Abstract
Background
Renal podocyte damage plays a crucial role in the development of diabetic nephropathy. Genistein is derived from a leguminous plant, and MyD88 and TRIF are adaptor molecules in the Toll-like receptor (TLR) signaling pathway, which may play a role in autophagy. In this study, we utilized an in vitro high glucose (HG)-treated podocyte model to investigate the effects and underlying mechanisms of Genistein and MyD88 or TRIF siRNA induced autophagy and renal protection.
Material/Methods
An immortalized mouse podocyte cell line was treated with HG, Genistein, chloroquine, and/or transfected with specific Myd88 and TRIF siRNAs. The formation of autophagosomes and related autophagic vacuoles were monitored by transmission electron microscopy. The expression of autophagy-related factors and podocyte structure and functional markers, including LC3, p62, p-mTOR, synaptopodin, and nephrin, were measured by Western blot, and LC3 and p-mTOR expression were also assessed by immunofluorescence.
Results
We showed that HG transiently (after 6-h exposure) induced expression of the autophagy activation marker LC3-II in podocytes. Genistein treatment induced autophagy in both normal and HG-treated podocytes through inactivating mTOR signaling. Moreover, Genistein protected podocytes against chloroquine in HG-cultured conditions in vitro by maintaining the level of autophagy-related proteins. In addition, MyD88 siRNA downregulated expression of autophagy-related proteins, whereas Genistein treatment reversed these effects.
Conclusions
This study demonstrated that Genistein-induced autophagy could be a potential treatment strategy for glomerular diseases.
MeSH Keywords: Autophagy, Diabetic Nephropathies, Genistein, Podocytes
Background
Diabetes mellitus is a significant health problem worldwide, and the incidence rate of diabetes has reached alarming levels [1,2]. Diabetic nephropathy is the most common microvascular complication in both type 1 and type 2 diabetes, and it is a major cause of chronic renal failure and end-stage renal disease. The rising prevalence of diabetic nephropathy has increased diabetes-related mortality and morbidity. Thus, prevention and management of diabetes-induced complications are critical for prolonging lifespan in diabetes patients.
Podocytes wrap around capillaries of the glomerulus to form the Bowman’s capsule in the kidney, which filters blood to produce urine [3,4]. Altered morphology and reduced podocytes are the earliest pathological manifestations of diabetic nephropathy [5]. Cell autophagy is the orderly degradation and recycling of cellular components, which utilizes a lysosomal protein degradation pathway [6]. This catabolic process is a normal cell function that plays a crucial role in maintenance of cell homeostasis and integrity by removing unnecessary or dysfunctional components or organelles [7]. In this regard, autophagy primarily exerts a protective effect under physiological conditions and ensures cell survival under stress conditions. This “waste management” mechanism is especially important in terminally differentiated podocytes that have a very limited capacity for cell division and renewal [4]; thus, podocytes exhibit a high basal level of autophagic activity [8,9].
Molecularly, amino acids, growth factors, and reactive oxygen species (ROS) can activate protein kinases such as mTOR to downregulate autophagy [10–12]. In autophagy, a cytosolic truncated protein (LC3-I) is converted to its autophagosomal membrane-associated, phosphatidylethanolamine-conjugated form (LC3-II) to form an autophagosome. Since the autophagy substrates LC3B-II and p62 are both degraded with the autophagic cargo in the autolysosome, accumulation of LC3B-II and p62 aggregates is regarded as a robust marker of impaired autophagic flux. Furthermore, Toll-like receptor (TLR) signaling was recently implicated in autophagy [13]. Knockdown of TLR4 expression can inhibit cell proliferation, but the autophagy-induced survival mechanism is an evolutionarily conserved catabolic pathway that is involved in several physiological processes, such as cell metabolism, survival, and host defense [14,15]. TLR4 is expressed in podocytes and upregulated in inflammatory glomerular diseases. Mechanistically, activation of TLR4 through an adaptor molecule, myeloid differentiation primary response 88 (MyD88) or TIR-domain-containing adapter-inducing interferon-β (TRIF), leads to nuclear factor kappa B (NF-κB) translocation into the nuclei and subsequent upregulation of pro-inflammatory cytokines and chemokines. Despite increasing knowledge regarding the role of autophagy in inflammation, little is known about the mechanism underlying MyD88/TRIF-induced autophagy and inflammation.
Genistein is an isoflavone present in soy and is known to have multiple molecular effects, such as inhibition of inflammation, promotion of apoptosis, and modulation of steroidal hormone receptors and metabolic pathways [16,17]. Since these molecular effects affect carcinogenesis, cancer propagation, obesity, osteoporosis, and metabolic syndromes, Genistein plays an important role in preventing and treating common disorders. Recently, it was reported that Genistein might prevent or delay diabetic nephropathy progression by inhibiting inflammation via inactivating NF-κB and monocyte chemotactic protein-1 (MCP-1) pathways [18]. However, it is unclear whether the underlying mechanism of Genistein in the treatment of diabetic nephropathy is associated with podocyte autophagy. We utilized the in vitro high glucose-induced cell model to investigate the effects and underlying mechanisms of Genistein on renal protection and autophagy induction. Our findings provide insight into the role of Genistein in modulating autophagic activity and protection of podocytes.
Material and Methods
Cell culture and treatment
A thermosensitive SV-40-immortalized mouse podocyte cell line, H-2Kb-tsA58, was obtained from the Chinese National Cell Resource Center (Beijing, China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin (North China Pharmaceutical Company, Shijiazhuang, China) at 33°C (permissive conditions) in a 5% CO2 incubator. Cells were treated with mouse recombinant interferon-γ (IFN-γ) at a dose of 10 U/ml (Sigma, St Louis, MO, USA). After cells reached approximately 80% confluency, podocytes were cultured at 37°C (non-permissive conditions) without IFN-γ for 10 to 14 days to induce differentiation and were used for our experiments. For culture in high glucose (HG) condition, cells were incubated in fresh culture medium containing D-glucose at a normal concentration of 5.5 mmol/L (normal glucose; NG) or at a high D-glucose concentration of 30 mmol/L (HG). In addition, cells cultured in NG medium (5.5 mmol/l D-glucose) were supplemented with 19.5 mmol/L D-mannitol to maintain the osmotic effects of a HG concentration.
Small interfering RNA and cell transfection
MyD88 siRNA was purchased from Invitrogen (Carlsbad, CA, USA) and transfected into podocytes according to the manufacturer’s instructions. Briefly, podocytes were seeded into 6-well plates at a density of 2×105 per well in an antibiotic-free DMEM supplemented with 10% FBS and grown for 24 h at 37°C and then serum-starved for 24 h. After siRNA transfection, the cells were washed with DMEM once and cultured with DMEM containing 10% FBS for 24 h and then with DMEM containing high D-glucose or gremlin for an additional 48 h. Thereafter, the cells were harvested for protein extraction and other experimental procedures as described below. All experiments were done in triplicate and repeated at least twice.
Western blot
Podocytes were treated with Genistein (20 μM) or siRNA for 6 h. The cells were homogenized and lysed in an ice-cold radioimmunoprecipitation assay buffer (RIPA; Solarbio, Beijing, China) and then centrifuged. Protein concentrations were assayed using Coomassie Brilliant Blue (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Protein samples were then fractionated in 10% or 12% denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred onto polyvinylidene fluoride membranes (PVDF; Millipore, Billerica, MA, USA). For Western blot analysis, the membranes were blocked in 5% fat-free dry milk in Tris-buffered saline-Tween 20 (TBST) for 2 h, followed by incubation with a primary antibody at 4°C overnight. Primary antibodies included: anti-LC3 (Abgent, Suzhou, China), anti-p62 (Abgent), anti-p-mTOR (Cell Signaling Technology, Danvers, MA, USA), anti-mTOR (Cell Signaling Technology), anti-Myd88 (Novus Biologicals, Littleton, CO, USA), anti-TRIF (Abcam, Cambridge, MA, USA), anti-synaptopodin (Proteintech, Chicago, IL, USA), anti-nephrin (Abcam), and anti-β-actin (Blue Gene, Shanghai, China). On the next day, the membranes were washed with TBST 3 times and then incubated with goat anti-rabbit or mouse IgG conjugated with horseradish peroxidase (Zhongshan Gold-Bridge, Beijing, China). Positive protein bands were detected using an enhanced chemiluminescence (ECL) reagent (Tiangen, Beijing, China) and quantified relative to β-actin using Image J software (National Institutes of Health, Bethesda, MD, USA).
Immunofluorescence
Following Genistein treatment or siRNA transfection, podocytes were seeded onto 6-well chamber slides, grown overnight, and then fixed with frozen acetone at −4°C for 10 min. After washing with tap water, the chamber slides were permeabilized with 0.3% Triton X-100 at room temperature for 10 min and subsequently incubated with a primary antibody against LC3 and p-mTOR at 4°C overnight. On the next day, the slides were washed with TBS and then incubated with a FITC-conjugated secondary antibody at 37°C for 1 h. After washing, the slides were counterstained with 4, 6-diamidino-2-phenylindole (DAPI) to visualize cell nuclei, and images were captured under a fluorescence microscope (Olympus BX63, Olympus Tokyo, Japan).
Transmission electron microscopy
Podocytes were cultured in 6-cm cell culture dishes in DMEM containing HG (30 mmol/l) for 6 h, detached with trypsin, centrifuged, and fixed in 2.5% glutaraldehyde solution (Sigma) at 4°C overnight. Next, the cells were washed with 0.1 M phosphate-buffered saline (PBS) and post-fixed with 1% (w/v) osmium tetroxide (Sigma) at 20°C for 2 h at room temperature. The samples were then dehydrated and solidified for ultrathin-sectioning and staining and viewed under a Hitachi transmission electron microscope (Tokyo, Japan).
Statistical analysis
Data were analyzed using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). Quantitative data are summarized as mean ± standard deviation (SD) from 3 independent experiments and analyzed using one-way analysis of variance (ANOVA) for differences among multiple groups. P<0.05 was considered statistically significant.
Results
High glucose induces transient induction of LC3-II expression in podocytes
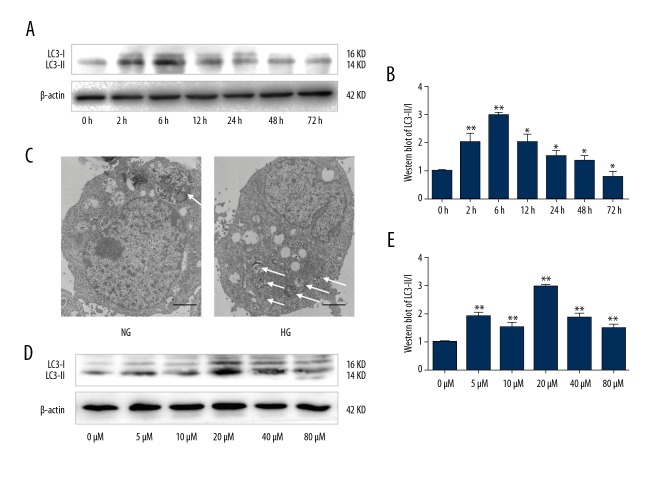
To determine the effect of HG on autophagy activation in podocytes, we measured expression of LC3-II, a biomarker for autophagy. We first grew podocytes in either HG or NG containing DMEM (30 mM vs. 5.5 mM glucose, respectively) for various periods of time (0, 2, 6, 12, 24, 48, and 72 h) and then performed Western blot analysis (Figure 1A, 1B). LC3-II expression increased in podocytes after 6 h of HG treatment compared to the NG group and then returned to basal levels. To confirm HG-induced autophagy in podocytes, we performed morphological analysis using transmission electron microscopy. We monitored the appearance of autophagosomes and related autophagic vacuoles. As shown in Figure 1C, there was only 1 obvious autophagic vacuole in podocytes exposed to NG, whereas several autophagic vacuoles were visible in podocytes after HG treatment for 6 h.
Figure 1.
High glucose induced LC3-II expression and autophagy in podocytes (A) Western blot. Podocytes were cultured in HG (30 mM) or NG (5.5 mM) for various periods of time (0, 2, 6, 12, 24, 48, and 72 h) and subjected to Western blot analysis of LC3-II expression. (B) Quantified data of A. Values are presented as mean ±SD from 3 independent experiments. * P<0.05 and ** P<0.01 vs. control (0 h). (C) Transmission electron microscopy. Podocytes were cultured in HG (30 mM) or NG (5.5 mM) for 6 h and subjected to transmission electron microscopy. Magnification, ×5000. (D) Western blot. Podocytes were cultured in Genistein at various concentrations (0, 5, 10, 20, 40 and 80 μM) and subjected to Western blot analysis of LC3-II expression. (E) Quantified data of D. The values are expressed as mean ±SD of 3 independent experiments. ** P<0.01 vs control (0).
Genistein induces autophagy in podocytes by inhibiting the mTOR pathway in vitro
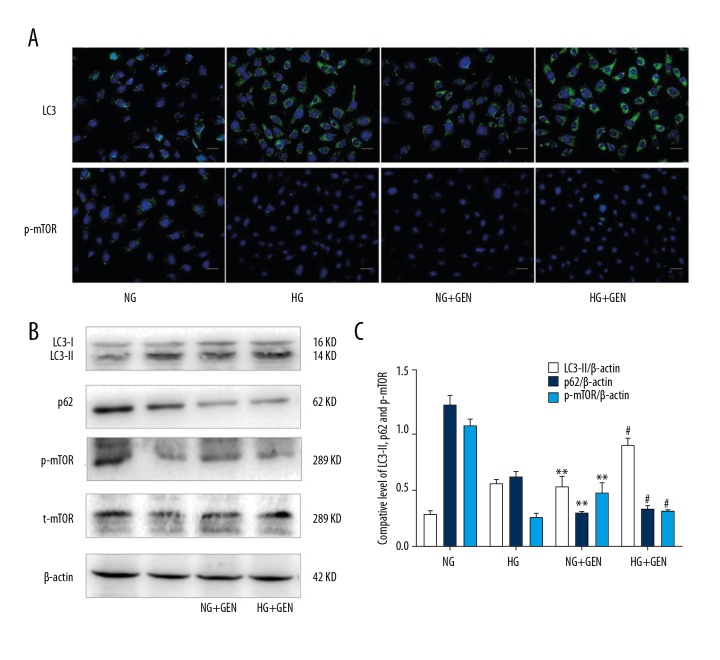
To determine the effects of Genistein on LC3-II expression, we first grew podocytes in NG containing DMEM (5.5 mM glucose) with various concentrations of Genistein (0, 5, 10, 20, 40, or 80 μM) and then performed Western blot analysis. Our data showed that 20 μM of Genistein most effectively induced LC3-II expression in podocytes (Figure 1D, 1E) after 6 h of HG treatment compared to control. We then investigated the molecular mechanism underlying Genistein-induced autophagy in podocytes. We first measured LC3 and p-mTOR expression in podocytes using double immunostaining and LC3, p62, p-mTOR, and total mTOR expression using Western blot. Immunostaining data showed that the fluorescence intensity of LC3 was stronger in the Genistein (20 μM)-treated and HG-induced podocytes compared to the non-Genistein-treated podocytes (Figure 2), whereas we observed the opposite effects on p-mTOR levels in the Genistein-treated podocytes (Figure 2). Western blot data further showed that p-mTOR and total mTOR levels were downregulated in Genistein-treated cells. Moreover, LC3-II and p62, which are mTOR downstream genes, were significantly upregulated by Genistein treatment (P<0.01; Figure 2). These data suggest that Genistein-induced autophagy negatively regulates mTOR signaling in podocytes.
Figure 2.
Genistein induced autophagy through inhibiting the mTOR pathway in podocytes. (A) Immunofluorescence. Podocytes were cultured in HG (30 mM) or NG (5.5 mM) for 6 h and treated with Genistein (20 μM) for 6 h and then subjected to immunofluorescence detection of LC3 and p-mTOR (green). Nuclei are labeled with DAPI (blue). Magnification, ×200. (B) Western blot. Podocytes were cultured in HG (30 mM) or NG (5.5 mM) for 6 h and treated with Genistein (20 μM) for 6 h and then subjected to Western blot analysis. (C) Quantified data of B. Values are expressed as mean ±SD of 3 independent experiments. * P<0.05 and ** P<0.01 vs. NG. # P < 0.01 vs. HG.
Genistein protects podocytes against chloroquine-induced inhibition in high glucose-cultured podocytes
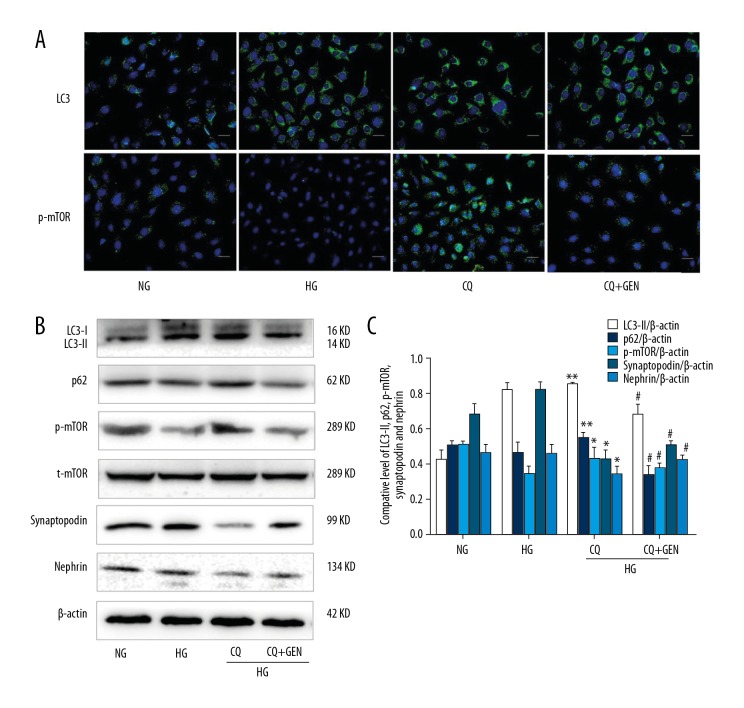
To further investigate if Genistein protects podocytes against chloroquine-induced inhibition of autophagy, we measured autolysosomal degradation to determine the upstream autophagic activation. As shown in Figure 3, immunostaining and Western blot analysis demonstrated that Genistein treatment reversed the decrease in synaptopodin and nephrin expression (functional protein markers in podocytes) after treatment with chloroquine. Furthermore, the levels of p62 and p-mTOR also decreased in podocytes after treatment with both Genistein and chloroquine. These results suggest that Genistein induces autophagy and protects podocytes.
Figure 3.
Genistein attenuated autophagy inhibition caused by chloroquine in high glucose-induced podocytes. (A) Immunofluorescence. Podocytes were cultured in NG (5.5 mM), HG (30 mM), HG plus chloroquine (30 mM HG +1 μM CQ), and HG plus Genistein (30 mM HG +20 μM genistein +1 μM chloroquine, CQ, CQ+GEN) for 6 h and then subjected to immunofluorescence analysis of LC3 (green) and p-mTOR (green) in podocytes. Nuclei are labeled with DAPI (blue). (B) Western blot. The same cells were subjected to Western blot analysis. (C) Quantified data of B. Values are expressed as mean ±SD of 3 independent experiments. ** P<0.01 vs. HG; # P<0.05 vs. CQ.
However, an increased LC3 level may not directly correspond to an increase in autophagic flux, but could instead indicate autophagosomal accumulation at the lysosomal level [19,20]. Thus, we measured p62 expression in podocytes following chloroquine treatment and found an increase in p62 levels, whereas Genistein (in combination with chloroquine) reduced p62 levels. Taken together, these results indicate that Genistein upregulates degradation of autophagic proteins and protects podocytes.
MyD88 stimulates autophagy in high glucose-induced podocytes in vitro
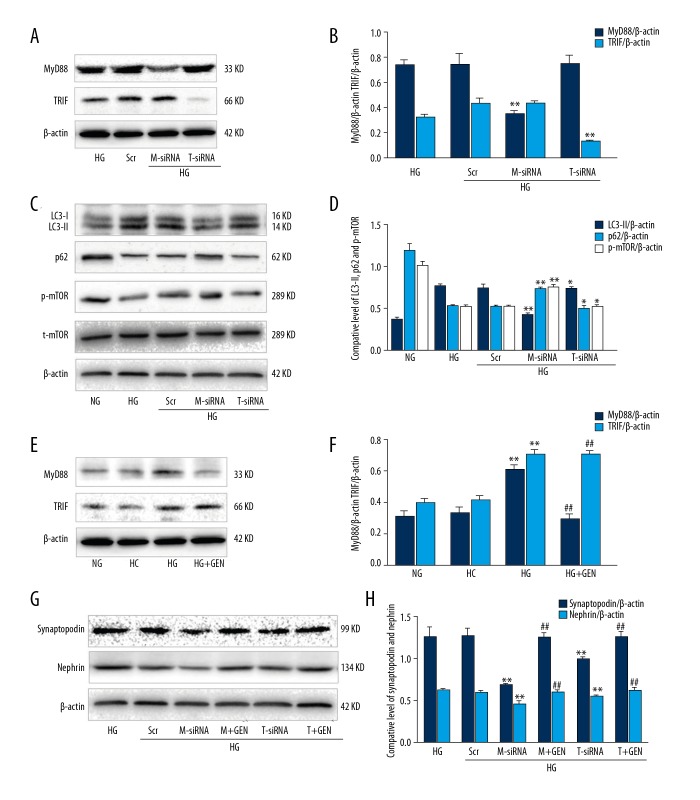
TLR4 signals through both MyD88 and TRIF, while TLR3 exclusively signals through TRIF, and other TLRs use MyD88 as their major adaptor to transduce signals to their downstream effectors [4]. To investigate if MyD88 or TRIF regulated autophagosome formation in our cell model, we knocked down MyD88 or TRIF using siRNA in podocytes. We then measured LC3, p62, and mTOR protein levels using Western blot analysis. We found that MyD88 or TRIF siRNA effectively reduced expression of both proteins in podocytes in vitro (Figure 4A, 4B). Knockdown of MyD88 expression downregulated LC3-II expression, but upregulated p62 and p-mTOR levels (Figure 4C, 4D). However, knockdown of TRIF had no obvious effect on these proteins (Figure 4C, 4D).
Figure 4.
MyD88 and TRIF triggered autophagy in high glucose-induced podocytes. (A) Western blot. Podocytes were incubated with HG (30 mM), HG plus negative control siRNA (Scr), HG plus MyD88 siRNA (M-siRNA), or HG plus TRIF siRNA (T-siRNA) and then subjected to Western blot analysis. (B) Quantified data of A. (C) Podocytes were incubated with HG (30 mM), HG plus negative control siRNA (Scr), HG plus MyD88 siRNA (M-siRNA), HG plus MyD88 siRNA and GEN (M+GEN), HG plus TRIF siRNA (T-siRNA), or HG plus TRIF siRNA and GEN (T+GEN) and then subjected to Western blot. (D) Quantified data of C. Values are expressed as mean ±SD of 3 independent experiments. * P>0.05 and ** P<0.05 vs. HG. (E) Western blot. Podocytes were incubated with NG (5.5 mM), HC (5.5 mM glucose +24.5 mM mannitol), and HG plus genistein (30 mM HG +20 μM genistein, HG+GEN) for 6 h and then subjected to Western blot analysis. (F) Quantified data of E. ** P<0.05 vs. NG; # P>0.05 and ## P<0.05 vs. HG. (G) Podocytes were incubated with HG (30 mM), HG plus negative control siRNA (Scr), HG plus MyD88 siRNA (M-siRNA), HG plus MyD88 siRNA and GEN (M+GEN), HG plus TRIF siRNA (T-siRNA), or HG plus TRIF siRNA and GEN (T+GEN) and then subjected to Western blot analysis. (H) Quantified data of G. Values are expressed as mean ±SD of 3 independent experiments. * P>0.05 and ** P<0.05 vs. HG; ## P<0.05 vs. M-siRNA or T-siRNA.
Furthermore, we investigated whether Genistein protects podocytes after knockdown of MyD88 or TRIF. We found that, compared to the TRIF siRNA-transfected group, MyD88 siRNA-transfected podocytes had a significant decrease in the functional podocyte protein markers synaptopodin and nephrin; however, Genistein treatment reversed this trend. Specifically, our Western blot data demonstrated that LC3-II levels were downregulated in the MyD88 siRNA-treated cells compared to the podocytes only cultured in HG for 6 h (P<0.01), whereas p62 and p-mTOR levels were upregulated in these podocytes (Figure 4C, 4D). In contrast, TRIF siRNA had no significant effect on these measures (P>0.05; Figure 4C, 4D). Moreover, MyD88 siRNA and TRIF siRNA downregulated expression of synaptopodin and nephrin, whereas Genistein treatment upregulated levels of synaptopodin and nephrin in these podocytes (Figure 4E, 4F, 4G, 4H). These data suggest that MyD88 upregulates autophagy, whereas Genistein reverses the effect of MyD88 siRNA on podocytes.
Discussion
Autophagy is critical for proper maintenance of intracellular homeostasis in all cell types [21,22], including podocytes. Basal autophagy in podocytes was reported to be highest among all renal cells [7,23]. Diabetic nephropathy is characterized by impaired renal filtration, mainly due to alterations in podocyte morphology and function, and previous studies confirmed the effects of diabetes on altering podocyte physiology [24,25]. Indeed, levels of autophagy markers, such as LC3-II, and the number of autophagic vacuoles have been shown to be decreased in diabetic animals, models of experimental diabetes, and in patients [26]. Thus, in the present study, we assessed the effects of Genistein and My88 on autophagy and renal protection and then explored the underlying molecular mechanisms using an in vitro HG-treated podocyte model. We found that short-term HG exposure promoted podocyte autophagy, whereas long-term (up to 72 h in our current study) HG exposure suppressed autophagy, which is consistent with previous studies [27]. Genistein treatment induced autophagy-related protein expression in both NG and HG-treated podocytes through inactivating mTOR signaling. Moreover, Genistein protected podocytes by maintaining autophagy levels following chloroquine inhibition in HG-cultured podocytes in vitro. In addition, knockdown of MyD88 expression downregulated autophagy, whereas Genistein treatment reversed the effect of My88 siRNA on podocytes. Our study demonstrates that Genistein-induced autophagy protects podocytes against chloroquine- or My88 siRNA-induced damage. Future studies will investigate the effects of Genistein on podocytes in vivo.
Genistein is one of the most active natural flavonoids and exerts antioxidative, antiproliferative, anti-cancer, and other biological effects [28]. Previous studies showed that Genistein inhibited renal inflammation, fibrosis, and early podocyte abnormalities in fructose-fed insulin-resistant rats. Moreover, Genistein combined with resveratrol was beneficial against oxidative stress in HG-treated Madin-Darby canine kidney epithelial cells [29]. Another study reported that Genistein prevented kidney damage in nephrotic syndrome in Sprague-Dawley rats [30]. Furthermore, oral Genistein reduced type 1 diabetes incidence and prolonged the onset time in female NOD mice [31]. Genistein protected against diabetes-induced renal damage by regulating oxidative stress and inflammation, but also possessed a protective effect by reducing renal inflammation, oxidative stress, and apoptosis in diabetic mice [32]. In the present study, we demonstrated that Genistein induces podocyte autophagy and protects podocytes from chloroquine inhibition in response to HG conditions in vitro. Specifically, chloroquine-mediated inhibition of autophagy decreased synaptopodin and nephrin levels in podocytes, whereas Genistein reversed this effect. The present data further support the protective role of Genistein in the kidney against various insults.
Proper activation of the inflammasome is an important first-line defense of innate immunity, but aberrant inflammasome activation has now been shown to contribute to the pathogenesis of numerous diseases, including diabetic nephropathy [14,33,34]. Inflammatory responses have also been shown to affect autophagy, but there is no clear understanding of the complex relationships among the immune system, autophagy, and glomerular diseases. Previous studies showed a possible relationship between autophagy and the inflammasome. For example, autophagy negatively regulated inflammasome activation, whereas induction of autophagy depended on the presence of specific inflammasome sensors [13,35]. TLRs play an important role in innate and adaptive immunity, and the major TLR signaling pathways in podocytes are initiated via 2 key adaptor proteins: myeloid differentiation factor (MyD88-dependent) and Toll-Interleukin 1 Receptor (TIR) domain-containing adapter-inducing interferon-β (TRIF, MyD88-independent) [36,37]. Autophagic receptors accumulate signaling proteins, including MyD88 and TRIF, to form cytosolic aggregates, ultimately degrading them through selective autophagy [38,39]. In the present study, we focused on the downstream signaling of TLRs in the regulation of autophagy and found that in the early stage of HG stimulation, MyD88 induces autophagy in podocytes, which was similar to Genistein treatment. We also showed that TLR signaling via its adaptor proteins increased p-mTOR, leading to autophagy.
Recent studies demonstrated that LC3-II expression and the number of autophagic vesicles were decreased in podocytes of diabetic patients and animal models. Furthermore, long-term exposure of primary rat podocytes to HG promoted podocyte injury, and attenuation of autophagy further deteriorated this process [40]. Taken together, these studies support that maintenance of autophagy in podocytes is essential to conserve podocyte survival. The central importance of autophagy in immunity is further underscored by the multitude of immune-related signaling molecules that regulate autophagy. Genistein has also been shown to modulate humoral and cellular immunity at multiple levels, such as inhibiting inflammatory cell migration through diminishing leukocytes adherence to endothelial cells [41, 42]. The present study not only further verified the protective effect of autophagy on podocytes, but also revealed that Genistein is an immunomodulator and a key factor in TLR signaling (MyD88) during autophagy at the early stage of HG stimulation in podocytes. Our findings could provide a novel therapeutic target for future treatment of incipient diabetic nephropathy.
Conclusions
Genistein-induced autophagy and alleviation of podocyte injury caused by HG were mediated by inactivating mTOR signaling in podocytes. This study is just proof-of-principle and further research will attempt to verify autophagy induction as a novel strategy for treatment of glomerular diseases.
Acknowledgement
We would like to thank the staff in the Department of Pathology, Hebei Medical University (Shijiazhuang, China) for technical assistance.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.World Health Organization. Global Report on Diabetes. Geneva: 2016. [Google Scholar]
- 2.Zhu H, Yu W, Xie Y, et al. Association of Pentraxin 3 gene polymorphisms with susceptibility to diabetic nephropathy. Med Sci Monit. 2017;23:428–36. doi: 10.12659/MSM.902783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wickelgren I. First components found for new kidney filter. Science. 1999;286(5438):225–26. doi: 10.1126/science.286.5438.225. [DOI] [PubMed] [Google Scholar]
- 4.Lowik MM, Groenen PJ, Levtchenko EN, et al. Molecular genetic analysis of podocyte genes in focal segmental glomerulosclerosis – a review. Eur J Pediatr. 2009;168(11):1291–304. doi: 10.1007/s00431-009-1017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maezawa Y, Takemoto M, Yokote K. Cell biology of diabetic nephropathy: Roles of endothelial cells, tubulointerstitial cells and podocytes. J Diabetes Investig. 2015;6(1):3–15. doi: 10.1111/jdi.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147(4):728–41. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Kitada M, Ogura Y, Monno I, Koya D. Regulating autophagy as a therapeutic target for diabetic nephropathy. Curr Diab Rep. 2017;17(7):53. doi: 10.1007/s11892-017-0879-y. [DOI] [PubMed] [Google Scholar]
- 8.Magee C, Grieve DJ, Watson CJ, Brazil DP. Diabetic nephropathy: A Tangled Web to Unweave. Cardiovasc Drugs Ther. 2017;31(5–6):579–92. doi: 10.1007/s10557-017-6755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cellesi F, Li M, Rastaldi MP. Podocyte injury and repair mechanisms. Curr Opin Nephrol Hypertens. 2015;24(3):239–44. doi: 10.1097/MNH.0000000000000124. [DOI] [PubMed] [Google Scholar]
- 10.Lamb CA, Yoshimori T, Tooze SA. The autophagosome: Origins unknown, biogenesis complex. Nat Rev Mol Cell Biol. 2013;14(12):759–74. doi: 10.1038/nrm3696. [DOI] [PubMed] [Google Scholar]
- 11.Russell RC, Yuan HX, Guan KL. Autophagy regulation by nutrient signaling. Cell Res. 2014;24(1):42–57. doi: 10.1038/cr.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan EY. Regulation and function of uncoordinated-51 like kinase proteins. Antioxid Redox Signal. 2012;17(5):775–85. doi: 10.1089/ars.2011.4396. [DOI] [PubMed] [Google Scholar]
- 13.Into T, Inomata M, Takayama E, Takigawa T. Autophagy in regulation of Toll-like receptor signaling. Cell Signal. 2012;24(6):1150–62. doi: 10.1016/j.cellsig.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Kuballa P, Nolte WM, Castoreno AB, Xavier RJ. Autophagy and the immune system. Annu Rev Immunol. 2012;30:611–46. doi: 10.1146/annurev-immunol-020711-074948. [DOI] [PubMed] [Google Scholar]
- 15.Tey SK, Khanna R. Host immune system strikes back: Autophagy-mediated antigen presentation bypasses viral blockade of the classic MHC class I processing pathway. Autophagy. 2012;8(12):1839–41. doi: 10.4161/auto.21860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eo H, Lee HJ, Lim Y. Ameliorative effect of dietary genistein on diabetes induced hyper-inflammation and oxidative stress during early stage of wound healing in alloxan induced diabetic mice. Biochem Biophys Res Commun. 2016;478(3):1021–27. doi: 10.1016/j.bbrc.2016.07.039. [DOI] [PubMed] [Google Scholar]
- 17.Tang C, Zhang K, Zhao Q, Zhang J. Effects of dietary genistein on plasma and liver lipids, hepatic gene expression, and plasma metabolic profiles of hamsters with diet-induced hyperlipidemia. J Agric Food Chem. 2015;63(36):7929–36. doi: 10.1021/acs.jafc.5b01590. [DOI] [PubMed] [Google Scholar]
- 18.Kim MJ, Lim Y. Protective effect of short-term genistein supplementation on the early stage in diabetes-induced renal damage. Mediators Inflamm. 2013;2013:510212. doi: 10.1155/2013/510212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klionsky DJ, Abdalla FC, Abeliovich H, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8(4):445–44. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanida I, Waguri S. Measurement of autophagy in cells and tissues. Methods Mol Biol. 2010;648:193–214. doi: 10.1007/978-1-60761-756-3_13. [DOI] [PubMed] [Google Scholar]
- 21.Kamitani M, Miyatsuka T, Miura M, et al. Heterogeneity of autophagic status in pancreatic beta cells under metabolic stress. Biochem Biophys Res Commun. 2018;496(2):328–34. doi: 10.1016/j.bbrc.2018.01.070. [DOI] [PubMed] [Google Scholar]
- 22.Ke D, Fu X, Xue Y, et al. IL-17A regulates the autophagic activity of osteoclast precursors through RANKL-JNK1 signaling during osteoclastogenesis in vitro. Biochem Biophys Res Commun. 2018;497(3):890–96. doi: 10.1016/j.bbrc.2018.02.164. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Gao L, Lin H, et al. Mangiferin prevents diabetic nephropathy progression and protects podocyte function via autophagy in diabetic rat glomeruli. Eur J Pharmacol. 2018;824:170–78. doi: 10.1016/j.ejphar.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Liu N, Xu L, Shi Y, Zhuang S. Podocyte autophagy: A potential therapeutic target to prevent the progression of diabetic nephropathy. J Diabetes Res. 2017;2017:7024024. doi: 10.1155/2017/3560238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding Y, Choi ME. Autophagy in diabetic nephropathy. J Endocrinol. 2014;224(1):R15–30. doi: 10.1530/JOE-14-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang L, Zhou Y, Cao H, et al. Autophagy attenuates diabetic glomerular damage through protection of hyperglycemia-induced podocyte injury. PLoS One. 2013;8(4):e60546. doi: 10.1371/journal.pone.0060546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Audzeyenka I, Rogacka D, Piwkowska A, et al. Viability of primary cultured podocytes is associated with extracellular high glucose-dependent autophagy downregulation. Mol Cell Biochem. 2017;430(1–2):11–19. doi: 10.1007/s11010-017-2949-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukund V, Mukund D, Sharma V, et al. Genistein: Its role in metabolic diseases and cancer. Crit Rev Oncol Hematol. 2017;119:13–22. doi: 10.1016/j.critrevonc.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Palanisamy N, Kannappan S, Anuradha CV. Genistein modulates NF-κB-associated renal inflammation, fibrosis and podocyte abnormalities in fructose-fed rats. Eur J Pharmacol. 2011;667(1–3):355–64. doi: 10.1016/j.ejphar.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Javanbakht MH, Sadria R, Djalali M, et al. Soy protein and genistein improves renal antioxidant status in experimental nephrotic syndrome. Nefrologia. 2014;34(4):483–90. doi: 10.3265/Nefrologia.pre2014.Jun.12051. [DOI] [PubMed] [Google Scholar]
- 31.Guo TL, Germolec DR, Zheng JF, et al. Genistein protects female nonobese diabetic mice from developing type 1 diabetes when fed a soy- and alfalfa-free diet. Toxicol Pathol. 2015;43(3):435–48. doi: 10.1177/0192623314526318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elmarakby AA, Ibrahim AS, Faulkner J, et al. Tyrosine kinase inhibitor, genistein, reduces renal inflammation and injury in streptozotocin-induced diabetic mice. Vascular Pharmacology. 2011;55(5–6):149–156. doi: 10.1016/j.vph.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Deretic V. Autophagy as an innate immunity paradigm: Expanding the scope and repertoire of pattern recognition receptors. Curr Opin Immunol. 2012;24(1):21–31. doi: 10.1016/j.coi.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469(7330):323–35. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh JE, Lee HK. Modulation of pathogen recognition by autophagy. Front Immunol. 2012;3:44. doi: 10.3389/fimmu.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng Z, Taylor B, Ourthiague DR, Hoffmann A. Distinct single-cell signaling characteristics are conferred by the MyD88 and TRIF pathways during TLR4 activation. Sci Signal. 2015;8(385):ra69. doi: 10.1126/scisignal.aaa5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mudaliar H, Pollock C, Panchapakesan U. Role of Toll-like receptors in diabetic nephropathy. Clin Sci. 2014;126(10):685–94. doi: 10.1042/CS20130267. [DOI] [PubMed] [Google Scholar]
- 38.Into T, Inomata M, Niida S, et al. Regulation of MyD88 aggregation and the MyD88-dependent signaling pathway by Sequestosome 1 and histone Deacetylase 6. J Biol Chem. 2010;285(46):35759–69. doi: 10.1074/jbc.M110.126904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inomata M, Niida S, Shibata K, Into T. Regulation of Toll-like receptor signaling by NDP52-mediated selective autophagy is normally inactivated by A20. Cell Mol Life Sci. 2012;69(6):963–79. doi: 10.1007/s00018-011-0819-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tagawa A, Yasuda M, Kume S, et al. Impaired podocyte autophagy exacerbates proteinuria in diabetic nephropathy. Diabetes. 2016;65(3):755–67. doi: 10.2337/db15-0473. [DOI] [PubMed] [Google Scholar]
- 41.Yum MK, Jung MY, Cho D, Kim TS. Suppression of dendritic cells’ maturation and functions by daidzein, a phytoestrogen. Toxicol Appl Pharmacol. 2011;257(2):174–81. doi: 10.1016/j.taap.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Lee YW, Lee WH. Protective effects of genistein on proinflammatory pathways in human brain microvascular endothelial cells. J Nutr Biochem. 2008;19(12):819–25. doi: 10.1016/j.jnutbio.2007.10.006. [DOI] [PubMed] [Google Scholar]