Summary
Introduction
We described an outbreak of C. difficile that occurred in the Internal Medicine department of an Italian hospital and assessed the efficacy of the measures adopted to manage the outbreak.
Methods
The outbreak involved 15 patients and was identified by means of continuous integrated microbiological surveillance, starting with laboratory data (alert organism surveillance). Diarrheal fecal samples from patients with suspected infection by C. difficile underwent rapid membrane immuno-enzymatic testing, which detects both the presence of the glutamate dehydrogenase antigen and the presence of the A and B toxins. Extensive microbiological sampling was carried out both before and after sanitation of the environment, in order to assess the efficacy of the sanitation procedure.
Results
The outbreak lasted one and a half month, during which time the Committee for the Prevention of Hospital Infections ordered the implementation of multiple interventions, which enabled the outbreak to be controlled and the occurrence of new cases to be progressively prevented. The strategies adopted mainly involved patient isolation, reinforcement of proper hand hygiene techniques, antimicrobial stewardship and environmental decontamination by means of chlorine-based products. Moreover, the multifaceted management of the outbreak involved numerous sessions of instruction/training for nursing staff and socio-sanitary operatives during the outbreak. Sampling of environmental surfaces enabled two sites contaminated by C. difficile to be identified.
Conclusions
Joint planning of multiple infection control practices, together with effective communication and collaboration between the Hospital Infections Committee and the ward involved proved to be successful in controlling the outbreak.
Keywords: C. difficile, Outbreak, Infection control practices, Implementation
Introduction
C. difficile is a Gram-positive anaerobic bacterium. Its vegetative cells are capable of forming spores, which confer resistance to heating, drying and chemical agents, including disinfectants. The pathogenic strains of C. difficile produce large exotoxin proteins, toxin A (TcdA) and toxin B (TcdB), which constitute the principal virulence factors of the microorganism [1, 2].
Disease caused by C. difficile can range in severity from mild diarrhea to fulminant pseudomembranous colitis and, without suitable treatment, toxic megacolon and death [3]. A recent prevalence survey of healthcare-associated infections (HAI) conducted in 183 hospitals determined that C. difficile was the most frequently reported infectious agent, being responsible for 12.1% of all HAI [4, 5].
Clostridium difficile has increased in prevalence since 2000, and has caused outbreaks of nosocomial diarrhea worldwide [6]. The main cause of most outbreaks of Clostridium difficile infection is NAP1/BI/027: a more virulent ribotype that has been associated with significantly higher morbidity and mortality as a result of more severe complications [7]. It is characterized by an in vitro overproduction of toxins A and B and by the production of binary toxins [2].
The principal risk factor in Clostridium difficile infection (CDI) is antibiotic use, and antibiotics from almost all classes have been associated with infection [7]. Other well-described risk factors are: advanced age, extensive comorbidity, and prolonged hospital stay leading to asymptomatic carriage, recurrent diarrhea, pseudomembranous colitis, or death [7-10].
Patients suffering from C. difficile infection shed large amounts of spores that are resistant to disinfectants and regular cleaning procedures, contaminating their surroundings and the hands of nurses, medical staff and others who come into contact with them; hence, contaminated environmental surfaces play a major role in the transmission of C. difficile in hospitals [11, 12].
The mortality associated with CDI is high, particularly in older adults with comorbid conditions, severe disease and illness caused by the NAP1 strain of C. difficile [13]. Mortality is at least 6% within 3 months of diagnosis and 13% in patients >80 years of age [14].
The economic impact of CDI on the healthcare system is significant, as it doubles the average length of hospitalization and increases the cost of treatment [6, 15]. Nosocomial transmission highlights the importance of rigorous infection control practices for preventing the spread of C. difficile [14, 16].
The aims of the present study were to describe an outbreak of C. difficile that occurred from 29 December 2015 to 15 February 2016 in the Internal Medicine department of an Italian hospital and to assess the efficacy of the measures adopted to manage the outbreak.
Methods
The outbreak occurred in a nationally renowned, highly specialized hospital in northern Italy, organized in accordance with treatment intensity. The facility is composed of separate pavilions with a total of 431 beds. The ward directly involved was female internal medicine, which has 26 beds.
Hospital infection cases were defined as patients with positive toxin assays > 48 hours after hospital admission.
The outbreak, which involved 15 patients from 29 December 2015 to 15 February 2016, was identified by means of continuous integrated microbiological surveillance, starting with laboratory data (alert organism surveillance). Following laboratory identification of an epidemiologically important microorganism, the dedicated software of the surveillance system automatically e-mails the data to all the members of the Hospital Infections Committee (made up of members of the hospital’s healthcare administration, physicians, microbiologists, infectious disease specialists, epidemiologists), who then implement the interventions deemed necessary, with particular regard to the application of isolation measures. A validated report is simultaneously sent through the laboratory information system to the hospital facility involved.
For patients with a diagnosis of Clostridium difficile, information on age, history of hospitalizations, antibiotic treatments, duration of hospitalization and outcome were collected.
MICROBIOLOGICAL ANALYSIS
Diarrheal fecal samples from patients with suspected infection by C. difficile underwent rapid membrane immuno-enzymatic testing by means of the TECHLAB C. diff Chek Quick Complete® (AlereTM) kit, which detects both the presence of the glutamate dehydrogenase (GDH) antigen, as a means of screening for C. difficile, and the presence of the A and B toxins.
ENVIRONMENTAL INVESTIGATION
Extensive microbiological sampling was carried out both before and after sanitation of the environment, in order to assess the efficacy of the sanitation procedure. Sampling was carried out at 14 sites of high-frequency contact; the sampling points were selected in accordance with the checklist of the CDCs reported in the APIC guidelines “Guide to Preventing Clostridium difficile Infections” [17], which specifies the critical points to be examined in the event of an outbreak. Monitoring therefore included critical surfaces in proximity to the patient’s bed (e.g. personal light switch and call button) and other surfaces at high risk of contact with hospital personnel (e.g. medicine trolley, light switch, curtains between the beds, etc) or patients.
In accordance with the methods of Best et al. [18] and Ali et al. [19], specimens were taken by using 25-cm2 sponge swabs pre-moistened with neutralizing solution (Medical Wire & Equipment, England). The swabs were then placed aseptically into sterile Stomacher bags containing 50 ml of Ringer solution (Oxoid) and homogenized manually by vigorously massaging the bag between the fingertips for 1 min. Liquid from the bag was passed through a 0.45-mm filter (Millipore), which was then placed aseptically onto Brazier’s Clostridium difficile selective agar (Oxoid). Plates were then incubated at 37°C under anaerobic conditions for 48 h prior to reading.
C. difficile was initially identified on the basis of the macroscopic appearance of colonies and microscopic characteristics, and confirmed to be C. difficile by means of latex agglutination testing (Oxoid C. difficile Test kit).
Results
DESCRIPTION OF THE OUTBREAK
Following the analysis of patients’ records, a possible index case was identified: an 86-year-old woman hospitalized on 16 December 2015 in the ward where the outbreak originated. This patient had already been admitted to the same hospital in the previous month (geriatric ward) for bronchopneumopathy.
On 12 December she was taken to the Emergency Department with bruising to the pelvis after a fall at home. A bilateral pleural effusion and respiratory insufficiency were diagnosed. She was therefore hospitalized in the Sub-intensive Care Unit and, after being stabilized, was transferred to the Internal Medicine Department three days later.
Table I reports the characteristics of the patients involved in the outbreak. Their mean age was 82.13 years (range 70-90 years), the mean Charlson index was 7 (range 4-13) and the mean duration of hospitalization before the first isolation of C. difficile was 16 days (range 4-34 days). With regard to outcome, 5 patients died (4 attributable to C. difficile), 6 were transferred to other healthcare facilities and/or wards, and 4 were discharged.
Tab. I.
Characteristics of patients involved in the outbreak
| Patient | Clostridium difficile toxins | Days of hospitalization | Age | Charlson index | Bed | Outcome |
|---|---|---|---|---|---|---|
| 1* | A and B | 16 | 85 | 7 | 23 | Transferred |
| 2 | A and B | 11 | 80 | 7 | 24 | Transferred |
| 3 | A and B | 9 | 70 | 12 | 6 | Died |
| 4 | A and B | 27 | 88 | 6 | 5 | Died |
| 5 | A and B | 26 | 79 | 4 | 8 | Discharged |
| 6 | Weak positivity | 13 | 84 | 6 | 9 | Discharged |
| 7 | A and B | 16 | 75 | 7 | 1 | Died |
| 8 | A and B | 12 | 85 | 5 | 10 | Discharged |
| 9 | A and B | 15 | 90 | 8 | 7 | Died |
| 10 | A and B | 4 | 84 | 5 | 1 | Discharged |
| 11 | A and B | 34 | 80 | 6 | 14 | Died |
| 12 | A and B | 16 | 77 | 4 | 23 | Transferred |
| 13 | Weak positivity | 12 | 80 | 7 | 10 | Transferred |
| 14 | A and B | 17 | 88 | 6 | 6 | Transferred |
| 15 | A and B | 12 | 87 | 13 | 26 | Transferred |
*Suspected index case
In 86.67% of cases, the C. difficile strain responsible for the infection produced both toxin A and toxin B; in the remaining cases, weak positivity to the immuno-enzymatic test was recorded. The patients were treated with metronidazole and, in the event of failure, vancomycin.
Figure 1 describes distribution of Clostridium difficile infected patients as a function of time
Fig. 1.
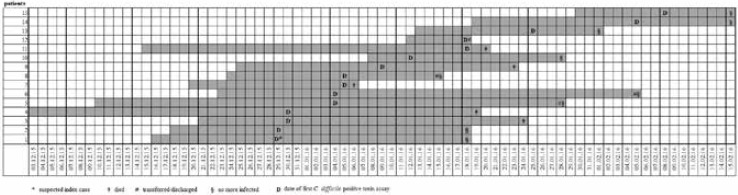
Distribution of Clostridium difficile infected patients as a function of time.
INFECTION CONTROL PRACTICES DURING THE OUTBREAK
Figure 2 shows the epidemic curve of the outbreak from 29 December 2015 to 15 February 2016. The timescale of the interventions implemented by the Committee for the Prevention of Hospital Infections is also indicated. From the moment when the first two cases of infection were diagnosed an antimicrobial stewardship program and the following interventions were implemented:
Fig. 2.
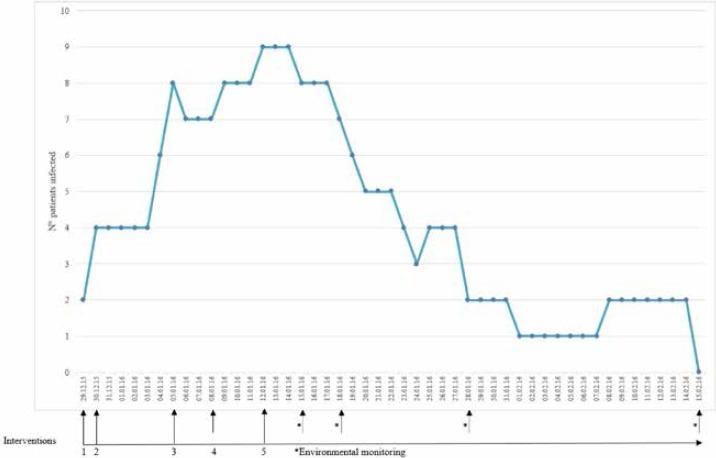
Epidemic curve of the C. difficile outbreak and the timescale of the interventions implemented.
Intervention 1: from 29 December 2015:
Specification of the measures to be taken in order to contain risk of infection by Clostridium difficile, considering all patients to be potentially infected; written instructions delivered to all healthcare personnel involved.
Testing for C. difficile toxins in all symptomatic patients.
Isolation in cohorts of infected patients; assistance to cohorts (dedicated operators); use of dedicated small devices (e.g. oximeter, hemoglucotest device, etc) for infected patients.
Ad hoc environmental sanitation for Clostridium difficile in the entire department (with 20% concentrations of chlorine-based detergent), including decontamination of telephones and computer keyboards and screens (ready-to-use sodium hypochlorite solution). In order to facilitate adequate daily sanitation, bedside tables were kept clear of all but indispensable objects (bottle of water and glass).
Checking to ensure that healthcare personnel complied with hand hygiene protocols. In addition, the hands of all non-self-sufficient patients were washed more frequently and self-sufficient patients were instructed on how to wash their hands properly.
Checking to ensure that gloves were used properly and were changed after assisting each individual patient, and that hands were washed immediately after the removal of gloves.
Operators involved in direct assistance were instructed to change their overalls daily and were encouraged to use microfiber overalls, which are more protective of the hygiene of infected patients, and disposable nonwoven gowns.
Staff were forbidden to use personal mobile phones while assisting infected patients.
Correct patient hygiene practices were emphasized; soiled underwear was placed in an impermeable bag labeled with the patient’s name, which was then placed in a dedicated container inside the room/cubicle. If a patient lift was used, the sling cover was changed for each patient and sent for disinfection as if it were certainly infected; the same approach was adopted towards minor aids, for which disposable protective covers were also used.
The number of visitors was reduced, and a specific information leaflet concerning the behavior of visitors to infected patients was distributed; this provided instructions on hand washing and interpersonal contact.
The day after implementation of intervention 1, another two cases of infection were discovered. Following a meeting to update and instruct nursing staff and social/healthcare workers, the second phase of intervention was implemented.
Intervention 2: from 30 December 2015:
Simulation of donning and removing personal protection devices (PPD).
Reiteration of procedures for the proper sanitation of stands for i.v. drips, commode chairs, infusion pumps, PCs and telephones.
Meals served in heat-sealed containers for all patients.
Checking of proper isolation of infected patients (e.g. collocation of the patient, supply of hand-washing requisites, availability of disposable overalls, materials and dedicated devices, etc.); this revealed the need to supply some types of medical devices for dedicated use (e.g. stethoscope and sphigmomanometer).
Urgent processing of fecal samples for culture tests; prompt telephone communication of positive reports to the expert consultants of the Committee for the Prevention of Hospital Infections, for immediate application of the necessary measures.
Periodic checks on compliance with the measures recommended.
Intervention 3: from 5 January 2016
Ward staff increased on both day shifts and night shifts.
Intervention 4: 8 January 2016
Review of cases following the administration of antibiotic treatment and implementation of the control measures; assessment of the need to institute further briefings/training for medical and nursing staff.
Intervention 5: from 12 January 2016
Structural, logistical and organizational segregation of infected patients (left side of the ward) from uninfected patients (right side), and consequent reorganization of the activities of sanitation and assistance.
Direct observation to ensure proper implementation of the measures to contain the risk of infection, and institution of “on the job” staff training with regard to: donning and removal of personal protection devices; the hygiene of infected patients, with particular regard to the hands; decontamination of the patient-unit; use of personalized devices for each patient; decontamination of the environment, materials and medical devices; functional isolation of cohorts; institution of a differential pathway from “clean” to “dirty”; proper collection and conservation of fecal samples prior to analysis; application of medication to CVC with maintenance of asepsis in infected patients; healthcare education of visitors, with simulation of hand hygiene.
From this date onwards, thanks to the set of control measures adopted, the number of cases of infection progressively diminished, and the last two cases recorded on 8 February 2016 were resolved.
ENVIRONMENTAL MONITORING
The microbiological results of environmental monitoring conducted on 15 January 2016 revealed contamination by Clostridium difficile on the curtain separating two beds that had been occupied by patients involved in the outbreak (beds 23 and 24) and on the call button of bed 24. The curtain was promptly removed and disposed of, and the entire environment was thoroughly disinfected. Subsequent monitorings, carried out after environmental sanitation, revealed no contamination by C. difficile.
Discussion
Several reports suggest that the incidence and severity of C. difficile infection have been increasing in recent years across the United States, Canada and Europe. Recent data from 28 community hospitals in the southern United States suggest that C. difficile has replaced methicillin-resistant Staphylococcus aureus as the most common cause of healthcare-associated infection [20, 21]. The burden of healthcare-associated CDIs in acute-care hospitals in the EU/EEA has been estimated at 123,997 cases annually. In the ECDC point prevalence survey of healthcare-associated infections in European acute-care hospitals 2011-2012, C. difficile was the 8th most frequently detected microorganism among HAIs [22].
In the present study, we documented the occurrence of 15 cases of C. difficile infection in an internal medicine department in an Italian hospital. During the outbreak the Committee for the Prevention of Hospital Infections ordered the implementation of multiple interventions, which enabled the outbreak to be controlled and the occurrence of new cases to be progressively prevented.
The outbreak described in this paper started and finished in a single ward, involved a relatively small number of patients, and lasted one and a half month. Wong-McClure et al. [23] described an outbreak due to C. difficile that involved three wards and 389 patients, and which lasted for several months. More recently, van Beurden et al. [6] described an outbreak that involved 19 wards and 72 patients, and which lasted for a year.
As pointed out by several studies, there may not be a single method that is effective in minimizing exposure to C. difficile, and a multifaceted approach is usually required [24]. Indeed, the management of CDI in hospitals requires just such a multidisciplinary approach, which begins with infection prevention. A previous study by Weiss et al. [25] showed that a multi-pronged intervention strategy is most effective in reducing the rate of healthcare CDI.
Strategies for the prevention and control of C. difficile infections are aimed at promptly identifying, isolating and efficaciously treating patients affected by CDI (in order to reduce the dissemination of spores and prevent secondary cases) and at minimizing preventable risk factors through the implementation of protocols of behavior, environmental sanitation and antibiotic stewardship [26].
In accordance with this approach, the strategies adopted for the control of the Clostridium difficile outbreak described here mainly involved patient isolation, reinforcement of proper hand hygiene techniques, antimicrobial stewardship and environmental decontamination by means of chlorine-based products.
Indeed, the presence of other patients with infection, hand carriage on the part of healthcare personnel and contaminated environmental surfaces are considered to be major factors in the transmission of pathogens in hospitals [27-29], including C. difficile.
When there is an infected patient in hospital, the hospital environment is contaminated by spores within a few hours of the onset of diarrhea; other patients may therefore be infected and the patient himself/herself may be reinfected. Moreover, C. difficile spores are highly resistant to many commonly used disinfectants and may persist for months in hospital environments [30].
Environmental contamination with C. difficile spores occurs at as many as 34-58% of sites, despite cleaning, with surfaces of fomites being most frequently contaminated [18].
Frequenly touched surfaces in near patient areas are rapidly contaminated by the microorganisms disseminated by the infected patient occupying the room, and may remain contaminated for extended periods of time [31]. Consequently, C. difficile can be found on hospital floors, on bedrails, windowsills, commodes, toilets, call buttons, blood pressure cuffs, electronic thermometers, bedsheets and anything that comes into contact with contaminated hands [32]. Thus, thorough disinfection of the contaminated hospital environment is essential in order to prevent the transmission of this nosocomial pathogen, and the choice of hospital decontamination protocols can markedly affect the prevalence and environmental distribution of C. difficile contamination [33].
The scientific evidence supports the use of detergents containing chlorine (at least 1000 ppm of active chlorine) in endemic situations or during epidemic outbreaks [1]. A study by Fawley et al. [33] compared the efficacy of five different cleaning agents against epidemic and non-epidemic C. difficile strains. They found that only chlorine-based germicides were able to inactivate C. difficile spores.
Contamination of the hands of healthcare staff and patients with C. difficile is a major route of transmission of the infection, and there is a close correlation between hand contamination and the degree of environmental contamination. For this reason, proper hand hygiene is crucial to preventing the transmission of C. difficile in the hospital setting [32].
During the outbreak described in this paper, various interventions were undertaken in order to ensure adherence to hand hygiene protocols on the part of healthcare staff and patients; those visiting infected patients were also taught to wash their hands and to limit contact only to the patient being visited. Indeed, checking the staff’s compliance with hand hygiene has been deemed a more effective strategy than microbiological testing of the hands by means of sampling.
A recommendation common to many guidelines on the prevention and control of healthcare-related infections concerns the training of healthcare personnel, visitors, caregivers and patients themselves. The multifaceted management of the outbreak described here involved numerous sessions of instruction/training for nursing staff and socio-sanitary operatives during the course of the epidemic. By modifying risk behaviors, these interventions certainly helped to control the outbreak.
Equally important was environmental monitoring. Limited to times of outbreak, rather than being part of routine practice, this can provide a valuable estimate of the level of contamination on surfaces such as walls, work surfaces, floors and equipment [34] and is currently recommended by the Centers for Disease Control and Prevention [35]. In the present case, sampling of environmental surfaces enabled two sites contaminated by C. difficile to be identified, one of which was a soft plastic-coated curtain separating two beds that had previously been occupied by infected patients. As this curtain would have been very difficult to disinfect, it was removed and disposed of immediately after the detection of contamination; this measure may well have enabled an environmental reservoir of the microorganism to be eliminated, a hypothesis that is also supported by the trend in the epidemic curve after the implementation of environmental monitoring.
In conclusion, joint planning of multiple infection control practices, together with effective communication and collaboration between the Hospital Infections Committee and the ward involved proved to be successful in controlling the outbreak.
Acknowledgements
All authors declare that there is no conflict of interest.
Footnotes
Authors’ contributions
MLC conceived and designed the study. AB collected data. BC performed the data quality control. ES performed environmental controls. MS validating and analysing the data. MLC and AMS wrote the paper. GLP revised the manuscript. All Authors revised the manuscript and gave their contribution to improve the paper. All authors read and approved the final manuscript.
References
- [1].Cristina ML, Spagnolo AM, Sartini M, Panatto D, Perdelli F. Clostridium difficile infections: An emerging problem in healthcare facilities. Rev Med Microbiol 2012;23:67-75. [Google Scholar]
- [2].Jia H, Du P, Yang H, Zhang Y, Wang J, Zhang W, Han G, Han N, Yao Z, Wang H, Zhang J, Wang Z, Ding Q, Qiang Y, Barbut F, Gao GF, Cao Y, Cheng Y, Chen C. Nosocomial transmission of Clostridium difficile ribotype 027 in a Chinese hospital, 2012-2014, traced by whole genome sequencing. BMC Genomics 2016;17:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Secore S, Wang S, Doughtry J, Xie J, Miezeiewski M, Rustandi RR, Horton M, Xoconostle R, Wang B, Lancaster C, Kristopeit A, Wang SC, Christanti S, Vitelli S, Gentile MP, Goerke A, Skinner J, Strable E, Thiriot DS, Bodmer JL, Heinrichs JH. Development of a novel vaccine containing binary toxin for the prevention of clostridium difficile disease with enhanced efficacy against NAP1 Strains. PLoS One 2017;12(1):e0170640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SKEmerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014;370:1198-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Morfin-Otero R, Garza-Gonzalez E, Aguirre-Diaz SA, Escobedo-Sanchez R, Esparza-Ahumada S, Perez-Gomez HR, Petersen-Morfin S, Gonzalez-Diaz E, Martinez-Melendez A, Rodriguez-Noriega E; Hospital Civil de Guadalajara, Fray Antonio Alcalde Clostridium difficile Team. Clostridium difficile outbreak caused by NAP1/BI/027 strain and non-027 strains in a Mexican hospital. Braz J Infect Dis 2016;20:8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].van Beurden YH, Bomers MK, van der Werff SD, Pompe EA, Spiering S, Vandenbroucke-Grauls CM, Mulder CJ. Cost analysis of an outbreak of Clostridium difficile infection ribotype 027 in a Dutch tertiary care centre. J Hosp Infect 2017;95:421-5. [DOI] [PubMed] [Google Scholar]
- [7].van Beurden YH, Dekkers OM, Bomers MK, Kaiser AM, van Houdt R, Knetsch CW, Girbes AR, Mulder CJ, Vandenbroucke-Grauls CM. An outbreak of Clostridium difficile ribotype 027 associated with length of stay in the intensive care unit and use of selective decontamination of the digestive tract: A Case Control Study. PLoS One 2016;11:e0160778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kumar N, Miyajima F, He M, Roberts P, Swale A, Ellison L, Pickard D, Smith G, Molyneux R, Dougan G, Parkhill J, Wren BW, Parry CM, Pirmohamed M, Lawley TD. Genome-based Infection tracking reveals dynamics of Clostridium difficile transmission and disease recurrence. Clin Infect Dis 2016;62:746-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Visconti V, Brunetti G, Cuomo MR, Giordano A, Raponi G. Nosocomial-acquired and community-onset Clostridium difficile infection at an academic hospital in Italy: Epidemiology, recurrences and toxin genes distribution. J Infect Chemother 2017; doi: 10.1016/j.jiac.2017.08.002. [DOI] [PubMed] [Google Scholar]
- [10].Vonberg RP, Kuijper EJ, Wilcox MH, Barbut F, Tüll P, Gastmeier P, European C difficile-Infection Control Group; European Centre for Disease Prevention and Control (ECDC) van den Broek PJ, Colville A, Coignard B, Daha T, Debast S, Duerden BI, van den Hof S, van der Kooi T, Maarleveld HJ, Nagy E, Notermans DW, O’Driscoll J, Patel B, Stone S, Wiuff C. Infection control measures to limit the spread of Clostridium difficile. Clin Microbiol Infect 2008;14:2-20. [DOI] [PubMed] [Google Scholar]
- [11].Morales L, Rodríguez C, Gamboa-Coronado MD. Molecular detection of Clostridium difficile on inert surfaces from a Costa Rican hospital during and after an outbreak. Am J Infect Control 2016;44:1517-9. [DOI] [PubMed] [Google Scholar]
- [12].Cristina ML, Spagnolo AM, Orlando P, Perdelli F. The role of the environment in the spread of emerging pathogens in at-risk hospital wards. Rev Med Microbiol 2013;104-12. [Google Scholar]
- [13].Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis 2015;60:S66-71. [DOI] [PubMed] [Google Scholar]
- [14].Jullian-Desayes I, Landelle C, Mallaret MR, Brun-Buisson C, Barbut F. Clostridium difficile contamination of health care workers’ hands and its potential contribution to the spread of infection: Review of the literature. Am J Infect Control 2017;45:51-8. [DOI] [PubMed] [Google Scholar]
- [15].Bouza E. Consequences of Clostridium difficile infection: understanding the healthcare burden. Clin Microbiol Infect 2012;18:5-12. [DOI] [PubMed] [Google Scholar]
- [16].Planche T, Aghaizu A, Holliman R, Riley P, Poloniecki J, Breathnach A, Krishna S. Diagnosis of Clostridium difficile infection by toxin detection kits: a systematic review. Lancet Infect Dis 2008;8:777-84. [DOI] [PubMed] [Google Scholar]
- [17].APIC Implementation Guide 2013. Guide to Preventing Clostridium difficile Infections. 2013. http://apic.org/Resource_/EliminationGuideForm/59397fc6-3f90-43d1-9325-e8be75d86888/File/2013CDiffFinal.pdf. Accessed 30 Dec 2015.
- [18].Best EL, Fawley WN, Parnell P, Wilcox MH. The potential for airborne dispersal of Clostridium difficile from symptomatic patients. Clin Infect Dis 2010;50:1450-7. [DOI] [PubMed] [Google Scholar]
- [19].Ali S, Muzslay M, Wilson P. A novel quantitative sampling technique for detection and monitoring of Clostridium difficile contamination in the clinical environment. J Clin Microbiol 2015;53:2570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lessa FC, Gould CV, McDonald LC. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis 2012;55:S65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile Infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol 2011;32:387-90. [DOI] [PubMed] [Google Scholar]
- [22].ECDC. Clostridium difficile infections. 2016. http://antibiotic.ecdc.europa.eu/en/healthtopics/Healthcare-associated_infections/Clostridium-difficile-infections/Pages/Clostridium-difficile-infections.aspx Accessed 3 Apr 2017.
- [23].Wong-McClure RA, Ramírez-Salas E, Mora-Brenes N, Aguero-Sandí L, Morera-Sigler M, Badilla-Vargas X, Hernández-de Merzerville M, O’Shea M, Bryce E. Long term effect of infection control practices and associated factors during a major Clostridium difficile outbreak in Costa Rica. J Infect Dev Ctries 2013;7:914-21. [DOI] [PubMed] [Google Scholar]
- [24].Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol 2010;31:431-55. [DOI] [PubMed] [Google Scholar]
- [25].Weiss K, Boisvert A, Chagnon M, Duchesne C, Habash S, Lepage Y, Letourneau J, Raty J, Savoie M. Multipronged intervention strategy to control an outbreak of Clostridium difficile infection (CDI) and its impact on the rates of CDI from 2002 to 2007. Infect Control Hosp Epidemiol 2009;30:156-62. [DOI] [PubMed] [Google Scholar]
- [26].SIMPIOS. Prevenzione e controllo delle infezioni da Clostridium difficile. 2009. http://www.simpios.it/public/ufiles/Prevenzione%20e%20controllo%20delle%20infezioni%20da%20Clostridium%20Difficile.pdf Accessed 30 Dec 2015.
- [27].Perdelli F, Dallera M, Cristina ML, Sartini M, Ottria G, Spagnolo AM, Orlando P. A new microbiological problem in intensive care units: environmental contamination by MRSA with reduced susceptibility to glycopeptides. Int J Hyg Environ Health 2008;211:213-8. [DOI] [PubMed] [Google Scholar]
- [28].Orlando P, Cristina ML, Dallera M, Ottria G, Vitale A, Badolati G. Surface disinfection: evaluation of the efficacy of a nebulization system spraying hydrogen peroxide. J Prev Med Hyg 2008;49:116-9. [PubMed] [Google Scholar]
- [29].Spagnolo AM, Orlando P, Panatto D, Amicizia D, Perdelli F, Cristina ML. Staphylococcus aureus with reduced susceptibility to vancomycin in healthcare settings. J Prev Med Hyg 2014;55:137-44. [PMC free article] [PubMed] [Google Scholar]
- [30].Kuijper EJ, Coignard B, Tüll P. Emergence of Clostridium difficile-associated disease in North America and Europe. Clin Microbiol Infect 2006;12:2-18. [DOI] [PubMed] [Google Scholar]
- [31].Ali S, Muzslay M, Bruce M, Jeanes A, Moore G, Wilson AP. Efficacy of two hydrogen peroxide vapour aerial decontamination systems for enhanced disinfection of meticillin-resistant Staphylococcus aureus, Klebsiella pneumoniae and Clostridium difficile in single isolation rooms. J Hosp Infect 2016;93:70-7. [DOI] [PubMed] [Google Scholar]
- [32].Martinez FJ, Leffler DA, Kelly CP. Clostridium difficile outbreaks: prevention and treatment strategies. Risk Manag Healthc Policy 2012;5:55-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fawley WN, Underwood S, Freeman J, Baines SD, Saxton K, Stephenson K, Owens RC, Jr, Wilcox MH. Efficacy of hospital cleaning agents and germicides against epidemic Clostridium difficile strains. Infect Control Hosp Epidemiol 2007;28:920-5. [DOI] [PubMed] [Google Scholar]
- [34].Ottria G, Dallera M, Aresu O, Manniello MA, Parodi B, Spagnolo AM, Cristina ML. Environmental monitoring programme in the cell therapy facility of a research centre: preliminary investigation. J Prev Med Hyg 2010;51:133-8. [PubMed] [Google Scholar]
- [35].Sehulster L, Chinn R. CDC HICPAC. Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 2003;52:1-42. [PubMed] [Google Scholar]


