (Table 1; Table S1, supporting information). The studies included a total of 3537 women with MIBC undergoing BCS: 2677 with tumours defined as MF, 292 as MC, and 568 not defined as either MF or MC, but reported as MIBC. Assessment of the observational studies using the NOS showed that three20 27, 43 were of high quality (score at least 7) and 1421, 22, 23 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42 of moderate quality (score 4–6) (Table S2, supporting information).
Table 1.
Summary of study characteristics, treatments and clinical outcomes of primary studies comparing breast‐conserving surgery with mastectomy
| Reference Study interval and location NOS score | Cancers included FU (months)* | Group differences in CP | No of patients (MF; MC) Other treatments Pathology | Outcomes | ||
|---|---|---|---|---|---|---|
| BCS | Mastectomy | BCS‡ | Mastectomy | |||
| Nos et al. 32 1983–1989 France 1 centre NOS 4 | n.a. FU 101 (86–129) | Significant differences in age and T category | 56 (56; 0) Radiotherapy, chemotherapy, endocrine IDC 79%, ILC 13% | 132 Radiotherapy, chemotherapy, endocrine IDC 83%, ILC 8% | 5‐year LRR: 11% 10‐year LRR: 23% 5‐year DM: 18% 10‐year DM: 28% 5‐year OS: 94% 10‐year OS: 73% | 5‐year LRR: 11% 10‐year LRR: 14% 5‐year DM: 18% 10‐year DM: 35% 5‐year OS: 89% 10‐year OS: 65% |
| Kaplan et al. 33, † 1989–2002 USA 2 centres NOS 4 | MIBC, diagnosed before surgery FU 45 (1–143) | No significant differences | 36 Radiotherapy, chemotherapy, endocrine IDC 72%, ILC 19%, DCIS 8% | 19 Radiotherapy, chemotherapy, endocrine IDC 68%, ILC 21%, DCIS 11% | 5‐year LRR: 1 of 36 (3%) (P = 0.54) DM: 1 of 36 (3%) (P = 0·20) OS: 36 of 36 (100%) | 5‐year LRR: 0 of 19 (0%) DM: 1 of 19 (5%) OS: 19 of 19 (100%) |
| Lim et al. 34 1990–2003 South Korea 1 centre NOS 6 | MF FU 59 (1–177) Mastectomy: 65 (6–196) | HER2+ (P = 0·007) T2 (P = 0·006) | 147 (147; 0) Radiotherapy, endocrine IDC 97%, ILC 3% | 331 Radiotherapy, endocrine IDC 96%, ILC 4% | 5‐year LRR: 3 of 147 (2.0%) (P = 0·38) 5‐year DFS: 89% (P = 0·45) 5‐year OS: 93·4% (P = 0·21) | 5‐year LRR: 3 of 331 (0·9%) 5‐year DFS: 92% 5‐year OS: 94·5% |
| Kadioğlu et al. 35 2002–2011 Turkey 1 centre NOS 5 | MF, diagnosed by histology FU 55 (10–102) | No. of foci (P = 0·001) LVI (P = 0·04) LN positivity (P = 0·002) TNM stage (P = 0·01) HER2+ (P = 0·03) | 119 (119; 0) Radiotherapy, chemotherapy, endocrine IDC 71%, ILC 12% | 103 Radiotherapy, chemotherapy, endocrine IDC 69%, ILC 8% | 5‐year LRR: 6 of 119 (5·0%) (P = 0·06) 5‐year OS: 92%, median 95 (range 91–99) months (P < 0·001) | 5‐year LRR: 6 of 103 (5·8%) 5‐year OS: 72%, median 73 (range 68–78) months |
| Neri et al. 20, † Italy 1991–2005 1 centre NOS 7 | MF diagnosed before surgery FU 88 (11–248) | n.a. | 36 (36; 0) Radiotherapy, chemotherapy endocrine | 155 Radiotherapy, chemotherapy, endocrine | 7‐year LR: 3 of 36 (8%) 7‐year LLR: 5 of 36 (14%) 7‐year RR: 2 of 36 (6%) DM: 7 of 36 (19%) | 7‐year LR: 12 of 155 (7·7%) 7‐year LLR: 23 of 155 (14·8%) 7‐year RR: 11 of 155 (7·1%) DM: 42 of 155 (27·1%) |
| Yerushalmi et al. 43, † 1989–2005 South Korea 5 regional centres NOS 7 | MIBC, diagnosed before surgery FU 93 | T, N category (P < 0·001) EIC (P < 0·001) Positive margins (P < 0·001) | 300 Radiotherapy, chemotherapy, endocrine IDC 88%, ILC 12% | 887 Radiotherapy, chemotherapy, endocrine IDC 92%, ILC 6% | 17 of 300 (5·7%) | 58 of 887 (6·5%) |
Values are mean (range). All studies were retrospective;
prospective database.
P values are for comparison of breast‐conserving surgery (BCS) versus mastectomy. NOS, Newcastle–Ottawa Scale; FU, follow‐up; CP, clinical pathology; MF, multifocal; MC, multicentric; n.a., not available; IDC, invasive ductal cancer; ILC, invasive lobular cancer; LRR, locoregional recurrence; DM, distant metastasis; OS, overall survival; MIBC, multiple ipsilateral breast cancers; DCIS, preinvasive ductal cancer in situ; HER2, human epidermal growth factor receptor 2; T2, tumour size 20–50 mm; DFS, disease‐free survival; LVI, lymphovascular invasion; LN, lymph node; LR, local recurrence; RR, regional recurrence; EIC, extensive preinvasive cancer or DCIS. Further details of the studies can be found in Tables S1–S3 (supporting information).
Participants
Seventeen studies20 21, 27 28, 32 34, 35, 36, 37 40, 41, 42 44, 46, 47, 48, 49 were from single centres, four33 38, 39 45 involved two centres, and there were three multicentre studies22 23, 43 comprising a regional cancer registry (British Columbia Cancer Agency)43, a German regional study group (BRENDA Study Group: Breast Cancer Care under Evidence‐based Guidelines)23 and a substudy within three multicentre RCTs of neoadjuvant chemotherapy (German Breast Group (Gepar) trials)22 (Table 1; Table S1, supporting information).
Studies included patients recruited between 1968 and 2010, with only two22 35 including patients treated after 2000. The median group size of patients with MIBC receiving BCS was 61 (i.q.r. 35–169) in comparative studies and 22 (14–43) in case series. Diagnostic methods for evaluating MIBC were recorded in six of the seven case series44, 45, 46, 47, 48, 49 and nine27 32, 33 35, 36, 37, 38, 39, 40 of the 17 comparative studies. In these 15 studies, 258 of 592 patients (43·6 per cent) were diagnosed by postoperative pathology and 294 (49·7 per cent) before surgery: clinical examination (129, 21·8 per cent), radiology (103, 17·4 per cent) or undefined methods (62, 10·5 per cent). The remaining 40 patients (6·8 per cent) were classified as having MIBC based on lesion detection at surgery32 38, 39. Where preoperative diagnostic methods were defined, ultrasound imaging was reported in four27 33, 40 46 and MRI in three35 47, 49 studies.
Data were collected retrospectively in the case series, whereas six comparative studies20, 21, 22 27, 33 43 used a prospective database to identify patients with MIBC treated by BCS. In the 11 other comparative studies, data were derived from postoperative histology reports based on pathologically determined as opposed to clinically detected disease. Ataseven and co‐workers22 omitted prerequisite pathological confirmation of each cancer. Studies reported varied inclusion criteria for MIBC. Twelve studies20 23, 27 32, 34 36, 37 40, 42 43, 48 49 based inclusion on TNM staging, with three of these23 32, 42 mandating inclusion by size of the largest cancer. Another six studies21 28, 39 44, 46 47 used the feasibility of BCS to define eligibility. The remaining studies included: macroscopically separate tumours33 35, 45, synchronous ipsilateral breast cancers on gross pathological review over and above microscopy38, patients with DCIS undergoing BCS41 and recruitment within the Gepar trials22.
Clinical and pathological characteristics
Significant differences between groups in clinical and pathological characteristics (age, tumour type, DCIS, TNM stage, receptor status (oestrogen receptor, progesterone receptor, HER2), lymphovascular invasion, margins) were reported in four studies32 34, 35 43 of BCS versus mastectomy for MIBC, and in four studies27 37, 39 43 of BCS for MIBC versus unifocal cancers (Table S3, supporting information). Intergroup comparisons showed that prognostic factors were worse among patients who underwent mastectomy than those who underwent BCS, and among those with MIBC compared with those who had unifocal disease. Yerushalmi and colleagues43 used a matched analysis (3 : 1) of clinicopathological characteristics, accounting for three times more unifocal cancers than MIBC. Retrospective study designs made it difficult to compare clinical data, compounded by significantly larger numbers of unifocal cancers20, 21, 22, 23 41, 42. With the exception of one recent study22, no studies reported the distribution of molecular subtypes (luminal A, luminal B, non‐luminal HER2, basal and triple‐negative). Overall, five studies23 27, 34 35, 43 used statistical regression methodologies adjusting for baseline co‐variables.
Definitions of MIBC
Definitions of MF or MC tumours were described in 2120, 21, 22, 23 27, 28 32, 34 35, 37 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 of 24 studies. Eleven studies assessed MIBC as a single group33 36, 37, 38, 39, 40 42, 43, 44, 45 47, and 1320, 21, 22, 23 27, 28 32, 34 35, 41 46, 48 49 evaluated BCS in MF and MC cancers separately (Table 1; Table S3, supporting information).
Clinical outcomes
Clinical outcomes are summarized in Table 1 and detailed in Table S4 (supporting information). Eleven21 22, 27 36, 37, 38, 39, 40, 41, 42, 43 of 17 comparative studies evaluated clinical outcomes of BCS for both MIBC and unifocal cancers; only four studies23 32, 33 35 exclusively compared BCS with mastectomy for MIBC. Lim and colleagues34 and Neri et al. 20 evaluated outcomes of both MIBC and unifocal cancers independent of the type of surgery. Of the 24 studies, five20 23, 27 32, 43 reported actuarial 10‐year clinical outcomes, despite 10 years being the optimal period of follow‐up according to the Association of Breast Surgery guidelines50. Overall, the studies reported a median follow‐up of 60 (i.q.r. 53–73) months.
Interventions
Various descriptions of the type of BCS were used (Table 1; Table S1, supporting information). Fourteen studies21, 22, 23 28, 32 34, 35 39, 40, 41, 42, 43, 44 47 referred to BCS and three36, 37, 38 to wide local excision. Others referred to partial mastectomy20 45, quadrantectomy46 48, segmentectomy27 or lumpectomy33 49. The extent of acceptable microscopic margin status following BCS was defined in 1821, 22, 23 27, 28 33, 34, 35, 36, 37, 38, 39, 40, 41 44, 45, 46 48 actuarial studies. Differing definitions of microscopically clear radial cancer margins were used. Commonly, this comprised radial margins of at least 1 mm23 27, 28 33, 34 40, 44 48, although two studies5 46 used 2 mm or more and one39 required margins of at least 5 mm. Other studies referred to ‘grossly excised’36, 37, 38, ‘tumour‐free margins’22 and ‘clear at the inked margin’41. In one study21, margins were defined as ‘close’ if less than 2 mm from the ‘cut edges’, and another35 based margin re‐excisions on clinicians' assessments.
Adjuvant treatments
Adjuvant radiotherapy
All 24 studies reported using postoperative RT after BCS. Completion of RT after BCS was described in 15 studies20 21, 23 27, 32 35, 36 38, 39, 40, 41 43, 44, 45, 46. In three studies22 37, 42 it was not possible to define the extent of RT or dose fractionation used. The RT regimen was described in 11 studies23 32, 36, 37, 38, 39, 40 44, 45, 46, 47, with a tumour bed boost RT reported in eight23 32, 36 37, 39 40, 45 46 of these. There was no mention of more than one lumpectomy bed receiving a tumour bed RT boost in MC cancers; however, there were only 223 MC cancers treated by BCS (Table 1; Table S1, supporting information).
Adjuvant endocrine therapy
Twenty studies20, 21, 22, 23 27, 28 32, 33, 34, 35 39, 41, 42, 43, 44, 45, 46, 47, 48, 49 reported using adjuvant endocrine treatments. Eighteen20 21, 27 28, 32, 33, 34, 35 39, 41, 42, 43, 44, 45, 46, 47, 48, 49 reported patient compliance for endocrine therapy, with a median of 68 (i.q.r. 43–87) per cent. Four studies34 47, 48, 49 reported endocrine treatment of oestrogen receptor‐positive cancers, with a median compliance rate of 92 (78–96) per cent.
Adjuvant chemotherapy
Twenty studies20 22, 23 27, 28 32, 33 35, 37, 38, 39, 40 42, 43, 44, 45, 46, 47, 48, 49 described the use of chemotherapy, with 18 reporting percentages of patients who received it; this varied widely. Three studies22 39, 40 reported that all patients received some form of chemotherapy. The overall median percentage of patients receiving chemotherapy was 57 (i.q.r. 43–77) per cent20 22, 27 28, 32 33, 35 38, 39, 40 42, 43, 44, 45, 46, 47, 48, 49. Eight studies22 35, 38, 39, 40 44, 45 47 described chemotherapy schedules and proportions of women treated, and 11 others20 21, 27 28, 32 33, 42 43, 46 48, 49 reported proportions of patients alone, without chemotherapy regimens. Only two studies22 40 described neoadjuvant chemotherapy.
Clinical cancer outcomes in cohort studies
Reported clinical outcomes varied widely regarding clinical endpoints and duration of follow‐up (Table 1; Table S4, supporting information). Clinical outcomes reported were: LRR20, 21, 22 27, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, overall survival20 27, 32, 33, 34, 35 38, 40 43, occurrence of distant metastases20 32, 33 36 and DFS22 23, 27 34. Eleven studies21 32, 33, 34, 35, 36, 37, 38, 39, 40 42 reported outcomes at 5 years or just over; three32 41, 43 reported outcomes at 5 and 10 years; one23 reported at 10 years only; and the other three studies reported outcomes at 3 years22, 7 years20 and 9 years27. The median duration of follow‐up in all studies was 60 (i.q.r. 53–73) months.
Clinical cancer outcomes in case series
Six28 44, 45, 46, 47 49 of seven case series evaluated clinical outcomes after BCS for MIBC with rates of ‘local recurrences’ (LRR, local relapse‐free and cumulative local events) ranging from 0 to 5·1 per cent over a median follow‐up of 53 (i.q.r. 34–72) months (Table S4, supporting information). The 5‐year LRR data were weaker, with Gentilini and colleagues28 reporting a 6‐year actuarial LRR rate of 5·1 per cent (24 of 476) after BCS, without a comparator group. Distant metastasis rates ranged from 4·5 to 11 per cent during follow‐up. Median overall survival rates in four studies28 44, 48 49 ranged from 89 to 100 per cent.
Clinical cancer outcomes after breast‐conserving surgery versus mastectomy for MIBC
Six20 32, 33, 34, 35 43 of seven studies reported clinical outcomes for BCS versus mastectomy for MIBC, which was the primary aim of the review, with a median follow‐up of 59·5 (i.q.r. 56–81) months (Table 1; Table S4, supporting information). The largest of the seven studies was part of the multicentre BRENDA cohort study23, but did not provide raw data for comparison. This was scored as having moderate quality based on analyses of clinical subgroups, judged to be adherent to German guidelines or not. Adherence to guidelines meant that BCS was contraindicated for MC cancers23. Non‐conformance with guidelines resulted in 12·9 per cent of MC cancers (60 of 464) being treated with BCS, compared with 46·8 per cent (217 of 464) undergoing mastectomy23. LRR was reported in five studies20 32, 33, 34, 35, distant metastases in three20 32, 33, overall survival in four32, 33, 34, 35 and DFS in two23 34.
Local recurrence
Six studies20 32, 33, 34, 35 43 reported LRR rates ranging from 2 to 23 per cent after BCS, with apparently similar rates of LRR for BCS compared with mastectomy (risk ratio 0·94, 95 per cent c.i. 0·65 to 1·36) (Fig. 2). There was no heterogeneity in these studies, which may reflect similar case selection biases with surgeons choosing BCS for low‐risk patients and mastectomy for high‐risk cases. Overall, the results should be viewed with caution because they may be compromised by study quality.
Figure 2.
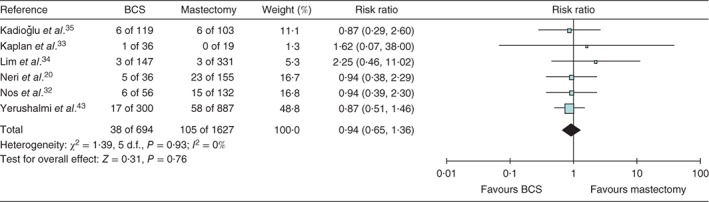
Risk ratio for locoregional recurrence after breast‐conserving surgery (BCS) versus mastectomy. An inverse‐variance fixed‐effect model was used for meta‐analysis. Risk ratios are shown with 95 per cent confidence intervals. Reference 23 was not included in this analysis as no raw data were available
The historical study of Yerushalmi and colleagues43 reported the potential clinical equivalence of mastectomy in 887 patients compared with standard BCS in 300 patients, with 10‐year LRR rates of 6·5 per cent (58 of 887) versus 5·7 per cent (17 of 300) respectively (P = 0·95). Five‐year LRR rates of MIBC in this study were 4·5 per cent after mastectomy versus 2·5 per cent after BCS43.
Survival
Wolters and colleagues23 concluded that treatment of MF cancers according to German guidelines by BCS (683 of 1398, 48·9 per cent) versus mastectomy (329 of 1398, 23·5 per cent) showed no significant differences in 5‐year recurrence‐free survival. Neri and co‐workers20 showed that MF cancers were a significant independent predictor of worse breast cancer‐specific survival for BCS (hazard ratio (HR) 3·88, 95 per cent c.i. 1·06 to 14·12; P = 0·026) and mastectomy (HR 2·72, 1·15 to 6·48; P = 0·023). Kadioğlu et al.35 reported significantly better 5‐year survival of 92 per cent (median 95 (range 91–99) months) after BCS in 119 patients, compared with 72 per cent (median 73 (68–78) months) after mastectomy in 103 patients (P < 0·001). Multivariable analyses in the latter study, accounting for intergroup differences, subsequently showed no significant effects on outcomes between types of surgery (P = 0·07)35. Similarly, Nos and colleagues32, Kaplan and co‐workers33 and Lim et al.34 reported no differences in overall survival, DFS or distant metastases by type of surgery.
Clinical outcomes after breast‐conserving surgery for MIBC versus unifocal cancers
Table S4 (supporting information) provides data on outcomes after BCS for MIBC versus unifocal cancers.
Local recurrence
Twelve of 13 studies reported LRR rates after BCS for MIBC ranging from 2 to 40 per cent at a median follow‐up of 64 (i.q.r. 57–73) months. Two historical studies36 37 showed significantly higher LRR rates after BCS for MIBC compared with those for unifocal cancers. More recently, Chung et al.27 reported significantly worse 9‐year LRR rates of 6·1 per cent (10 of 164) in patients with MIBC compared with 0·6 per cent (6 of 999) in patients with unifocal disease (P = 0·001). Using a matched‐pair analysis, Yerushalmi and colleagues43 reported equivalent actuarial 10‐year local recurrence rates of 5·6 per cent for MIBC (MF and MC) among 300 patients treated by BCS versus 4·3 per cent for unifocal cancer among 11 683 patients after BCS (HR 1·09, 95 per cent c.i. 0·55 to 2·16; P = 0·78). Furthermore, no differences were shown for 10‐year LRR between the groups (Table S4, supporting information). Neri et al.20 reported significantly worse 7‐year LRR for MIBC treated by BCS (2 of 36, 6 per cent) compared with BCS for unifocal cancers (8 of 491, 1·6 per cent) (P = 0·050). This study reported 304 disease recurrences at a median follow‐up of 7·3 years (range 11–248 months), with a median time to relapse of 32 months, and 172 deaths from breast cancer20.
Survival
Nine‐year DFS was reported in two studies27 34 (Table S4, supporting information). Chung and co‐workers27 reported a significantly worse 9‐year DFS rate of 89·3 per cent (14 breast cancer‐related events among 164 patients) for MIBC treated by BCS versus 97·7 per cent (17 of 999) following BCS for unifocal cancers, with a HR of 5·86 (95 per cent c.i. 2·57 to 13·3; P < 0·001) in multivariable analysis. A substudy22 within the GeparTrio randomized trials evaluated the impact of neoadjuvant chemotherapy on clinical outcomes of patients with unifocal, MF and MC cancers. The choice of either BCS or mastectomy was based on clinical cancer responses and the surgeon's discretion. This substudy demonstrated that 5‐year local relapse‐free survival (LRFS) rates in all patients were significantly adversely affected by focality; MC cancers alone had the worst LRFS rates of 90·4 per cent, compared with 95·1 per cent for MF and 92·9 per cent for unifocal cancers. Lower LRFS rates persisted for MC cancers after mastectomy (P = 0·030), although this group presented with significantly worse disease in terms of TNM stage and HER2 positivity (P < 0·001)22. Survival outcomes (LRFS) were not inferior for MC (P = 0·844) and MF (P = 0·430) cancers compared with unifocal cancers after complete pathological responses to neoadjuvant chemotherapy22. Neri and colleagues20 identified MIBC as a significant independent prognostic factor for breast cancer‐specific survival (HR 1·64, 95 per cent c.i. 1·05 to 2·57; P = 0·029) using multivariable analyses. Overall actuarial breast cancer‐specific survival rates (independent of type of surgery) for MIBC were 89·7 per cent at 5 years and 79·8 per cent at 10 years.
Distant metastases were reported in two articles20 36. Leopold et al.36 reported a 5‐year rate of distant metastasis of 40 per cent (4 of 10) for MIBC treated by BCS. In both papers, distant disease was more common in the MIBC group. The results reported by Neri and co‐workers20 were statistically significant (19 versus 11·2 per cent for MIBC versus unifocal cancers respectively; P = 0·030).
Overall survival was reported in six studies20 27, 34 38, 40 43. Two studies demonstrated significantly worse survival for MIBC compared with unifocal cancers at a mean follow‐up of 88 months (HR 3·88, 95 per cent c.i. 1·06 to 14·12; P = 0·020)20 and 112 months (85·8 versus 98·4 per cent; P < 0·001)27. Of the remaining four studies, two34 38 reported worse outcomes, and two40 43 better outcomes for MIBC, with no significant differences. Although not statistically significant, a further six studies21 38, 39 41, 42, 43 reported trends towards worse outcomes after BCS for MIBC compared with unifocal cancers.
Discussion
This systematic review attempted to appraise the published literature critically regarding the impact of BCS in treating MIBC, compared with the standard of mastectomy with or without breast reconstruction. Overall, there was limited evidence of moderate quality supporting the clinical equivalence of BCS versus mastectomy for treating MIBC. Factors limiting the quality of evidence were study designs, heterogeneous clinical outcomes, and few if any representative studies of use of BCS to treat MC tumours compared with MF cancers. The inclusion of exclusively pathological diagnosis in some studies and the complexity of surgical case selection were major limiting factors inherent in the study designs. Other factors were non‐comparability of statistical methodologies, low patient numbers by type of surgery (particularly for MC cancers) and limited duration of follow‐up. In the context of current treatments, most studies were historical, with poor reporting of adjuvant chemotherapy22. Two studies22 40 evaluated neoadjuvant chemotherapy and BCS in MIBC. Most did not address the primary aim of this review, but compared BCS for MIBC versus unifocal cancers. The apparent lack of significant intergroup differences in the rates of LRR may allow clinical equipoise and support the rationale for a randomized trial. A National Institute for Health Research (NIHR)‐funded randomized trial would evaluate the non‐inferiority in terms of LRR after BCS for MF and/or MC cancers (MIBC) compared with mastectomy.
Most studies included in this review were at high risk of bias30 31, 51 52. Although one‐quarter of studies used a prospective database, none of these published their protocols or defined their core clinical outcome sets52 53, or reported study size calculations for the surgical groups54. Other markers of study quality were lacking, such as ethical approvals and conflicts of interest30 31, 51.
Older studies did not report therapeutic mammaplasty (TM) techniques55, 56, 57. TM as a form of BCS for treating unifocal cancers has become more common over the past 5 years. However, evidence is lacking for its use in the treatment of MIBC55, 56, 57. TM techniques comprise either extended breast tissue excisions for cancer(s) with simple reapproximation of breast tissue (level 1 reconing) or a therapeutic reduction mammaplasty (level 2)56 57. Currently, TM is the standard best practice for optimizing cosmetic outcomes after extended breast tissue excisions relative to breast volume. Recently, a small case series58 (68 patients) describing BCS for 20 patients with MF cancers was reported. In principle, treating MC cancers using two or more separate wide local excisions combined with TM merits future investigation, particularly in the context of RT boost(s) to one or more tumour beds. A meta‐analysis56 of a non‐randomized comparison between 3165 TM procedures with standard BCS in 5494 patients with unifocal cancers showed that the former significantly reduced rates of cancer margin positivity (P < 0·001) and surgical re‐excisions (P < 0·001). Recently, the St Gallen panel2 recommended a minimal acceptable surgical margin of ‘no ink on invasive tumour or DCIS’. Other interventions significantly reducing intraoperative tumour margin positivity have been described: digital specimen radiology (P = 0·012 for digital versus conventional mammography)59, tumour margin cavity shaves60 and real‐time cancer margin assessments61 62. Ataseven and colleagues22 reported that neoadjuvant chemotherapy‐induced pathological complete cancer response rates increased the surgical options for BCS without compromising clinical outcomes, an approach requiring future investigation. Future TM approaches for treating MIBC should recommend standardized operating procedures, involving tumour bed clips to facilitate image‐guided RT63, 64, 65, 66, 67, 68, 69. A minority of studies (8 of 24) in the present review referred to tumour bed boost RT following BCS for MIBC. The feasibility of administering one or more tumour bed RT boosts after TM in MC cancers will be evaluated in future63 69.
MIBC may more frequently be associated with poor prognostic factors compared with unifocal disease5 20, 22 23, 26. Coombs and Boyages10 recommended using aggregate cancer dimensions, thereby upstaging most MIBC to more advanced stages, with rates of lymph node positivity stage‐for‐stage comparable to those of unifocal cancers. Positive lymph node involvement was reported in 44–50 per cent of MIBC cases, compared with 38 per cent of unifocal cancers5 10, 20 23, 67 70. Dual‐localization SLNB is accurate diagnostically in MIBC70. A subset of women with MIBC (342, 8·5 per cent) in the European Organisation for Research and Treatment of Cancer 10981‐22023 AMAROS (After Mapping of the Axilla: Radiotherapy Or Surgery) trial had a 51 per cent rate of SLNB positivity, compared with 28 per cent of those with unifocal cancers70 71.
Clinical surveillance informing cancer outcomes should optimally extend to at least 10 years2 3. The Association of Breast Surgery guidelines50 recommend a target of 5‐year breast cancer LRR rates of 5 per cent or less from diagnosis. In future studies, the primary outcome measure for evaluating the impact of extent of surgery in MIBC should be 5‐year LRR, ideally with follow‐up to 10 years72, with disease‐specific and all‐cause mortality as important secondary outcomes53 72. The results of meta‐analyses72 including a total of 10 800 patients from 17 randomized trials of RT versus no RT after BCS underscore this; RT reduced the 10‐year risk of any first recurrence (LRR and distant) by 16 per cent and breast cancer death by 4 per cent. Yerushalmi and colleagues43 reported a 5‐year LRR rate for MIBC of 2·5 per cent after BCS compared with 4·5 per cent after mastectomy. The discordant LRR rates in favour of BCS suggest biased case selection, with clinically more aggressive disease selected for mastectomy. A similar phenomenon is likely in six reported studies that compared LRR rates in women with MIBC treated with BCS versus mastectomy and were analysed here using a forest plot; this analysis showed no intrastudy heterogeneity and no apparent effect by type of surgery. Preliminary calculations suggest that, in future, comparable surgical groups should comprise at least 1000 patients each, based on predicted 5‐year LRR rates of 2·5 per cent43, highlighting that the currently available studies were underpowered.
Some of the reviewed studies22 27 reported worse DFS and overall survival for MIBC than for single cancers, yet other studies21 43 noted similar outcomes. MC cancers (but not MF cancers) were distinguished by significantly worse overall survival (P = 0·009) and DFS (P < 0·001) compared with unifocal cancers22. However, this was negated by a complete pathological response after neoadjuvant chemotherapy, independent of type of surgery22. Similarly, Wolters et al.23 reported a significant association between MIBC and relapse‐free survival in a study of 1862 MIBC compared with 7073 unifocal cancers (P = 0·007); however, this finding related to clinical non‐adherence to German guidelines. Weissenbacher and colleagues19 confirmed a significant association between MIBC and overall breast cancer recurrence (P = 0·001) in matched‐pair multivariable analyses of MIBC compared with unifocal cancers (288 in each group). These conflicting reports support a future review of current TNM staging for MIBC.
Molecular subtyping in breast cancers provides therapeutic and prognostic stratification3 17. There is limited evidence on associations between MIBC and five molecular subtypes, compared with the subtype distribution in unifocal cancers5. A comprehensive IHC subtyping algorithm (six biomarkers) that can distinguish luminal B from luminal A cancers, and basal from triple‐negative disease, has potential clinical implications15 17, 18. Luminal cancers had a lower risk of 5‐year LRR than HER2‐positive or triple‐negative unifocal disease after BCS in 12 500 patients73. Ataseven and co‐workers22 reported increased associations between oestrogen receptor‐positive and HER2‐positive genotypes in MIBC, compared with unifocal cancers (P < 0·001). Similarly, Moon et al.74 reported fewer triple‐negative MIBC than unifocal cancers. Lynch and colleagues21 showed no significant associations between MIBC (906 patients) and molecular subtypes. Given the growing appreciation of intertumoral heterogeneity in MIBC, molecular characterization of a single focus may underestimate the molecular landscape12. Standard phenotyping and genotyping of each cancer in MIBC should underpin future treatment recommendations.
The true biological and clinical significance of MIBC remains uncertain, with current expert consensus based on limited evidence. The studies reviewed here have historical limitations and were not adequately powered for conclusive treatment recommendations to be drawn based on LRR or survival after BCS compared with mastectomy. Meta‐analyses of existing historical prospective data sets or early randomized trials are likely to be beset by poor‐quality data on pathological focality. Despite the potential for use of TM to treat MIBC, the evidence base for readily adopting this treatment is poor. Valuable data on 5‐year effect size for LRR could derive from a current registry study (ACOSOG (American College of Surgeons Oncology Group) Z11102)4 75. Based on this, an international collaboration guided by the IDEAL (Idea, Development, Exploration, Assessment, Long‐term Follow‐up) framework51 has suggested the need for a multicentre randomized trial (MIAMI trial)75. This trial should use a pragmatic classification of MF and MC cancers. In addition, the similarity or heterogeneity of genomic profiling for individual cancers is likely to supersede existing anatomical or surgical definitions of MIBC in the future.
Supporting information
Appendix S1 Search strategy
Appendix S2 Data extraction pro‐forma
Table S1 Summary of characteristics of papers reviewed and overall quality
Table S2 Newcastle–Ottawa scale scoring
Table S3 Clinical‐pathology characteristics and treatments
Table S4 Clinical outcomes
Acknowledgements
Z.E.W. and J.H. contributed equally to this review and are joint first authors. No grant funding was received for this work. The authors thank P. Sinai, who is funded by the EORTC and Abreast Cancer Research, for preparation of this manuscript. R. Davidson prepared the tables for the purposes of publication and Abreast Cancer Research funded these.
Funding information No funding
Presented to the Association of Breast Surgery Conference, Bournemouth, UK, June 2015, and the San Antonio Breast Cancer Symposium, San Antonio, Texas, USA, December 2015; published in abstract form as Eur J Surg Oncol 2015; 41: S37, and Breast 2016, 26: 149–150
References
- 1. Early Breast Cancer Trialists' Collaborative Group (EBCTCG) , Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M et al Effect of radiotherapy after breast‐conserving surgery on 10‐year recurrence and 15‐year breast cancer death: meta‐analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet 2011; 378: 1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart‐Gebhart M et al; Panel Members. Tailoring therapies – improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer. Ann Oncol 2015; 26: 1533–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart‐Gebhart M, Thürlimann B et al; Panel Members. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013; 24: 2206–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boughey JC, Rosenkranz K, Nelson H. Multiple ipsilateral breast cancers: can the breast be preserved? Bull Am Coll Surg 2012; 97: 43–45. [PubMed] [Google Scholar]
- 5. Bendifallah S, Werkoff G, Borie‐Moutafoff C, Antoine M, Chopier J, Gligorov J et al Multiple synchronous (multifocal and multicentric) breast cancer: clinical implications. Surg Oncol 2010; 19: e115–e123. [DOI] [PubMed] [Google Scholar]
- 6. Joergensen LE, Gunnarsdottir KA, Lanng C, Moeller S, Rasmussen BB. Multifocality as a prognostic factor in breast cancer patients registered in Danish Breast Cancer Cooperative Group (DBCG) 1996–2001. Breast 2008; 17: 587–591. [DOI] [PubMed] [Google Scholar]
- 7. Katz A, Strom EA, Buchholz TA, Theriault R, Singletary SE, McNeese MD. The influence of pathologic tumor characteristics on locoregional recurrence rates following mastectomy. Int J Radiat Oncol Biol Phys 2001; 50: 735–742. [DOI] [PubMed] [Google Scholar]
- 8. Desmedt C, Fumagalli D, Pietri E, Zoppoli G, Brown D, Nik‐Zainal S et al Uncovering the genomic heterogeneity of multifocal breast cancer. J Pathol 2015; 236: 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. College of American Pathologists . Protocol for the Examination of Specimens From Patients With Invasive Carcinoma of the Breast; 2017. http://www.cap.org/ShowProperty?nodePath=/UCMCon/Contribution%20Folders/WebContent/pdf/cp-breast-invasive-17protocol-4000.pdf http://www.cap.org/web/home/resources/cap-guidelines [accessed 18 February 2018]. [Google Scholar]
- 10. Coombs NJ, Boyages J. Multifocal and multicentric breast cancer: does each focus matter? J Clin Oncol 2005; 23: 7497–7502. [DOI] [PubMed] [Google Scholar]
- 11. Hilton JF, Bouganim N, Dong B, Chapman JW, Arnaout A, O'Malley F et al Do alternative methods of measuring tumor size, including consideration of multicentric/multifocal disease, enhance prognostic information beyond TNM staging in women with early stage breast cancer: an analysis of the NCIC CTG MA.5 and MA.12 clinical trials. Breast Cancer Res Treat 2013; 142: 143–151. [DOI] [PubMed] [Google Scholar]
- 12. American Joint Committee on Cancer . Breast AJCC Cancer Staging Manual (5th edn). Lippincott‐Raven: Philadelphia, 1997; 127–133. [Google Scholar]
- 13. Buggi F, Folli S, Curcio A, Casadei‐Giunchi D, Rocca A, Pietri E et al Multicentric/multifocal breast cancer with a single histotype: is the biological characterization of all individual foci justified? Ann Oncol 2012; 23: 2042–2046. [DOI] [PubMed] [Google Scholar]
- 14. Pekar G, Gere M, Tarjan M, Hellberg D, Tot T. Molecular phenotype of the foci in multifocal invasive breast carcinomas: intertumoral heterogeneity is related to shorter survival and may influence the choice of therapy. Cancer 2014; 120: 26–34. [DOI] [PubMed] [Google Scholar]
- 15. Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK et al Basal‐like breast cancer defined by five biomarkers has superior prognostic value than triple‐negative phenotype. Clin Cancer Res 2008; 14: 1368–1376. [DOI] [PubMed] [Google Scholar]
- 16. Boros M, Marian C, Moldovan C, Stolnicu S. Morphological heterogeneity of the simultaneous ipsilateral invasive tumor foci in breast carcinoma: a retrospective study of 418 cases of carcinomas. Pathol Res Pract 2012; 208: 604–609. [DOI] [PubMed] [Google Scholar]
- 17. Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J et al Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 2009; 101: 736–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nielsen TO , Perou CM. CCR 20th anniversary commentary: the development of breast cancer molecular subtyping. Clin Cancer Res 2015; 21: 1779–1781. [DOI] [PubMed] [Google Scholar]
- 19. Weissenbacher TM, Zschage M, Janni W, Jeschke U, Dimpfl T, Mayr D et al Multicentric and multifocal versus unifocal breast cancer: is the tumor‐node‐metastasis classification justified? Breast Cancer Res Treat 2010; 122: 27–34. [DOI] [PubMed] [Google Scholar]
- 20. Neri A, Marrelli D, Megha T, Bettarini F, Tacchini D, De Franco L et al Clinical significance of multifocal and multicentric breast cancers and choice of surgical treatment: a retrospective study on a series of 1158 cases. BMC Surg 2015; 15: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lynch SP, Lei X, Hsu L, Meric‐Bernstam F, Buchholz TA, Zhang H et al Breast cancer multifocality and multicentricity and locoregional recurrence. Oncologist 2013; 18: 1167–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ataseven B, Lederer B, Blohmer JU, Denkert C, Gerber B, Heil J et al Impact of multifocal or multicentric disease on surgery and locoregional, distant and overall survival of 6134 breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol 2015; 22: 1118–1127. [DOI] [PubMed] [Google Scholar]
- 23. Wolters R, Wockel A, Janni W, Novopashenny I, Ebner F, Kreienberg R et al; BRENDA Study Group. Comparing the outcome between multicentric and multifocal breast cancer: what is the impact on survival, and is there a role for guideline‐adherent adjuvant therapy? A retrospective multicenter cohort study of 8935 patients. Breast Cancer Res Treat 2013; 142: 579–590. [DOI] [PubMed] [Google Scholar]
- 24. Houssami N, Ciatto S, Macaskill P, Lord SJ, Warren RM, Dixon JM et al Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta‐analysis in detection of multifocal and multicentric cancer. J Clin Oncol 2008; 26: 3248–3258. [DOI] [PubMed] [Google Scholar]
- 25. Sardanelli F, Boetes C, Borisch B, Decker T, Federico M, Gilbert FJ et al Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer 2010; 46: 1296–1316. [DOI] [PubMed] [Google Scholar]
- 26. Yerushalmi R, Kennecke H, Woods R, Olivotto IA, Speers C, Gelmon KA. Does multicentric/multifocal breast cancer differ from unifocal breast cancer? An analysis of survival and contralateral breast cancer incidence. Breast Cancer Res Treat 2009; 117: 365–370. [DOI] [PubMed] [Google Scholar]
- 27. Chung AP, Huynh K, Kidner T, Mirzadehgan P, Sim MS, Giuliano AE. Comparison of outcomes of breast conserving therapy in multifocal and unifocal invasive breast cancer. J Am Coll Surg 2012; 215: 137–146. [DOI] [PubMed] [Google Scholar]
- 28. Gentilini O, Botteri E, Rotmensz N, Da Lima L, Caliskan M, Garcia‐Etienne CA et al Conservative surgery in patients with multifocal/multicentric breast cancer. Breast Cancer Res Treat 2009; 113: 577–583. [DOI] [PubMed] [Google Scholar]
- 29. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Higgins JPT, Green S. (eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. http://handbook-5-1.cochrane.org/ [accessed 13 February 2018]. [Google Scholar]
- 31. Deeks JJ, Dinnes J, D'Amico R, Sowden AJ, Sakarovitch C, Song F et al; International Stroke Trial Collaborative Group; European Carotid Surgery Trial Collaborative Group. Evaluating non‐randomised intervention studies. Health Technol Assess 2003; 7: iii–x, 1–173. [DOI] [PubMed] [Google Scholar]
- 32. Nos C, Bourgeois D, Darles C, Asselain B, Campana F, Zafrani B et al [Conservative treatment of multifocal breast cancer: a comparative study.] Bull Cancer 1999; 86: 184–188. [PubMed] [Google Scholar]
- 33. Kaplan J, Giron G, Tartter PI, Bleiweiss IJ, Estabrook A, Smith SR. Breast conservation in patients with multiple ipsilateral synchronous cancers. J Am Coll Surg 2003; 197: 726–729. [DOI] [PubMed] [Google Scholar]
- 34. Lim W, Park EH, Choi SL, Seo JY, Kim HJ, Chang MA et al Breast conserving surgery for multifocal breast cancer. Ann Surg 2009; 249: 87–90. [DOI] [PubMed] [Google Scholar]
- 35. Kadioğlu H, Yücel S, Yildiz S, Bozkurt S, Ersoy YE, Sağlam E et al Feasibility of breast conserving surgery in multifocal breast cancers. Am J Surg 2014; 208: 457–464. [DOI] [PubMed] [Google Scholar]
- 36. Leopold KA, Recht A, Schnitt SJ, Connolly JL, Rose MA, Silver B et al Results of conservative surgery and radiation therapy for multiple synchronous cancers of one breast. Int J Radiat Oncol Biol Phys 1989; 16: 11–16. [DOI] [PubMed] [Google Scholar]
- 37. Kurtz JM, Jacquemier J, Amalric R, Brandone H, Ayme Y, Hans D et al Breast‐conserving therapy for macroscopically multiple cancers. Ann Surg 1990; 212: 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wilson LD, Beinfield M, McKhann CF, Haffty BG. Conservative surgery and radiation in the treatment of synchronous ipsilateral breast cancers. Cancer 1993; 72: 137–142. [DOI] [PubMed] [Google Scholar]
- 39. Okumura S, Mitsumori M, Yamauchi C, Kawamura S, Oya N, Nagata Y et al Feasibility of breast‐conserving therapy for macroscopically multiple ipsilateral breast cancer. Int J Radiat Oncol Biol Phys 2004; 59: 146–151. [DOI] [PubMed] [Google Scholar]
- 40. Oh JL, Dryden MJ, Woodward WA, Yu TK, Tereffe W, Strom EA et al Locoregional control of clinically diagnosed multifocal or multicentric breast cancer after neoadjuvant chemotherapy and locoregional therapy. J Clin Oncol 2006; 24: 4971–4975. [DOI] [PubMed] [Google Scholar]
- 41. Rakovitch E, Pignol JP, Hanna W, Narod S, Spayne J, Nofech‐Mozes S et al Significance of multifocality in ductal carcinoma in situ: outcomes of women treated with breast‐conserving therapy. J Clin Oncol 2007; 25: 5591–5596. [DOI] [PubMed] [Google Scholar]
- 42. Cabioglu N, Ozmen V, Kaya H, Tuzlali S, Igci A, Muslumanoglu M et al Increased lymph node positivity in multifocal and multicentric breast cancer. J Am Coll Surg 2009; 208: 67–74. [DOI] [PubMed] [Google Scholar]
- 43. Yerushalmi R, Tyldesley S, Woods R, Kennecke HF, Speers C, Gelmon KA. Is breast‐conserving therapy a safe option for patients with tumor multicentricity and multifocality? Ann Oncol 2012; 23: 876–881. [DOI] [PubMed] [Google Scholar]
- 44. Hartsell WF, Recine DC, Griem KL, Cobleigh MA, Witt TR, Murthy AK. Should multicentric disease be an absolute contraindication to the use of breast‐conserving therapy? Int J Radiat Oncol Biol Phys 1994; 30: 49–53. [DOI] [PubMed] [Google Scholar]
- 45. Cho LC, Senzer N, Peters GN. Conservative surgery and radiation therapy for macroscopically multiple ipsilateral invasive breast cancers. Am J Surg 2002; 183: 650–654. [DOI] [PubMed] [Google Scholar]
- 46. Chie EK, Kim K, Han W, Dong‐Young NOH, Do‐Youn OH, Seock‐Ah IM et al Results of breast‐conserving therapy for multifocal or multicentric breast cancers. Asia‐Pac J Clin Oncol 2009; 5: 200–205. [Google Scholar]
- 47. Bauman L, Barth RJ, Rosenkranz KM. Breast conservation in women with multifocal–multicentric breast cancer: is it feasible? Ann Surg Oncol 2010; 17(Suppl 3): 325–329. [DOI] [PubMed] [Google Scholar]
- 48. Eryilmaz MA, Muslumanoglu M, Ozmen V, Igci A, Koc M. Breast conserving surgery in patients with multifocal/multicentric breast cancer. J BUON 2011; 16: 450–453. [PubMed] [Google Scholar]
- 49. Kapoor NS, Chung A, Huynh K, Giuliano AE. Preliminary results: double lumpectomies for multicentric breast carcinoma. Am Surg 2012; 78: 1345–1348. [PubMed] [Google Scholar]
- 50. Association of Breast Surgery . Oncoplastic Breast Reconstruction Guidelines for Best Practice; 2014. http://www.associationofbreastsurgery.org.uk/media/23851/final_oncoplastic_guidelines_for_use.pdf [accessed 8 August 2016]. [Google Scholar]
- 51. Ergina PL, Barkun JS, McCulloch P, Cook JA, Altman DG; IDEAL Group . IDEAL framework for surgical innovation 2: observational studies in the exploration and assessment stages. BMJ 2013; 346: f3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Williamson PR, Altman DG, Blazeby JM, Clarke M, Devane D, Gargon E et al Developing core outcome sets for clinical trials: issues to consider. Trials 2012; 13: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hudis CA, Barlow WE, Costantino JP, Gray RJ, Pritchard KI, Chapman JA et al Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol 2007; 25: 2127–2132. [DOI] [PubMed] [Google Scholar]
- 54. Cook JA, Hislop J, Adewuyi TE, Harrild K, Altman DG, Ramsay CR et al Assessing methods to specify the target difference for a randomised controlled trial: DELTA (Difference ELicitation in TriAls) review. Health Technol Assess 2014; 18: v–vi, 1–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chakravorty A, Shrestha AK, Sanmugalingam N, Rapisarda F, Roche N, Querci Della Rovere G et al How safe is oncoplastic breast conservation? Comparative analysis with standard breast conserving surgery. Eur J Surg Oncol 2012; 38: 395–398. [DOI] [PubMed] [Google Scholar]
- 56. Losken A, Dugal CS, Styblo TM, Carlson GW. A meta‐analysis comparing breast conservation therapy alone to the oncoplastic technique. Ann Plast Surg 2014; 72: 145–149. [DOI] [PubMed] [Google Scholar]
- 57. McIntosh J, O'Donoghue JM. Therapeutic mammoplasty – a systematic review of the evidence. Eur J Surg Oncol 2012; 38: 196–202. [DOI] [PubMed] [Google Scholar]
- 58. Bamford R, Sutton R, McIntosh J. Therapeutic mammoplasty allows for clear surgical margins in large and multifocal tumours without delaying adjuvant therapy. Breast 2015; 24: 171–174. [DOI] [PubMed] [Google Scholar]
- 59. Kim SH, Cornacchi SD, Heller B, Farrokhyar F, Babra M, Lovrics PJ. An evaluation of intraoperative digital specimen mammography versus conventional specimen radiography for the excision of nonpalpable breast lesions. Am J Surg 2013; 205: 703–710. [DOI] [PubMed] [Google Scholar]
- 60. Chagpar AB, Killelea BK, Tsangaris TN, Butler M, Stavris K, Li F et al A randomized, controlled trial of cavity shave margins in breast cancer. New Engl J Med 2015; 373: 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schnabel F, Boolbol SK, Gittleman M, Karni T, Tafra L, Feldman S et al A randomized prospective study of lumpectomy margin assessment with use of MarginProbe in patients with nonpalpable breast malignancies. Ann Surg Oncol 2014; 21: 1589–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Clinical Trials.gov . The LightPath™ Breast Cancer Study. https://clinicaltrials.gov/ct2/show/NCT02666079 [accessed 14 March 2016]. [Google Scholar]
- 63. Bartelink H, Maingon P, Poortmans P, Weltens C, Fourquet A, Jager J et al; European Organisation for Research and Treatment of Cancer Radiation Oncology and Breast Cancer Groups. Whole‐breast irradiation with or without a boost for patients treated with breast‐conserving surgery for early breast cancer: 20‐year follow‐up of a randomised phase 3 trial. Lancet Oncol 2015; 16: 47–56. [DOI] [PubMed] [Google Scholar]
- 64. Buchholz TA, Somerfield MR, Griggs JJ, El‐Eid S, Hammond ME, Lyman GH et al Margins for breast‐conserving surgery with whole‐breast irradiation in stage I and II invasive breast cancer: American Society of Clinical Oncology endorsement of the Society of Surgical Oncology/American Society for Radiation Oncology consensus guideline. J Clin Oncol 2014; 32: 1502–1506. [DOI] [PubMed] [Google Scholar]
- 65. Kirby AN, Jena R, Harris EJ, Evans PM, Crowley C, Gregory DL et al Tumour bed delineation for partial breast/breast boost radiotherapy: what is the optimal number of implanted markers? Radiother Oncol 2013; 106: 231–235. [DOI] [PubMed] [Google Scholar]
- 66. Kirwan CC, Al Sarakbi W, Loncaster J, Chan HY, Thompson AM, Wishart GC. Tumour bed clip localisation for targeted breast radiotherapy: compliance is proportional to trial‐related research activity: tumour bed clip localisation in breast radiotherapy. Eur J Surg Oncol 2014; 40: 158–162. [DOI] [PubMed] [Google Scholar]
- 67. Schaverien MV, Stallard S, Dodwell D, Doughty JC. Use of boost radiotherapy in oncoplastic breast‐conserving surgery – a systematic review. Eur J Surg Oncol 2013; 39: 1179–1185. [DOI] [PubMed] [Google Scholar]
- 68. Verhoeven K, Kindts I, Laenen A, Peeters S, Janssen H, Van Limbergen E et al A comparison of three different radiotherapy boost techniques after breast conserving therapy for breast cancer. Breast 2015; 24: 391–396. [DOI] [PubMed] [Google Scholar]
- 69. Eaton BR, Losken A, Okwan‐Duodu D, Schuster DM, Switchenko JM, Mister D et al Local recurrence patterns in breast cancer patients treated with oncoplastic reduction mammaplasty and radiotherapy. Ann Surg Oncol 2014; 21: 93–99. [DOI] [PubMed] [Google Scholar]
- 70. Donker M, Straver ME, van Tienhoven G, van de Velde CJ, Mansel RE, Litière S et al Comparison of the sentinel node procedure between patients with multifocal and unifocal breast cancer in the EORTC 10981‐22023 AMAROS Trial: identification rate and nodal outcome. Eur J Cancer 2013; 49: 2093–2100. [DOI] [PubMed] [Google Scholar]
- 71. Donker M, van Tienhoven G, Straver ME, Meijnen P, van de Velde CJ, Mansel RE et al Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981‐22023 AMAROS): a randomised, multicentre, open‐label, phase 3 non‐inferiority trial. Lancet Oncol 2014; 15: 1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jatoi I, Proschan MA. Randomized trials of breast‐conserving therapy versus mastectomy for primary breast cancer: a pooled analysis of updated results. Am J Clin Oncol 2005; 28: 289–294. [DOI] [PubMed] [Google Scholar]
- 73. Lowery AJ, Kell MR, Glynn RW, Kerin MJ, Sweeney KJ. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat 2012; 133: 831–841. [DOI] [PubMed] [Google Scholar]
- 74. Moon HG, Han W, Kim JY, Kim SJ, Yoon JH, Oh SJ et al Effect of multiple invasive foci on breast cancer outcomes according to the molecular subtypes: a report from the Korean Breast Cancer Society. Ann Oncol 2013; 24: 2298–2304. [DOI] [PubMed] [Google Scholar]
- 75. Winters ZE, Benson JR; MIAMI (Multiple Ipsilateral breast conserving surgery versus mastectomy) Trial Management Group . Surgical treatment of multiple ipsilateral breast cancers. Br J Surg 2018; 105: 466–468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Search strategy
Appendix S2 Data extraction pro‐forma
Table S1 Summary of characteristics of papers reviewed and overall quality
Table S2 Newcastle–Ottawa scale scoring
Table S3 Clinical‐pathology characteristics and treatments
Table S4 Clinical outcomes


