Abstract
Background
It has been proven that phenotype shifting, from the contractile phenotype to the synthetic phenotype, of vascular smooth muscle cells (VSMCs), plays an important role in vascular diseases such as atherosclerosis, restenosis, and hypertension. Recently, accumulating evidence suggests that Klotho is associated with many cardiovascular diseases or damage. Through the estimation of the proliferation and migration of Ang II-induced VSMCs and the related intracellular signal transduction pathways, we researched the effects of Klotho on phenotype modulation in this study.
Material/Methods
A rat vascular smooth muscle cell line was grown in vitro with or without Ang II or Klotho, and cell proliferation and migration were evaluated.
Results
The dose-dependent inhibition of Ang II-induced proliferation and migration by Klotho was shown in VSMCs. The phenotype modulation was inhibited by Klotho co-treatment; this co-treatment promoted the expression of contractile phenotype marker proteins, including SM22α, and also the proliferation phenotype marker protein PCNA compared with Ang II alone, which was suppressed, and activated VSMCs. Furthermore, by reducing the expression of G0/G1-specific regulatory proteins such as cyclin D1, cyclin-dependent kinase (CDK) 4, cyclin E, and CDK2, cell cycle arrest was induced by Klotho at G0/G1 phase. Although Ang II strongly stimulated NF-κB, p65, Akt, and ERK phosphorylation, these activation events were diminished by co-treatment with Ang II and Klotho.
Conclusions
Phenotype modulation of Ang II-induced VSMCs and stimulation of the NF-κB, p65, Akt, and ERK signaling pathways were inhibited by Klotho, which suggests that Klotho may play an important role in the phenotype modulation of VSMCs.
MeSH Keywords: Angiotensin II, Cell Proliferation, Emigration and Immigration
Background
Vascular smooth muscle cells have the ability to shift from a contractile into a synthetic, proliferative, and migratory phenotype, which is known as phenotypic modulation [1]. This modulation has been reported to play a key role in many cardiovascular diseases such as atherosclerosis, restenosis, and hypertension [2]. Previous studies confirmed that many cytokines and growth factors, including platelet-derived growth factor (PDGF), transforming growth factor (TGF)-β, and tumor necrosis factor (TNF)-α [3–5], can induce VSMC proliferation and migration. In recent years, accumulating evidence has suggested that angiotensin II (Ang II), a main peptide hormone of the rennin-angiotensin system, also plays an important role in the pathogenesis of cardiovascular diseases [6]. Previous studies confirmed that Ang II can produce inflammation in VSMCs via activating the extracellular signal regulated kinase 1/2 (ERK1/2) pathway and the Toll-like receptor 4-dependent signaling pathway [7,8]. Although atherosclerosis is known to be a multifactorial disease, an increasing number of investigations have revealed that atherosclerosis is a chronic inflammatory and immunity-related disease [9–11]. Hence, anti-inflammatory treatments might attenuate the process of atherosclerosis.
Klotho, a gene identified in 1997 as coding for a novel anti-aging protein, has been reported to be involved in the suppression of several aging phenotypes [12]. Mice with Klotho gene defects show human-like aging, including short lifespan, infertility, and arteriosclerosis [13]. Similarly, Klotho gene polymorphisms in humans are associated with various cardiovascular events [14]. Ming Chang Hu et al. showed that in vitro, high phosphate induces Na+-dependent uptake of phosphate and mineralization, while Klotho suppressed this reaction and preserved the differentiation of vascular smooth muscle cells. This study also confirms that Klotho ameliorates vascular calcification by enhancing phosphaturia and directly inhibiting phosphate uptake by vascular smooth muscle [15]. Previous studies reported that HMG-CoA reductase inhibitors (statins) have pleiotropic vascular protective effects besides lowering cholesterol. Recently, Kuwahara et al. confirmed that statin treatment significantly improved arteriosclerotic lesions induced by NOS inhibition by preventing the reduction of Klotho expression induced by NOS inhibition, rather than by affecting blood pressure or lipid levels. These data suggested a novel vascular protective effect of statins might occur via enhancing anti-aging Klotho protein expression [16]. Many previous in vitro and in vivo studies confirmed that Klotho is relevant to atherosclerosis and vascular calcification. Furthermore, Wang et al. compared 215 patients with essential hypertension and 220 non-hypertensive subjects and found that the G-395A polymorphism in the promoter region of the human Klotho gene was associated with the prevalence of essential hypertension [17].
Klotho has been reported to have functions in energy metabolism, anti-inflammatory and anti-oxidative effects, modulate ion transport, and regulate mineral metabolism [18]. Most recently, Yu et al. reported that Klotho can inhibit Ang II-induced cardiomyocyte hypertrophy [19]. Therefore, Klotho has been shown to be related to the onset of cardiovascular diseases. However, the effects of exogenous treatment with Klotho on Ang II-induced proliferation and migration of VSMCs have not been identified. Therefore, we sought to determine the influence of anti-proliferative and anti-migratory Klotho on Ang II-induced VSMCs in the present study. We also investigated the cellular mechanisms by which Klotho inhibits cell cycle progression in Ang II-treated cells.
Material and Methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM) was purchased from HyClone (HyClone, Logan, CT, USA). Fetal bovine serum (FBS) was purchased from ABGENT (San Diego, CA, USA). We purchased penicillin, streptomycin, and Angiotensin II from Sigma-Aldrich (St. Louis, MO, USA). The Cell Counting Kit-8 (CCK8) was purchased from Dojindo (Kumamoto, Japan). The rat recombinant Klotho protein was from Cloud-Clone Corporation (Houston, TX, USA). The antibodies used in this study were as follows: mouse monoclonal PCNA (PC10), rabbit polyclonal NF-κB p65 (C-20), rabbit polyclonal p-NF-κB p65 (Ser 536), rabbit polyclonal ERK1/2 (K-23), rabbit polyclonal p-ERK1/2 (Thr202/Tyr204), rabbit polyclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (FL-335) (Santa Cruz Biotechnology, CA, USA); and rabbit polyclonal SM22α (Cloud-Clone Corp., Wuhan, China). Anti-CDK2, anti-CDK4, anti-cyclin D, and anti-cyclin E antibodies were purchased from Wanleibio, Shenyang, China, and anti-Akt and anti-phospho-Akt (Ser473) antibodies were from Cell Signaling (MA, USA). The reactivity with rat antigens is included in all of these antibodies. Through ImageJ software (NIH, USA), the signal intensity was quantified.
Cell culture
The rat vascular smooth muscle cell line, A7r5 (China Center for Type Culture Collection, Wuhan, China), was grown in 25-cm2 culture flasks [Corning Inc., Corning, NY, USA]. The cells were grown in DMEM containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 U/mL streptomycin and were maintained at 37°C in a 5% CO2 humidified incubator. For subsequent experiments (unless otherwise stated), cells at 80% confluence in culture wells were growth-arrested by serum-starvation for 24 h.
Cell proliferation
Cell number
Cell count experiments were performed as described previously [20]. A suspension of VSMCs (0.5×105 cells/ml) was pretreated with or without Klotho (200 ng/ml) for 1 h and then incubated with or without Ang II (10−7 M) for 24 h. Cells were counted in a hemocytometer by light microscopy.
CCK8 assay
Cells were grown in 96-well plates at a density of 5000 cells/well. After the indicated treatments, 10 μl of WST-8 solution (2-[2-methoxy-4-nitrophenyl]-3-[4-nitrophenyl]-5-[2,4-disulfophenyl]-2H-tetrazolium, monosodium salt) was added to each well. Then, we incubated the plates for 4 h at 37°C. We measured the absorbance value at 450 nm on a microplate reader.
Wound healing experiment (Cell migration experiment)
The wound healing experiment was carried out on 60-mm plates. We pretreated the synchronized cells with Klotho (200 ng/ml) in serum-free medium for 24 h and allowed the cells to reach 90% confluence. After 24 h of Ang II (10−7 M) stimulation, a single wound was created in the center of the cell monolayer by scratching using a sterile plastic pipette. The cells migrating into the wounded area or protruding from the border of the wound were imaged with an inverted microscope after 24 h of incubation.
The Boyden chamber migration experiment
Transwell chambers with fibronectin-coated, 8-μm-pore-size polycarbonate membranes (BD Biosciences, Bedford, MA) were utilized for the Boyden chamber migration experiment. Next, we added 600 μL of DMEM containing 0.5% FBS and Klotho to the lower compartment and 100 μL of cell suspension (5×104 cells/well) to the upper compartment. Then, we added Ang II (10−7 M) to the lower chambers, as instructed. After 24 h of incubation at 37°C, we used a cotton swab to remove the non-migrated cells from the upper membrane. We fixed the migrated cells with 4% paraformaldehyde and stained them with DAPI (Sigma). Then, we quantified the stained migratory cells by manual counting under an inverted light microscope (Olympus Corporation, Tokyo, Japan) in at least 5 randomly selected fields of view and performed a statistical analysis.
Analysis of cell morphology and actin filaments by laser confocal microscopy
VSMCs were fixed in 4% paraformaldehyde at room temperature for 10 min and then permeabilized with 1% Triton X-100 for 5 min. The cells were washed with PBS and then incubated in the dark with Alexa 546-conjugated rhodamine phalloidin (5 U/mL, 1: 100, Invitrogen, Carlsbad, CA, USA) for 30 min. Then, the nuclei were stained with DAPI (Sigma) for 3 min in the dark. Cell morphology and actin filaments were imaged by fluorescence microscopy (Leica).
DNA content analysis by flow cytometry (cell cycle analysis)
We used flow cytometry to analyze cellular DNA content. VSMCs were harvested, fixed in cold ethanol, washed twice with PBS, and incubated with 10 μg/mL RNase and 50 μg/mL propidium iodide solution for 30 min at 37°C. Through flow cytometry (Becton, Dickinson and Company, FL, USA), we captured fluorescence from 1.0×104 cells. By using the Modifit LT software, we calculated the percentage of cells in the G0/G1, S, and G2/M phases of the cell cycle.
Protein extraction and Western blot
The cells were lysed with RIPA lysis buffer, which contained PMSF protease inhibitors. By BCA analysis (Beyotime Institute of Biotechnology, China) and using BSA as a standard, we measured the protein concentrations. We boiled the samples at 100°C for 5 min in 5× sample buffer. Proteins (50 μg per sample) were separated by 8–12% SDS-PAGE and then transferred onto a PVDF membrane with 200 mA constant current. Membranes were blocked with 5% BSA in Tris-buffered saline-Tween 20 (TBS-T, pH 7.6) for 2 h at room temperature and then were probed with primary antibodies overnight at 4°C. Primary antibodies against BAG3, TLR4, NF-κB p65, phospho-NF-κB p65, PCNA, and SM22α were used at a dilution of 1: 500, and the results were normalized to those of GAPDH, for which the antibody was diluted 1: 1000. Then, the membranes were incubated with anti-rabbit or anti-mouse IgG secondary antibodies (1: 5000). ECL-Plus chemiluminescent detection HRP reagents (Beyotime Institute of Biotechnology, China) were then applied, and the Microchemi 4.2 Bio-imaging system was used to detect the immunoreactive bands after extensive washing. The relative gray value of the immunoreactive bands was compared by Gelpro32 software. We normalized the densitometry of a phosphorylated protein to its corresponding total protein for assessing protein activation. We repeated the experiments 3 times under the same conditions.
Statistical analyses
The experiments were repeated 3 times with similar results. In addition, we used the SPSS statistical software package (version 17.0 software SPSS Inc, Chicago, IL, US) for data analysis. We obtained all data from at least 3 independent experiments, and the data are expressed as the mean ±S.E.M. We analyzed the differences between groups by ANOVA, followed by the LSD test for normally distributed values. The reported p values are two-tailed, and we considered a p value <0.05 to indicate statistical significance and a p value <0.001 to be highly significant.
Results
The Ang II-induced proliferation of VSMCs was inhibited by Klotho
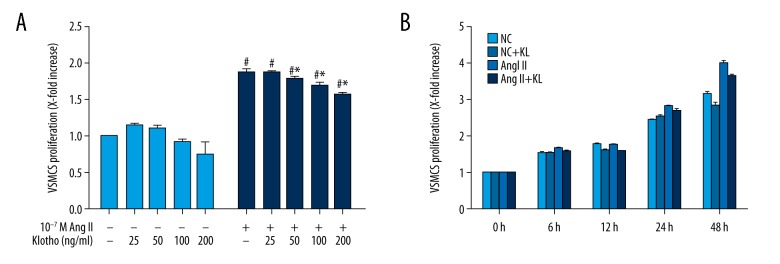
Through the CCK-8 assay, the effect of Klotho on the Ang II-induced proliferation of VSMCs was analyzed. The VSMCs were pretreated with Klotho (25, 50, 100, or 200 ng/ml) in serum-free medium for 24 h and then were stimulated with Ang II (10−7 M) for 24 h. Cell viability compared with untreated cells (Figure 1A) was significantly increased 1.84-fold by Ang II-treated cells. In addition, the Ang II-induced cell proliferation decreased upon treatment with Klotho (50, 100, and 200 ng/ml). Furthermore, compared with untreated cells (Figure 1A), the viability of VSMCs did not decrease in response to Klotho treatment in the absence of Ang II. As shown in Figure 1B, there was a time-dependent reduction in the Klotho-induced inhibitory effect on Ang II-induced VSMC proliferation at a fixed dose of 200 ng/ml. In Figure 2, the number of cells without Klotho pretreatment was significantly increased compared to that of VSMCs pretreated with Klotho, which indicated that Klotho inhibits the proliferation of Ang II-induced VSMCs.
Figure 1.
Effects of Klotho on Ang II-induced proliferation of VSMCs. (A) This was observed via CCK-8 assay. VSMCs were pretreated in serum-free medium in the presence or absence of Klotho (25, 50, 100, 200 ng/ml) for 24 h, and then stimulated with 10−7 M Ang II for a further 24 h. (B) VSMCs were treated with 200 ng/ml Klotho for 24h and then treated with Ang II for different time (6 h, 12 h, 24 h, 48 h). # p<0.05 versus untreated cells. * p<0.05 versus Ang II treatment.
Figure 2.
Representative of cell growth with and without Ang II (10−7 M) and with and without Klotho (200 ng/ml). Magnification 50×.
Ang II-induced VSMC migration was inhibited by Klotho
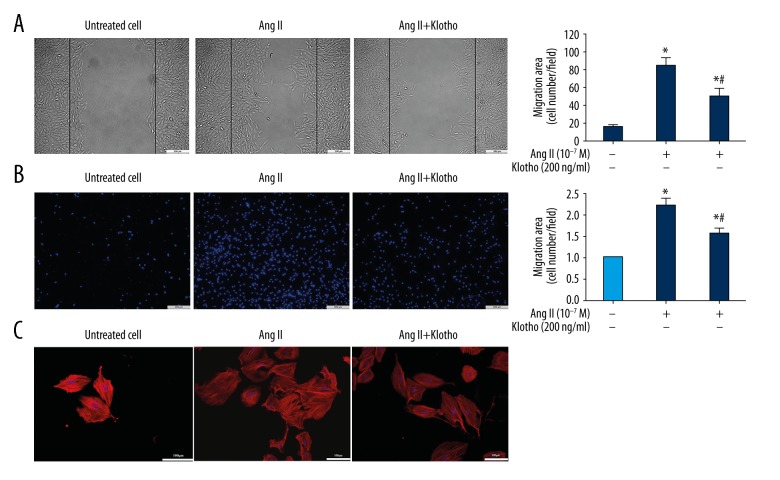
Through the wound healing experiment, the migration behavior after treatment with Klotho was observed to confirm the effect of Klotho on cell migration. As shown in Figure 3A, the migration of VSMCs was promoted by treatment with Ang II (10−7 M), while 200 ng/ml Klotho significantly reduced cell migration compared to Ang II. Furthermore, in order to confirm the inhibitory effect of Klotho on VSMC migration (Figure 3B), the transwell Boyden chamber experiment was utilized. The number of VSMCs that migrated through the transwell chamber was greatly increased by treatment with Ang II for 24 h. Despite Ang II stimulation, the number of migrated cells was significantly reduced by Klotho (200 ng/ml).
Figure 3.
Effects of Klotho on Ang II-induced migration of VSMC. (A) Wound healing assay. Conflunet VSMCs were scapes wounded and allowed to migrate for 24 h. (B) Transwell chemotactic assay. Klotho inhibited Ang II-induced VSMCs miagration. (C) Cell morphology and actin filaments analysis by laser confocal microscopy. VSMCs were incubated with Alexa 546-conjugated rhodamine phalloidin (red) and the nuclei were stained with DAPI (blue). * p<0.05 versus untreated cells. # p<0.05 versus Ang II treatmen.
The influence of Klotho on cell cycle progression of Ang II-induced cells
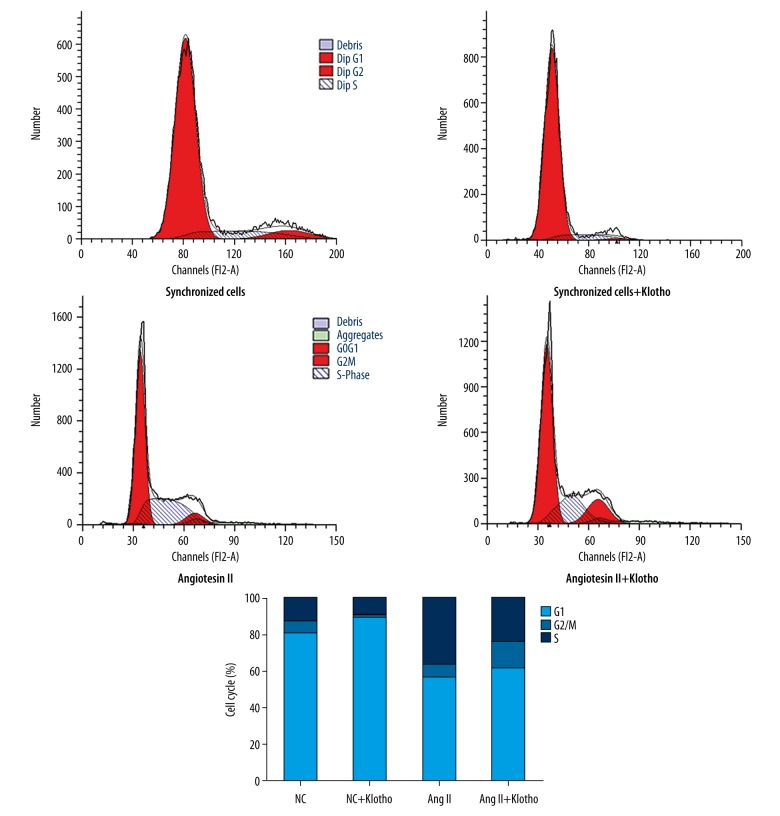
The influence of Klotho treatment on the cell cycle distribution of Ang II-stimulated VSMCs was analyzed by flow cytometry. Serum deprivation of VSMCs for 24 h caused 80.8% synchronization to the G0/G1 phase of cell cycle (Figure 4). With the concentration of cells in the G0/G1 phase reduced, the percentage of cells in S phase increased (from 12.41% to 36.24%) upon stimulation with Ang II, which showed that Ang II can activate cell proliferation. The reduction in the S phase population to approximately 23.02% and the increase in the fraction of cells in G0/G1 phase among Ang II-treated cells occurred upon treatment with 200 ng/ml Klotho. These data indicate that S phase entry in Ang II-induced VSMCs may be prevented by Klotho.
Figure 4.
Effects of Klotho on Ang II induced cell cycle progression in VSMCs. Cell cycle progression was assessed by flow cytometric analysis of DNA content. The cells were pretreated in the presence or absence of Klotho in serum-free medium for 24 h and then stimulated with 10−7 M Ang II. After 24 h, individual nuclear DNA content is reflected by the fluorescence intensity of incorporated propidium iodide. Each item is derived from representative experiments, where data from at least 10,000 events were obtained.
The influence of Klotho on cell cycle regulatory protein expression
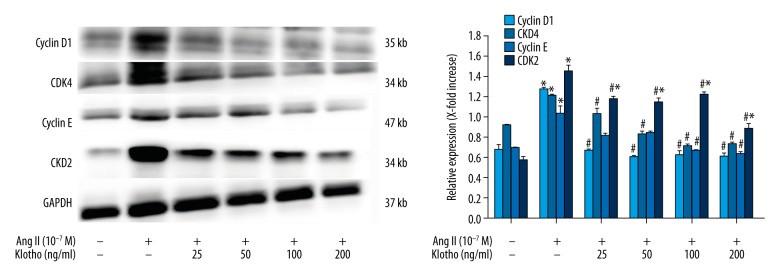
The phase-specific CDK-cyclins regulate progression through the cell cycle. We determined the effects of Klotho on cyclin D1, CDK4, cyclin E, and CDK2 expression through investigating the mechanism of cell cycle arrest by Klotho. Ang II greatly increased the cellular levels of cyclin D1 (1.90-fold), CDK4 (1.33-fold), cyclin E (1.50-fold), and CDK2 (2.54-fold) in VSMCs (P<0.05) (Figure 5). At 200 ng/ml Klotho, the expression of cyclin D1, CDK4, cyclin E, and CDK2 in Ang II-stimulated VSMCs was attenuated. Therefore, these data suggest that cell cycle progression through S phase via G0/G1 arrest was inhibited by Klotho.
Figure 5.
Effects of Klotho on Ang II-induced smooth muscle markers in VSMCs. Cell were pretreated in a serum-free medium in the presence or absence of Klotho for 24 h. Then treated with or without Ang II for 24 h. The protein expression levels of the smooth muscle markers SM22α and PCNA were determined by western blotting. GAPDH was used as an internal control. The graphs represent the relative expression of these proteins for three independent experiments. * p<0.05 versus untreated cells. # p<0.05 versus Ang II treatment.
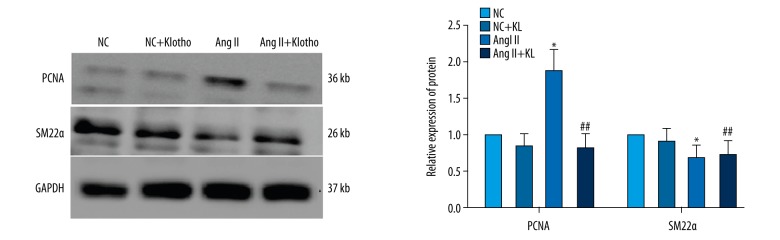
The influence of Klotho on the expression of Ang II-induced VSMC marker gene expression
When VSMCs enter a proliferative state in response to Ang II stimulation, they show high expression of PCNA and low expression of SM22α. The expression of VSMC marker genes was tested to determine the effect of Klotho. VSMCs were stimulated with Ang II (10−7 M) and 200 ng/ml Klotho alone or together for 24 h after starvation. Western blot data showed that the expression level of PCNA was markedly increased, while SM22α expression was significantly decreased with Ang II (P<0.05) (Figure 6), indicating that Ang II induces VSMC proliferation. However, this effect was attenuated by co-treatment with 200 ng/ml Klotho, which showed that the Ang II-induced change in VSMC marker gene expression (P<0.05) (Figure 6) can be inhibited by Klotho.
Figure 6.
Effects of Klotho on Ang II-induced cell cycle related-protein in VSMCs. Cell were pretreated in a serum-free medium in the presence or absence of Klotho for 24 h. The cells were lysed, and proteins were analyzed by Western Blot. GAPDH was used as an internal control. The graphs represent the relative expression of these proteins for three independent experiments. * p<0.05 versus untreated cells. # p<0.05 versus Ang II treatment.
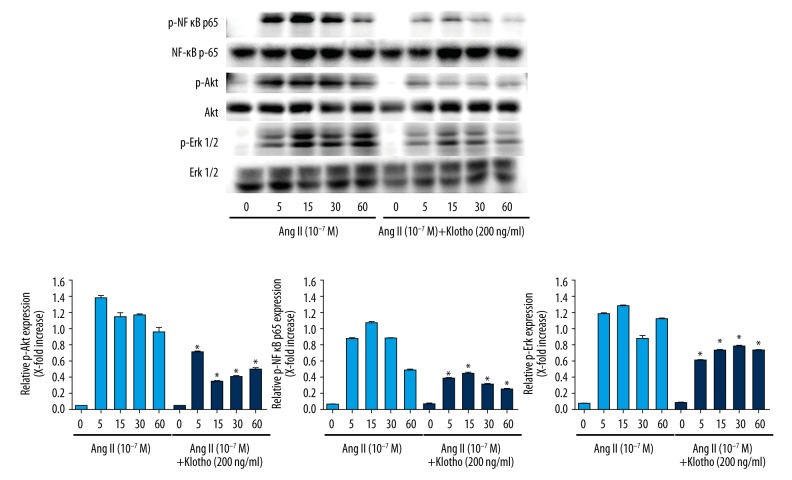
Klotho suppressed the Ang II signaling pathway
Previous studies demonstrated that the NF-κB, p65, Akt, and ERK signaling pathways have a considerable influence on the proliferation of Ang II-induced VSMCs. In the present study, we intended to estimate the activity of the NF-κB p65, Akt, and ERK signaling pathways in VSMCs stimulated with Ang II (10−7 M) with or without 200 ng/ml Klotho for specified lengths of time. As shown in Figure 7, we treated VSMCs with Ang II for 5, 10, 15, and 30 min with or without Klotho pretreatment. The phosphorylation of NF-κB, p65, Akt, and ERK1/2 was greatly increased by Ang II at 5 min; however, Ang II with Klotho significantly inhibited NF-κB, p65, Akt, and ERK1/2 phosphorylation.
Figure 7.
Klotho suppresses NF-κB p65, Akt and Erk 1/2 signaling pathways activated by Ang II in VSMCs. VSMC were stimulated with Ang II for indicated time periods with or without pretreatment by 200 ng/ml Klotho. Quantification of normalized densities for NF-κB p65, Akt and Erk1/2 activities is shown. The graphs represent the relative activity of these kinases for three independent experiments. * p<0.05 versus untreated cells. # p<0.05 versus Ang II treatment.
Discussion
Phenotype modulation in response to environmental stimuli is a characteristic of vascular smooth muscle cells [21]. This process is characterized by decreased gene expression of VSMC contractile markers and increased proliferation, migration, and matrix synthesis. Furthermore, the phenotypic switch was confirmed to be associated with many cardiovascular diseases, such as hypertension, atherosclerosis, and restenosis [22].
In the present study, we used Ang II to induce VSMC proliferation and migration. Many previous studies have demonstrated that Ang II plays a crucial role in endothelial dysfunction, VSMC proliferation and migration, monocyte chemoattraction, and inflammation in vascular tissue [23,24]. Furthermore, Ang II takes part in the vascular inflammatory response and cardiovascular disorders through receptor-mediated signaling and subsequent activation of multiple intracellular signaling pathways by the stimulation of VSMCs to produce cytokines and growth factors (e.g., PDGF, bFGF, and VEGF) [25]. Ji et al. demonstrated that Ang II induced VSMC phenotype switching by activating TLR4 through AT1 receptor and subsequent ERK1/2, NF-κB, and p65 activation in VSMCs [8,26–28]. In addition, many previous studies reported that Ang II inhibited VSMC marker gene expression by activating the Akt pathway [26,27]. Klotho was first identified as a novel anti-aging protein, but it has been recently implicated in multiple biological processes [28]. More recently, the involvement of Klotho in vascular protection through different mechanisms (e.g., inhibition of oxidative stress, modulation of inflammation, and attenuation of vascular calcification) has been demonstrated [15,29–31]. Therefore, Klotho has been suggested to be a master regulator of cardiovascular diseases [31]. In our study, we found that Ang II increased proliferation and migration and decreased SM22α, while PCNA expression was increased in VSMCs, indicating that Ang II regulates the phenotype modulation of VSMCs; however, the proliferation and migration of Ang II-induced VSMCs was inhibited by Klotho. First, we found that Klotho dose-dependently inhibited Ang II-induced proliferation of VSMCs. In addition, an increase in the expression of contractile proteins and a reduction in the expression of proliferative proteins, which corresponded to changes in the cytoskeleton in VSMCs, were observed upon co-treatment with Klotho and Ang II. Klotho could effectively suppress Ang II-induced VSMC proliferation, migration, and phenotypic modulation. Second, we found that cell cycle progression at S phase via G0/G1 arrest was probably inhibited by Klotho. Previous studies confirmed that cell cycle progression and cell cycle phases are coordinated by CDKs that form holoenzymes with their regulatory subunits, the cyclins [32,33], which may regulate cellular proliferation. In our study, we showed that with Ang II induction, Klotho inhibited the up-regulation of cyclin D1/CDK4 and cyclin E/CDK2. Therefore, Klotho mediated cell cycle arrest at G0/G1 phase by inhibiting the formation of active CDK/cyclin complexes through the suppression of cyclin D1 and cyclin E. In addition, we treated VSMCs with or without Klotho in order to evaluate the signal transduction pathways in Ang II-induced VSMCs. The phosphorylation levels of NF-κB, p65, Akt, and ERK1/2 were significantly upregulated in Ang II-stimulated VSMCs, but pretreatment with Klotho effectively attenuated these effects, suggesting that Klotho can suppress the phenotypic modulation of VSMCs induced by Ang II via down-regulation of the NF-κB, p65, Akt, and ERK1/2 pathways.
Conclusions
In the present study we aimed to determine the ability of Klotho to inhibit the Ang II-induced phenotypic modulation of VSMCs. For the first time, we demonstrate that Klotho has the potential to inhibit the proliferation and migration of VSMCs. Furthermore, we found that Klotho can inhibit VSMC proliferation and migration through modulation of the NF-κB, p65, Akt, and ERK1/2 pathways. This study provides a new perspective on exploiting the pathological mechanism of vascular phenotypic modulation and new findings regarding the mechanism by which the anti-aging protein Klotho protects against cardiovascular diseases.
Footnotes
Source of support: Departmental sources
References
- 1.Sandison ME, Dempster J, McCarron JG. The transition of smooth muscle cells from a contractile to a migratory, phagocytic phenotype: Direct demonstration of phenotypic modulation. J Physiol. 2016;594(21):6189–209. doi: 10.1113/JP272729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson JL. Emerging regulators of vascular smooth muscle cell function in the development and progression of atherosclerosis. Cardiovasc Res. 2014;103(4):452–60. doi: 10.1093/cvr/cvu171. [DOI] [PubMed] [Google Scholar]
- 3.Chen S, Liu B, Kong D, et al. Atorvastatin calcium inhibits phenotypic modulation of PDGF-BB-induced VSMCs via down-regulation the Akt signaling pathway. PLoS One. 2015;10(4):e122577. doi: 10.1371/journal.pone.0122577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rostam MA, Kamato D, Piva TJ, et al. The role of specific Smad linker region phosphorylation in TGF-beta mediated expression of glycosaminoglycan synthesizing enzymes in vascular smooth muscle. Cell Signal. 2016;28(8):956–66. doi: 10.1016/j.cellsig.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Meng L, Xu W, Guo L, et al. Paeonol inhibits the proliferation, invasion, and inflammatory reaction induced by TNF-alpha in vascular smooth muscle cells. Cell Biochem Biophys. 2015;73(2):495–503. doi: 10.1007/s12013-015-0686-5. [DOI] [PubMed] [Google Scholar]
- 6.Berk BC, Vekshtein V, Gordon HM, Tsuda T. Angiotensin II-stimulated protein synthesis in cultured vascular smooth muscle cells. Hypertension. 1989;13(4):305–14. doi: 10.1161/01.hyp.13.4.305. [DOI] [PubMed] [Google Scholar]
- 7.Peng N, Liu JT, Gao DF, et al. Angiotensin II-induced C-reactive protein generation: Inflammatory role of vascular smooth muscle cells in atherosclerosis. Atherosclerosis. 2007;193(2):292–98. doi: 10.1016/j.atherosclerosis.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Ji Y, Liu J, Wang Z, Liu N. Angiotensin II induces inflammatory response partly via toll-like receptor 4-dependent signaling pathway in vascular smooth muscle cells. Cell Physiol Biochem. 2009;23(4–6):265–76. doi: 10.1159/000218173. [DOI] [PubMed] [Google Scholar]
- 9.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340(2):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 10.Viola J, Soehnlein O. Atherosclerosis – A matter of unresolved inflammation. Semin Immunol. 2015;27(3):184–93. doi: 10.1016/j.smim.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Sorci-Thomas MG, Thomas MJ. Microdomains, inflammation, and atherosclerosis. Circ Res. 2016;118(4):679–91. doi: 10.1161/CIRCRESAHA.115.306246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mencke R, Hillebrands JL NIGRAM consortium. The role of the anti-ageing protein Klotho in vascular physiology and pathophysiology. Ageing Res Rev. 2017;35:124–46. doi: 10.1016/j.arr.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 14.Ding HY, Ma HX. Significant roles of anti-aging protein klotho and fibroblast growth factor23 in cardiovascular disease. J Geriatr Cardiol. 2015;12(4):439–47. doi: 10.11909/j.issn.1671-5411.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu MC, Shi M, Zhang J, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22(1):124–36. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuwahara N, Sasaki S, Kobara M, et al. HMG-CoA reductase inhibition improves anti-aging klotho protein expression and arteriosclerosis in rats with chronic inhibition of nitric oxide synthesis. Int J Cardiol. 2008;123(2):84–90. doi: 10.1016/j.ijcard.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 17.Wang HL, Xu Q, Wang Z, et al. A potential regulatory single nucleotide polymorphism in the promoter of the Klotho gene may be associated with essential hypertension in the Chinese Han population. Clin Chim Acta. 2010;411(5–6):386–90. doi: 10.1016/j.cca.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Hu MC, Kuro-o M, Moe OW. Renal and extrarenal actions of Klotho. Semin Nephrol. 2013;33(2):118–29. doi: 10.1016/j.semnephrol.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu L, Meng W, Ding J, Cheng M. Klotho inhibits angiotensin II-induced cardiomyocyte hypertrophy through suppression of the AT1R/beta catenin pathway. Biochem Biophys Res Commun. 2016;473(2):455–61. doi: 10.1016/j.bbrc.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 20.Xiao F, Puddefoot JR, Barker S, Vinson GP. Mechanism for aldosterone potentiation of angiotensin II-stimulated rat arterial smooth muscle cell proliferation. Hypertension. 2004;44(3):340–45. doi: 10.1161/01.HYP.0000140771.21243.ed. [DOI] [PubMed] [Google Scholar]
- 21.Xie C, Ritchie RP, Huang H, et al. Smooth muscle cell differentiation in vitro: Models and underlying molecular mechanisms. Arterioscler Thromb Vasc Biol. 2011;31(7):1485–94. doi: 10.1161/ATVBAHA.110.221101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84(3):767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz-Ortega M, Lorenzo O, Suzuki Y, et al. Proinflammatory actions of angiotensins. Curr Opin Nephrol Hypertens. 2001;10(3):321–29. doi: 10.1097/00041552-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Tunon J, Ruiz-Ortega M, Egido J. Regulation of matrix proteins and impact on vascular structure. Curr Hypertens Rep. 2000;2(1):106–13. doi: 10.1007/s11906-000-0067-2. [DOI] [PubMed] [Google Scholar]
- 25.Kranzhofer R, Schmidt J, Pfeiffer CA, et al. Angiotensin induces inflammatory activation of human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19(7):1623–29. doi: 10.1161/01.atv.19.7.1623. [DOI] [PubMed] [Google Scholar]
- 26.Shen YJ, Zhu XX, Yang X, et al. Cardamonin inhibits angiotensin II-induced vascular smooth muscle cell proliferation and migration by downregulating p38 MAPK, Akt, and ERK phosphorylation. J Nat Med. 2014;68(3):623–29. doi: 10.1007/s11418-014-0825-0. [DOI] [PubMed] [Google Scholar]
- 27.Chiou WF, Chen CC, Wei BL. 3,4-Di-O-Caffeoylquinic acid inhibits Angiotensin-II-induced vascular smooth muscle cell proliferation and migration by downregulating the JNK and PI3K/Akt signaling pathways. Evid Based Complement Alternat Med. 2011;2011:634502. doi: 10.1093/ecam/nep140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin-Nunez E, Donate-Correa J, Muros-de-Fuentes M, et al. Implications of Klotho in vascular health and disease. World J Cardiol. 2014;6(12):1262–69. doi: 10.4330/wjc.v6.i12.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rakugi H, Matsukawa N, Ishikawa K, et al. Anti-oxidative effect of Klotho on endothelial cells through cAMP activation. Endocrine. 2007;31(1):82–87. doi: 10.1007/s12020-007-0016-9. [DOI] [PubMed] [Google Scholar]
- 30.Maekawa Y, Ishikawa K, Yasuda O, et al. Klotho suppresses TNF-alpha-induced expression of adhesion molecules in the endothelium and attenuates NF-kappaB activation. Endocrine. 2009;35(3):341–46. doi: 10.1007/s12020-009-9181-3. [DOI] [PubMed] [Google Scholar]
- 31.Moe SM. Klotho: A master regulator of cardiovascular disease? Circulation. 2012;125(18):2181–83. doi: 10.1161/CIRCULATIONAHA.112.104828. [DOI] [PubMed] [Google Scholar]
- 32.Braun-Dullaeus RC, Mann MJ, Dzau VJ. Cell cycle progression: New therapeutic target for vascular proliferative disease. Circulation. 1998;98(1):82–89. doi: 10.1161/01.cir.98.1.82. [DOI] [PubMed] [Google Scholar]
- 33.Wei GL, Krasinski K, Kearney M, et al. Temporally and spatially coordinated expression of cell cycle regulatory factors after angioplasty. Circ Res. 1997;80(3):418–26. [PubMed] [Google Scholar]