Abstract
Background
Several hyaluronan preparations are available that have different dosage forms, origins, and concentrations. The objective of the study was to compare the efficacy of intra-articular chemically cross-linked hyaluronan (CCH) and avian-derived hyaluronan (ADH) injections in knee osteoarthritis (KOA) patients.
Material/Methods
In total, 258 patients were randomized into 2 groups of 129 each: patients who received CCH injection (CCH group) and patients who received ADH injection (ADH group). Radiographic Kellgren-Lawrence score, visual analog scale (VAS) pain score, Lequesne index score, the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) index, single-limb stance (SLS) test, and timed “Up-and-Go” (TUG) test were performed. The Mann-Whitney U test or independent t-test following Bonferroni adjustment was performed for statistical analysis at 95% of confidence level.
Results
The CCH group had improved VAS pain score (P<0.0001, q=54.803), total WOMAC score (P<0.0001, q=4.753), Lequesne index score (P<0.0001, q=3.208), and SLS time (P<0.0001, q=8.76) at the end of 6 months as compared to those in the ADH group. After 6 months of follow-up, the ADH group had improved TUG time (P=0.0148, q=3.385) as compared to baseline. Both groups of patients had the similar improvement in Kellgren-Lawrence score and mild to moderate adverse effects after 6 months.
Conclusions
CCH injection was superior to ADH injection.
MeSH Keywords: Hyaluronic Acid; Knee Joint; Osteoarthritis; Osteoarthritis, Knee; Viscosupplementation; Visual Analog Scale
Background
Osteoarthritis (OA) can cause disability, pain, and restriction of mobility [1]. Cartilage attrition, subchondral bone remodeling, osteophyte formation, and synovial inflammation are common characteristics of OA [2]. Chondrosenescence is associated with inflammation, disturbed interplay between autophagy and inflammasomes, a decrease in the efficacy of articular cartilage repair, and the prevalence of osteoarthritis [3]. Recombinant lubricin treatment protects articular cartilage and prevents the process of OA [4]. For knee osteoarthritis (KOA), the use of hyaluronan as a visco-supplementation is a well-known option. Hyaluronan is known to boost viscoelasticity of synovial fluid and reduced pain [5]. It has pharmacological actions such as antalgic [6], anabolic [7], anti-inflammatory [6], and antinociceptive action [5]. Endogenous hyaluronan synthesis is also promoted by stimulation of CD44 receptor binding [7]. However, the use of hyaluronan in KOA continues to be debated [5–8]. Most findings suggest a significant effect-to-good-effect [9–11]. A few studies have reported insignificant benefits of hyaluronan compared to placebos [12]. Several guidelines suggest hyaluronan injection for KOA [13].
At present, several hyaluronan preparations are available that have different dosage forms, origins, and concentrations. In most of the formulations, hyaluronan is derived from rooster comb tissue, and dosing schedules are typically 3, 4, or 5 intra-articular injections [5]. However, single hyaluronan injections have been reported associated with patient convenience, safety, and effectiveness [14]. For such scenarios, chemically cross-linked hyaluronan (CCH) and avian derived hyaluronan (ADH) injections could be prepared to have competitive activities and single dose regimen [5].
The primary aim of the study was to decrease knee pain by intra-articular hyaluronan injections in KOA patients. The secondary outcome of the work was to compare tolerability and efficacy of CCH and ADH injections.
Material and Methods
Materials
CCH (HYA-JOINT Plus) was purchased from SciVision Biotech, Taiwan. ADH (Hylan G-F 20) was purchased from Sanofi-Aventis, Pharma Beijing Co., Ltd., China.
Ethical consideration and consent to participate
The study was registered in the Research Registry (https://www.researchregistry.com), UID No.: researchregistry3500, dated November 21, 2015. The study was approved by the Review Board of the First Affiliated Hospital of Xiamen University, China. The protocol for the study (PR/CL/Re/16/81, dated November 15, 2015) was maintained under the law of PR China, CONSORT guidelines, and 2013 Declarations of Helsinki. All enrolled patients signed an informed consent regarding interventions, pathology, and publications of the study in all forms (electronic and hard) irrespective of time, place, and language, before the interventions.
Design of the study
In total, 258 patients were included in study using simple randomization (1: 1 ratio, computer generated), parallel, double-blind (patient and consultant blind) study with a 6-month follow-up period. OpenEpi 3.01-English (Epidemiologic Statistics for Public Health, USA) software was used for prior sample size calculation, which was found to be 129. The confidence limit was 95%. Population size (N) was 258, hypothesized percentage frequency of N was 79±5%, confidence limits was 5%, design effect for cluster surveys was 1. CONSORT flow diagram of the study is presented in Figure 1. Sequentially numbered randomization was performed by authors.
Figure 1.

A Randomized, double-blind trial chart. The population size (N) was 258, the confidence level was 95%; the hypothesized percentage frequency of N was 79±5%, the confidence limit was 5%, and the design effect for cluster surveys was 1. WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; TUG, Timed “Up-and-Go”; SLS, single-limb stance; VAS, visual analog scale.
Inclusion criteria
All patients admitted to the Department of Orthopedics, the First Affiliated Hospital of Xiamen University, Xiamen, China, who had KOA for more than 180 days (confirmed by radiography) for treatment purposes from December 1, 2016 to June 30, 2017 were included in the study. Patients who were older than 38 years of age and younger than 80 years of age were included in the study. Patients who had an average visual analog scale (VAS) pain score of more than 30 were included in the study. Patients who had a Kellgren-Lawrence score of 2 or 3 were included in the study. The demographic characteristics of enrolled patients at the time of enrollment (baseline; BL) are represented in Table 1. The enrolled patients had no significant differences regarding demographic characteristics (P>0.01).
Table 1.
The demographic characteristics of patients at the time of enrolment.
| Characteristics | Groups | Comparisons | ||
|---|---|---|---|---|
| CCH | ADH | |||
| Sample size | 129 | 129 | p-Value | |
| Age (year) | 64.82±9.23 | 62.02±11.25 | 0.579 | |
| Gender | Male | 30 (23) | 35 (27) | 0.566 |
| Female | 99 (77) | 94 (73) | ||
| Site of KOA | Left | 61 (47) | 70 (54) | 0.319 |
| Right | 68 (53) | 59 (46) | ||
| BMI (kg/m2) | 27.44±2.4 | 27.62±2.31 | 0.927 | |
| Duration of KOA (year) | <5 | 85 (66) | 81 (63) | 0.417 |
| 5≥ but 10< | 35 (27) | 37 (29) | ||
| ≥10 | 9 (7) | 11 (8) | ||
| Marital status | Married | 121 (94) | 125 (97) | 0.376 |
| Unmarried | 8 (6) | 4 (3) | ||
| Ethnicity | Chinese | 127 (98) | 128 (99) | 0.5 |
| Non-Chines | 2 (2) | 1 (1) | ||
| TUG (sec) | 12.66±1.53 | 12.71±1.61 | 0.88 | |
| SLS (sec) | 18.71±1.5 | 18.20±1.49 | 0.971 | |
KOA – knee osteoarthritis. Continuous data were represented as mean ±SD and constant data were represented as a number (percentage). BMI – body mass index. Chi-squared Test for Independence was used for statistical analysis. A p<0.01 was considered as significant. N/A – not applicable; TUG – timed “Up-and-Go”; SLA – single-limb stance test.
Exclusion criteria
Patients who had average VAS pain scores of 30 or less were excluded from the study. Patients who had encountered lower limb, spine, or any orthopedic surgery in the past 10-years were excluded from the final enrollment. Patients who had apparent joint effusion or remarkable knee instability or deformity were excluded from the study. Patients who had an allergy to avian proteins, were lactating, or were pregnant were excluded from the study. Patients who had received intra-articular injections to the knee for KOA within the last 6 months were excluded. Patients who had neoplasm, rheumatoid arthritis, hemiparesis, or active infection were excluded from the final enrollment. Patients who had Kellgren-Lawrence score of 1 were excluded from the study.
Interventions
Patients received a single intra-articular injection of CCH (60 mg/3 mL), the CCH group, or ADH (60 mg/6 mL) the ADH group. All injections were made using 1.5 inch and 21-gauge needle by well-trained nursing staff who were clinically involved in the research and blinded for the study. No painkiller was taken by the patients during enrollment and follow-up. Paracetamol was the only rescued drug allowed for pain [5]. All hyaluronan injections were performed under the guidance of a physiatrist who had more than 10 years of experience. The area of the skin of the targeted knee was prepared and the ultrasound (Accuvix XQ; Samsung Medison Co., Ltd., Korea) probe was put near the puncture side and the injection were made as guided by ultrasound and color jet. If the injection failed as an intra-articular injection, then the patient was reinjected with hyaluronan as per the guidance of a consultant [15].
Radiographic Kellgren-Lawrence score
Radiographic Kellgren-Lawrence score was detected by magnetic resonance imaging (MRI) at baseline and after 6 months of treatment. The MRI-based grading was as follows: grade 1 was doubtful narrowing of the joint space with possible osteophyte formation, grade 2 was possible narrowing of the joint space with definite osteophyte formation, and grade 3 was definite narrowing of joint space, moderate osteophyte formation, some sclerosis, and possible deformity of bony ends [16].
Knee pain measurement
The primary aim of the study was to decrease knee pain. The pain was measured in each enrolled patient by VAS score at baseline and at 1 month, 3 months, and 6 months of follow-up. In the VAS score, a value of zero was indicated if there was no reported pain and a value of 100 was indicated for the worst possible pain [17].
Secondary outcomes: the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC, Likert scale)
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC, Likert scale) is a questionnaire with 24 questions. It included 3 subscales for stiffness, physical function, and pain. The value of zero indicated a good condition and the value of 96 indicated the worst possible outcome [18].
Lequesne index score
The Lequesne index score was included as a measure of a prior week evaluation of activities of daily living, walking distance, and pain. The value of zero indicated normal function and the value of 24 indicated worst possible function [19].
Timed “Up-and-Go” (TUG) test
The Timed “Up-and-Go” (TUG) test was defined as the time for the patient to rise from a chair, walk 3 meters, turn around, walk back, and sit down in the chair [20].
Single-limb stance (SLS) test
The single-limb stance (SLS) test was defined as the time for the patient to raise the targeted foot without touching one’s lower extremity and maintaining balance as long as possible [10].
Patient satisfaction
Patient satisfaction was defined by use of a simple questionnaire. The satisfaction was graded between zero to 100. Zero was considered completely dissatisfied and 100 was considered completely satisfied [10].
Safety assessment
Safety assessment was assessed on adverse events including joint pain, joint swelling, joint stiffness, joint effusion, limb weakness, injection site paresthesia, back pain, and infection as reported during follow-up. The decision to consider an event as an adverse event was based on the decision of evaluator(s) (nursing staff). Any dispute of a decision was resolved by a discussion with other evaluators [5,11]. A total of 15 evaluators were involved in the present study, including the physiatrist who conducted the injections and the investigator who conducted the assessments.
Statistical analysis
InStat (GraphPad, USA) was used for the statistical analysis. Chi-squared test for independence was performed between demographical characteristics of enrolled patients between groups at baseline and for a radiographic Kellgren-Lawrence score between baseline and after 6 months of treatment. The Wilcoxon test following Bonferroni adjustment was performed for VAS analog, WOMAC score, Lequesne Index score, TUG test, and SLS test between baseline and after 1 month, 3 months, and 6 months follow-up [21]. Independent t-test [22] following Bonferroni adjustment [21] was performed for VAS analog, WOMAC score, Lequesne Index score, patient satisfaction, and adverse effects between groups at 1-month, 3-months, and 6-months follow-up. The Mann-Whitney U test following Bonferroni adjustment was performed for the TUG test and the SLS test between the groups at 6-month follow-up [21]. Results regarding drug actions were considered significant at 95% confidence level and demographic characters were considered significant at 99% confidence level.
Results
There were 3 cases of intra-articular injection failure. In such conditions, hyaluronan was reinjected. Fifteen patients were not available for the follow-up study. They were contacted by telephone, email, or other social media and their data were collected for statistical analysis. CCH injections decreased the VAS pain score (Table 2) and total WOMAC score (Table 3) more strongly than that of ADH injections. Moreover, CCH injections strongly decreased the Lequesne index score during follow-up compared to ADH injections (Table 4). Radiographic Kellgren-Lawrence score grading is shown in Figure 2. Both the CCH group and the ADH group had the same kind improvement in Kellgren-Lawrence score after 6-months (Table 5).
Table 2.
Comparison of Visual analog scale pain score.
| Sample size | Groups | Comparisons between group | |||
|---|---|---|---|---|---|
| 129 | 129 | p-Value | q-Value | ||
| Type of hyaluronan injections | Chemically cross-linked | Avian derived | |||
| VAS | CCH | ADH | |||
| BL | 58.82±1.4 | 58.58±1.49 | 0.876 | N/A | |
| 30 days | Pain score | 24.22±1.43 | 35.47±2.87 | <0.0001 | 46.216 |
| p-Value | <0.0001 | <0.0001 | |||
| q-Value | 170.35 | 89.051 | |||
| 90 days | Pain score | 23.45±1.67 | 35.02±1.89 | <0.0001 | 56.178 |
| p-Value | <0.0001 | <0.0001 | |||
| q-Value | 174.17 | 90.733 | |||
| 180 days | Pain score | 21.6±2.14 | 34.33±1.96 | <0.0001 | 54.803 |
| p-Value | <0.0001 | <0.0001 | |||
| q-Value | 183.29 | 93.39 | |||
VAS – visual analog scale; BL – baseline. Data were represented as mean ±SD. Wilcoxon test (within the group) or independent t-test (between group) following Bonferroni adjustment was used for statistical analysis. A p<0.05 and q>2.652 were considered as significant. Within-group p-value was respect to BL. A 0: No pain and 100: Worst possible pain. N/A – not applicable.
Table 3.
Comparison of the Western Ontario and McMaster Universities Osteoarthritis Index.
| Groups | Comparisons between groups | ||||
|---|---|---|---|---|---|
| Sample size | 129 | 129 | |||
| Type of hyaluronan injections | Chemically cross-linked | Avian derived | p-Value | q-Value | |
| WOMAC index | CCH | ADH | |||
| Pain score (0–20) | |||||
| BL | 10.5±0.97 | 10.46±1.01 | 0.7936 | N/A | |
| 30 days | Pain score | 6.29±0.45 | 6.33±0.51 | 0.0575 | N/A |
| p-Value | <0.0001 | <0.0001 | |||
| q-Value | 49.676 | 45.588 | |||
| 90 days | Pain score | 6.23±0.52 | 6.26±0.53 | 0.1807 | N/A |
| p-Value | <0.0001 | <0.0001 | |||
| q-Value | 50.317 | 46.455 | |||
| 180 days | Pain score | 5.9±0.61 | 5.91±0.64 | 0.6565 | N/A |
| p-Value | <0.0001 | <0.0001 | |||
| q-Value | 54.251 | 50.301 | |||
| Stiffness score (0–8) | |||||
| BL | 3.01±0.6 | 63.02±0.59 | 0.851 | N/A | |
| 30 days | Score | 2.31±0.46 | 2.31±0.47 | 1.102 | N/A |
| p-Value | <0.0001 | <0.0001 | |||
| q-Value | 13.315 | 13.63 | |||
| 90 days | Score | 2.16±0.36 | 2.08±0.27 | 0.0677 | N/A |
| p-Value | <0.0001 | <0.0001 | |||
| q-Value | 16.274 | 18.075 | |||
| 180 days | Score | 1.92±0.27 | 1.92±0.26 | 1.103 | N/A |
| p-Value | <0.0001 | <0.0001 | |||
| q-Value | 20.712 | 21.038 | |||
| Physical function score (0–68) | |||||
| BL | 35.89±2.53 | 35.98±2.55 | 0.7479 | N/A | |
| 30 days | Score | 25.7±1.5 | 27.57±0.96 | <0.0001 | 8.364 |
| p-Value | <0.0001 | <0.0001 | |||
| q-Value | 59.324 | 44.606 | |||
| 90 days | Score | 25.27±1.24 | 26.67±0.95 | <0.0001 | 6.629 |
| p-Value | <0.0001 | <0.0001 | |||
| q-Value | 61.805 | 49.303 | |||
| 180 days | Score | 24.69±1.11 | 25.67±0.95 | <0.0001 | 4.758 |
| p-Value | <0.0001 | <0.0001 | |||
| q-Value | 65.189 | 54.628 | |||
| Total score (0–96) | |||||
| BL | 49.5±2.54 | 49.67±2.7 | 0.8345 | N/A | |
| 30 days | Score | 34.29±1.64 | 36.21±1.22 | <0.0001 | 8.11 |
| p-Value | <0.0001 | <0.0001 | |||
| q-Value | 78.999 | 66.973 | |||
| 90 days | Score | 33.66±1.42 | 35.01±1.15 | <0.0001 | 6.237 |
| p-Value | <0.0001 | <0.0001 | |||
| q-Value | 82.324 | 77.044 | |||
| 180 days | Score | 32.51±1.3 | 33.50±1.19 | <0.0001 | 4.753 |
| p-Value | <0.0001 | <0.0001 | |||
| q-Value | 88.326 | 80.642 | |||
Data were represented as mean ±SD. Wilcoxon test (within the group) or independent t-test (between group) following Bonferroni adjustment was used for statistical analysis. A p<0.05 and q>2.652 were considered as significant. Within-group p-value was respect to BL. BL – baseline, N/A – not applicable. WOMAC – The Western Ontario and McMaster Universities Osteoarthritis Index (Likert scale). A 0: Good condition and 96: The worst possible outcome.
Table 4.
Comparison of Lequesne Index Score.
| Sample size | Groups | Comparisons | |||
|---|---|---|---|---|---|
| 129 | 129 | p-Value | q-Value | ||
| Type of hyaluronan injections | Chemically cross-linked | Avian derived | |||
| Lequesne Index | CCH | ADH | |||
| BL | 11.13±1.96 | 11.21±2.04 | 0.7569 | N/A | |
| 30 days | Pain score | 8.04±0.58 | 8.91±1.13 | <0.0001 | 3.072 |
| p-Value | <0.0001 | <0.0001 | |||
| q-Value | 21.213 | 16.471 | |||
| 90 days | Pain score | 8.13±0.34 | 8.81±0.9 | <0.0001 | 4.354 |
| p-Value | <0.0001 | 17.194 | |||
| q-Value | 23.322 | <0.0001 | |||
| 180 days | Pain score | 7.88±0.35 | 8.37±0.73 | <0.0001 | 3.208 |
| p-Value | <0.0001 | <0.0001 | |||
| q-Value | 25.25 | 20.366 | |||
VAS – visual analog scale. Data were represented as mean ±SD. Wilcoxon test (within the group) or independent t-test (between group) following Bonferroni adjustment was used for statistical analysis. A p<0.05 and q>2.652 were considered as significant. Within-group p-value was respect to BL. BL – baseline; N/A – not applicable. A 0: Normal function and 24: The worst possible function.
Figure 2.
Radiographic for Kellgren-Lawrence score grading (magnetic resonance imaging): (A) Grade 1; (B) Grade 2; (C) Grade 3. U – upper portion of the knee, L – lower portion of the knee.
Table 5.
Comparisons of radiographic Kellgren-Lawrence score.
| Characteristics | Groups | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CCH | ADH | Comparisons between CCH and ADH | |||||||
| Level | BL | EL | Comparisons between BL and EL | BL | EL | Comparisons between BL and EL | BL | EL | |
| Sample size | 129 | 122 | p-Value | 129 | 121 | p-Value | p-Value | p-Value | |
| Radiographic Kellgren-Lawrence score | 1 | 0 (0) | 35 (29) | <0.0001 | 0 (0) | 38 (31) | <0.0001 | 0.204 | 0.799 |
| 2 | 83 (64) | 54 (44) | 72 (56) | 51 (42) | |||||
| 3 | 46 (36) | 33 (27) | 57 (44) | 33 (27) | |||||
Chi-squared Test for Independence was used for statistical analysis. A p<0.05 was considered as significant. BL – baseline; EL – after six-months.
Since 15 (7 from the CCH group and 8 from the ADH group) were not available for follow-up study, the TUG and SLS data were not evaluated for these cases. However, there was no significant difference between TUG and SLS time at baseline between the 2 groups. After 6 months of follow-up, patients who received ADH injections had improved TUG test times (P=0.0148, q=3.385) as compared to baseline (Figure 3).
Figure 3.
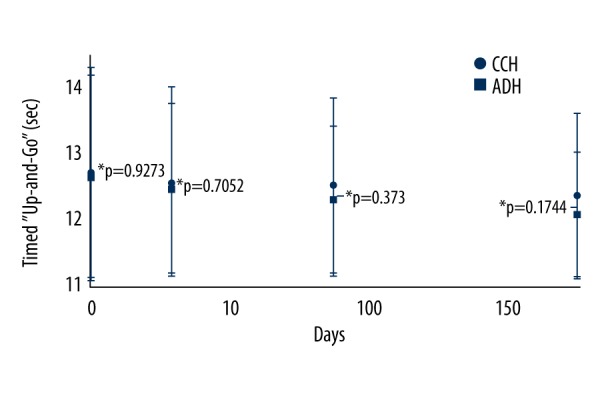
Timed “Up-and-Go” test. Data are represented as mean ±SD; n=122 for the CCH group and n=121 for the ADH group. Timed “Up-and-Go” was low in the ADH group after 6 months of follow-up as compared to baseline (BL) (P=0.0148, q=3.385). The Wilcoxon test (within the group) or the Mann-Whitney U test (between group) following Bonferroni adjustment was used for statistical analysis. P<0.05 and q>2.652 were considered significant. Within group P-value was with respect to BL. CCH – chemically cross-linked hyaluronan injections; ADH – avian derived hyaluronan injections.
After 6 months of follow-up, patients who received CCH injections had improved SLS times (P<0.0001, q=8.76) compared to patients who received ADH (Figure 4).
Figure 4.
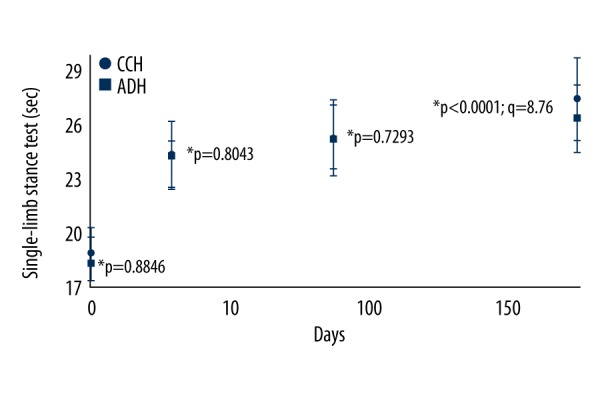
Single-limb stance test. Data are represented as mean ±SD; n=122 for the CCH group and n=121 for the ADH group. Single-limb stance time was higher in the CCH group than the ADH group after 6 months of follow-up (P<0.0001, q=8.76). The Wilcoxon test (within the group) or the Mann-Whitney U test (between group) following Bonferroni adjustment was used for statistical analysis. P<0.05 and q>2.652 were considered significant. Within group P-value was with respect to BL. CCH – chemically cross-linked hyaluronan injections; ADH – avian derived hyaluronan injections; BL – baseline.
Both groups of patients had equal patient satisfaction (Table 6) after 1 month, 3 months, and 6 months.
Table 6.
Patients’ satisfaction.
| Group | Comparisons between groups | ||
|---|---|---|---|
| Sample size | 129 | 129 | |
| Type of hyaluronan injections | Chemically cross-linked | Avian derived | p-Value |
| Period | CCH | ADH | |
| One month | 59.68±2.44 | 59.55±2.28 | 0.7028 |
| Three months | 60.67±2.40 | 60.55±2.27 | 0.7352 |
| Six months | 61.67±2.41 | 61.54±2.29 | 0.7189 |
Independent t-test was used for statistical analysis. A p<0.05 was considered as significant. Missing data were collected via electronic conversation. Data were represented as mean ±SD. A 0: Completely dissatisfied and 100: Completely satisfied.
Treatment-emergent adverse effects were the same in both groups after 6 months of follow-up (P≥0.05 or/and q≤2.4 1, Figure 5).
Figure 5.

Treatment-emergent adverse effects after 6 months of follow-up. For statistical analysis, the adverse event was considered as 1 and the absent of that was considered as 0. Independent t-test following Bonferroni adjustment was used for statistical analysis. A P<0.05 and q>2.41 were considered as significant. Missing data were collected via electronic conversation. CCH – chemically cross-linked hyaluronan injections; ADH – avian derived hyaluronan injections.
Discussion
Visco-supplement has been reported as an effective treatment for KOA [13]. This study compared 2 different ultrasound-guided hyaluronan injections in KOA patients. There are different visco-supplements used to treat KOA that differ according to the nature of origin and molecular weight [23,24]. This study provided data to inform the choice of hyaluronan injection in KOA.
After 6 months of follow-up, the WOMAC pain score was improved: 43.32±7.9% in the CCH group and 42.96±8.46% in the ADH group as compared to baseline. The threshold of the WOMAC pain score in case of OA, as accepted for clinical importance, is 12–18% [5,25]. In consideration of our study results for the WOMAC index, these finding were consistent with other available clinical trials on KOA.
At the end of the 6 months, the Lequesne index score was improved in the range of 13.77–40.21% for CCH group and 8.12–37.71% for ADH group as compared to baseline. For the effective form of the treatment, the threshold of the Lequesne index score is 30–40% in OKA [5,26]. The data on the Lequesne index in this study meet the requirement of therapy in OKA patients.
There was an improvement in TUG time in patients who received ADH injections at the end of 6 months compared to baseline. This was likely because of the excessive capsular distension effect of ADH on physical activity of patients [5]. There was an improvement in SLS time in patients who received CCH injections at the end of 6 months compared to those who received ADH injections. This was likely because of the efficacy of CCH injections in preventing catabolism of cartilage [27]. Further study is recommended to reveal the mechanism of actions for this finding.
Both types of hyaluronan injections had no reported harmful adverse effects. Our study data were in the line with available studies on different types of hyaluronan injections in KOA [5,6,14,28]. However, consideration should also include acute side effects like joint pain, joint swelling, and joint stiffness due to hyaluronan injection, the carrier of hyaluronan, the nature of origin, the injection technique(s) or the person who is administering the injection [5]. Further study is required to determine the cause of adverse effects.
To the best of our knowledge, there have been 2 studies that have compared different types of intra-articular hyaluronan injection in KOA patients. Both of these studies had follow-up periods of 26 weeks, but they had smaller sample size, did not use ultrasound guided intra-articular injections, and had no radiographic Kellgren-Lawrence score assessments [23,29]. It is a waste of resources if a study is carried out with a small sample size [30] and ultrasound is needed as a supportive tool in intra-articular injections [15]. The sample size and injection procedure in this study were chosen to prove the study hypothesis.
There were several limitations to our study. For example, the placebo-effect has been reported in KOA cases [12] but our study did not compare interventions with a placebo-control. In addition, oscillation of dose could affect therapeutic effect of hyaluronan injection [5]. However, the volume of the administered drug (but the same equivalent dose) of hyaluronan injection in the 2 groups was different. In addition, the cost of the treatment between the 2 groups was not compared.
Conclusions
This is the first study to look at the origin and efficacy of different hyaluronan injections in KOA cases. We demonstrated that CCH injection was superior to ADH injection in several ways. This suggests that formulations that consider the origin of the hyaluronan, and the characteristics of patients could be beneficial for effective KOA therapy. Further studies are required to evaluate the different effects based on the origin of hyaluronan with regards to different characteristics of patients.
Acknowledgements
Authors are thankful to the medical and non-medical staff of the First Affiliated Hospital of Xiamen University, Xiamen, China for their assistance within the research.
Footnotes
Conflict of Interest
None.
Source of support: Departmental sources
References
- 1.Musumeci G, Castrogiovanni P, Mazzone V, et al. Histochemistry as a unique approach for investigating normal and osteoarthritic cartilage. Eur J Histochem. 2014;58:107–11. doi: 10.4081/ejh.2014.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Musumeci G, Loreto C, Carnazza ML, Martinez G. Characterization of apoptosis in articular cartilage derived from the knee joints of patients with osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2011;19:307–13. doi: 10.1007/s00167-010-1215-0. [DOI] [PubMed] [Google Scholar]
- 3.Mobasheri A, Matta C, Zakany R, Musumeci G. Chondrosenescence: Definition, hallmarks and potential role in the pathogenesis of osteoarthritis. Maturitas. 2015;80:237–44. doi: 10.1016/j.maturitas.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Musumeci G, Castrogiovanni P, Trovato FM, et al. Physical activity ameliorates cartilage degeneration in a rat model of aging: A study on lubricin expression. Scand J Med Sci Sports. 2015;25:e222–30. doi: 10.1111/sms.12290. [DOI] [PubMed] [Google Scholar]
- 5.Sun SF, Hsu CW, Lin HS, et al. Comparison of single intra-articular injection of novel hyaluronan (HYA-JOINT Plus) with Synvisc-one for knee osteoarthritis: A randomized, controlled, double-blind trial of efficacy and safety. J Bone Joint Surg Am. 2017;99:462–71. doi: 10.2106/JBJS.16.00469. [DOI] [PubMed] [Google Scholar]
- 6.Vaquerizo V, Plasencia MA, Arribas I, et al. Comparison of intra-articular injections of plasma rich in growth factors (PRGF-Endoret) versus Durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: A randomized controlled trial. Arthroscopy. 2013;29:1635–43. doi: 10.1016/j.arthro.2013.07.264. [DOI] [PubMed] [Google Scholar]
- 7.Russo F, D’ Este M, Vadala G, et al. Platelet-rich plasma and hyaluronic acid blend for the treatment of osteoarthritis: Rheological and biological evaluation. PLoS One. 2016;11:e0157048. doi: 10.1371/journal.pone.0157048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutjes AW, Juni P, da Costa BR, et al. Viscosupplementation for osteoarthritis of the knee: A systematic review and meta-analysis. Ann Intern Med. 2012;157:180–91. doi: 10.7326/0003-4819-157-3-201208070-00473. [DOI] [PubMed] [Google Scholar]
- 9.Petrella RJ, Petrella M. A prospective, randomized, double-blind, placebo-controlled study to evaluate the efficacy of intraarticular hyaluronic acid for osteoarthritis of the knee. J Rheumatol. 2006;33:951–56. [PubMed] [Google Scholar]
- 10.Sun SF, Hsu CW, Lin HS, et al. Efficacy of intraarticular botulinum toxin A and intraarticular hyaluronate plus rehabilitation exercise in patients with unilateral ankle osteoarthritis: A randomized controlled trial. J Foot Ankle Res. 2014;7(1):9. doi: 10.1186/1757-1146-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chevalier X, Jerosch J, Goupille P, et al. Single, intra-articular treatment with 6 mL Hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: A randomised, multicentre, double-blind, placebo-controlled trial. Ann Rheum Dis. 2010;69:113–19. doi: 10.1136/ard.2008.094623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Weegen W, Wullems JA, Bos E, et al. No difference between intra-articular injection of hyaluronic acid and placebo for mild to moderate knee osteoarthritis: A randomized, controlled, double-blind trial. J Arthroplasty. 2015;30:754–57. doi: 10.1016/j.arth.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 13.McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:363–88. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Tammachote N, Kanitnate S, Yakumpor T, Panichkul P. Intra-articular, single-shot Hylan G-F 20 hyaluronic acid injection compared with corticosteroid in knee osteoarthritis: A double-blind, randomized controlled trial. J Bone Joint Surg Am. 2016;98:885–92. doi: 10.2106/JBJS.15.00544. [DOI] [PubMed] [Google Scholar]
- 15.Bum Park Y, Ah Choi W, Kim YK, et al. Accuracy of blind versus ultrasound-guided suprapatellar bursal injection. J Clin Ultrasound. 2012;40:20–25. doi: 10.1002/jcu.20890. [DOI] [PubMed] [Google Scholar]
- 16.Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence classification of osteoarthritis. Clin Orthop Relat Res. 2016;474:1886–93. doi: 10.1007/s11999-016-4732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glass N, Segal NA, Sluka KA, et al. Examining sex differences in knee pain: the multicenter osteoarthritis study. Osteoarthritis Cartilage. 2014;22:1100–16. doi: 10.1016/j.joca.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollard B, Johnston M, Dixon D. Exploring differential item functioning in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) BMC Musculoskelet Disord. 2012;13:265. doi: 10.1186/1471-2474-13-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clementi D, D’Ambrosi R, Bertocco P, et al. Efficacy of a single intra-articular injection of ultra-high molecular weight hyaluronic acid for hip osteoarthritis: A randomized controlled study. Eur J Orthop Surg Traumatol. :2017. doi: 10.1007/s00590-017-2083-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Ziegl A, Modre-Osprian R, Sanchez A, et al. Timed up-and-go device for unsupervised functional assessment of elderly patients. Stud Health Technol Inform. 2017;236:298–304. [PubMed] [Google Scholar]
- 21.Puente R, Illnait J, Mas R, et al. Comparison of the efficacy and tolerability of chondroitin plus glucosamine and D-002 (beeswax alcohols) in subjects with osteoarthritis symptoms. Rev Fac Cien Med Univ Nac Cordoba. 2017;74:107–18. [PubMed] [Google Scholar]
- 22.Jin Y, Chen X, Gao ZY, et al. The role of miR-320a and IL-1β in human chondrocyte degradation. Bone Joint Res. 2017;6:196–203. doi: 10.1302/2046-3758.64.BJR-2016-0224.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khanasuk Y, Dechmaneenin T, Tanavalee A. Prospective randomized trial comparing the efficacy of single 6-ml injection of Hylan G-F 20 and hyaluronic acid for primary knee arthritis: A preliminary study. J Med Assoc Thai. 2012;95:S92–97. [PubMed] [Google Scholar]
- 24.Raman R, Dutta A, Day N, et al. Efficacy of Hylan G-F 20 and Sodium Hyaluronate in the treatment of osteoarthritis of the knee-a prospective randomized clinical trial. Knee. 2008;15:318–24. doi: 10.1016/j.knee.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Doganay Erdogan B, Leung YY, et al. Minimal clinically important difference as applied in rheumatology: An OMERACT rasch working group systematic review and critique. J Rheumatol. 2016;43:194–202. doi: 10.3899/jrheum.141150. [DOI] [PubMed] [Google Scholar]
- 26.Konstantinidis GA, Aletras VH, Kanakari KA, et al. Comparative validation of the WOMAC osteoarthritis and Lequesne algofunctional indices in Greek patients with hip or knee osteoarthritis. Qual Life Res. 2014;23:539–48. doi: 10.1007/s11136-013-0490-x. [DOI] [PubMed] [Google Scholar]
- 27.Iannitti T, Elhensheri M, Bingol AO, Palmieri B. Preliminary histopathological study of intra-articular injection of a novel highly cross-linked hyaluronic acid in a rabbit model of knee osteoarthritis. J Mol Histol. 2013;44:191–201. doi: 10.1007/s10735-012-9457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper C, Rannou F, Richette P, et al. Use of intraarticular hyaluronic acid in the management of knee osteoarthritis in clinical practice. Arthritis Care Res (Hoboken) 2017;69:1287–96. doi: 10.1002/acr.23204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrella RJ, Emans PJ, Alleyne J, et al. Safety and performance of Hydros and Hydros-TA for knee osteoarthritis: A prospective, multicenter, randomized, double-blind feasibility trial. BMC Musculoskelet Disord. 2015;16:57. doi: 10.1186/s12891-015-0513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nayak BK. Understanding the relevance of sample size calculation. Indian J Ophthalmol. 2010;58:469–70. doi: 10.4103/0301-4738.71673. [DOI] [PMC free article] [PubMed] [Google Scholar]



