Abstract
Backgrounds
Lung adenocarcinoma (LAC) accounts for the majority of lung cancer, which is the leading cause of cancer-related mortality worldwide. Keratin 17 (KRT17) was reported to promote the tumor development of skin tumor and oral cancer. The aim of this study was to investigate the expression and function of KRT17 in LAC.
Material/Methods
Immunohistochemical staining and quantitative PCR were performed to explore the expression of KRT17 in both LAC tissues and adjacent normal liver tissues. Chi-square test, univariate analysis, and multivariate analysis were conducted to statistically evaluate the clinical significance of KRT17 in LAC. Proliferation, migration, and invasion capacities of LAC cells were assessed after overexpression or silencing KRT17.
Results
Both the RNA and protein levels of KRT17 were up-regulated in LAC tissues compared to normal lung tissues. High expression of KRT17 was correlated with advanced TNM stage and poor overall survival. Moreover, KRT17 was identified as a novel independent prognostic factor for LAC patients. Cellular studies showed that KRT17 can enhance the proliferation, migration, and invasion capacities of LAC cells, thereby promoting tumor progression.
Conclusions
High expression of KRT17 is frequent in LAC tissues, which promotes tumor proliferation and invasion, and is correlated with a poor overall survival. Targeting KRT17 may be a novel direction for LAC drug development.
MeSH Keywords: Adenocarcinoma, Prognosis, Survival Analysis
Background
Lung cancer is one of the most common tumor types worldwide, and is the leading cause of cancer-related mortality [1,2]. Non-small cell lung cancer (NSCLC) is the major type of lung cancer with poor prognosis, and the 5-year overall survival is around 15% [3]. Lung adenocarcinoma (LAC) accounts for 40% of lung cancers, and generally originates from peripheral lung tissues [4]. The most curative method for treating lung cancer is surgical treatment. Unfortunately, it is still hard to predict the clinical outcomes, even for those who underwent tumor resection [5]. The predominant factors for poor prognosis are disease metastases, early recurrence, and poor response to adjunctive therapy. Therefore, identification of novel biomarkers is essential to predict the prognostic patterns of NSCLC patients.
Keratin (KRT) is a protein family that is critical for hair formation and is abundant in the outer layer of human skin, thus protecting epithelial cells from damage. Based on the protein sequence, keratin is further divided into 2 types: type I keratin and type II keratin [6]. Mutation of keratin genes can cause several diseases, including epidermolysis bullosa simplex [7], ichthyosis bullosa of Siemens [7], and steatocystoma multiplex [8]. Keratin 17 (KRT17) is a kind of type I keratin, and a previous study showed that calcipotriol can inhibit expression of K RT17 in keratinocytes in a dose-dependent manner, which may help develop novel therapies for psoriasis [9]. Besides the dermatology, dysregulation of keratin was recently reported to participate in human systematic diseases. For example, KRT7 expression is an early marker of reflux-related columnar mucosa of the esophagus [10]. Long-lived esophageal progenitor cells with high KRT15 expressions are critical for homeostasis and regeneration [11]. An elevated serum concentration of caspase-cleaved KRT18 fragments was identified in patients undergoing alcohol withdrawal, and it thus help predict liver-related death in patients with alcoholic liver disease [12]. Additional roles of keratin proteins in cancer development have recently been reported. Papafotiou et al. reported that some bladder basal cells have high expression of KRT14, and are responsible for development of urothelial cancer [13]. High expression of KRT19 in hepatocellular carcinoma also induced a more aggressive tumor phenotype [14]. Interestingly, even the same KRT can show completely distinct roles in different tissues. For example, KRT8 exhibits a protecting role on colonic permeability and prevents colitis [15], while its upregulation promotes tumor metastasis of clear cell renal cell carcinoma [16]. Here, we focused on studying the tumor-related role of KRT17.
As a major skin protein, KRT17 was first reported to promote epithelial proliferation and skin tumor growth [17]. The mRNA level of KRT17 was also enriched in oral squamous cell carcinoma [18]. Consistent with the RNA screening, proteomic research revealed an overexpression of KRT17 protein in malignancies [19, 20], which was later validated by in situ transcript assay [21]. Along with more and more high-throughput discoveries on the abnormal expression of KRT17, its clinical significance was revealed in cervical squamous carcinoma [22] and gastric cancer [23]. Furthermore, the underlying mechanism by which it regulates tumor progression seems to be tissue-specific. For example, KRT17 stimulates the Akt/mTOR pathway and glucose uptake, thereby facilitating tumor growth of oral cancer [24], and another research group also revealed its anti-apoptotic function by inhibiting caspase-3 cleavage [25]. Low expression of KRT17 in cervical cancer can induce tissue-specific cytokine polarization, and is essential for recruitment of effector immune cells to lesion-prone cervical epithelia, thereby preventing cancer development [25]. However, there is no evidence on the expression and function of KRT17 in HCC.
In the present study, we first performed RT-qPCR and IHC experiments to explore the RNA and protein levels of KRT17 in clinically obtained HCC tissues. The associations between KRT17 and clinical characteristics, as well as risk factors for HCC survival, were identified by statistical analyses. We also conducted cellular studies to explore the functional mechanisms of KRT17 in HCC.
Material and Methods
Patients and tissue samples
This investigation was approved by the Yidu Central Hospital of Weifang and Linyi People’s Hospital. A total of 81 patients that were diagnosed with lung adenocarcinoma (LAC) according to histological examination were recruited from our hospital between January 2010 and December 2012, including 23 females and 58 males. LAC and adjacent normal lung tissue samples were flash-frozen and stored in liquid nitrogen within 2 h of surgical removal until total RNA extraction. All patients included in this study provided informed consent forms before samples analyses.
RNA extraction and QPCR assays
Total RNA was extracted from tissues using TRIZOL reagent (Invitrogen). Reverse-transcription PCR was performed using the PrimeScript RT reagent Kit (RR036A; TaKaRa, Dalian, China) according to the manufacturer’s instructions. Real-time PCR was carried out to quantify KRT17 transcript level using SYBR Premix Ex Taq II (RR420A; TaKaRa), according to a standard protocol [26]. β-actin was used as control for normalization of KRT17 expression with the 2−ΔΔCT method. The primers were: KRT17 forward: 5′-GTGATGGGTTTGAGATTAGAGGTAT-3′;
KRT17 reverse: 5′-AAACCACCAAATAAACATAACAAT-3′;
β-actin forward: 5′-GATCATTGCTCCTCCTGAGC-3′; and
β-actin reverse: 5′-ACTCCTGCTTGCTGATCCAC-3′.
Immunohistochemical (IHC) staining
IHC staining for KRT17 was performed on 81 LAC patients and matched adjacent normal lung tissue specimens. Paraffin-embedded tissue samples were cut into 4-μm-thick sections. After antigen retrieval with citrate buffer of pH 6.0 under high pressure for 5 min, the slides were incubated in blocking buffer (5% BSA) and then overnight in the primary antibody at 4°C. After washing, the sections were incubated with a secondary biotinylated antibody. Finally, the intensity and extent of staining were evaluated separately by 2 pathologists. The scores of KRT17 expression (overall score range, 1–16) was based on staining intensity and proportions of stained cells [27].
Cell culture and transfection
The LAC cell line A549 was purchased from the Shanghai Institute of Biochemistry and Cell Biology (China) and cultured in RPMI 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum (Hyclone), 100 U/ml penicillin, and 100 mg/ml streptomycin (Invitrogen). Lipofectamine 2000 (Invitrogen, Shanghai, China) were used for KRT17-siRNA transfection according to the manufacturer’s instructions. The sequence of KRT17-siRNA was AGAAAGAACCGGUGACCAC. Transfection of KRT17 plasmid or control vector were carried out with FuGENE 6 Transfection reagent (Roche) according to the protocol.
Western blot analysis
A549 cells were lysed with RIPA buffer supplemented with protease/phosphatase inhibitor (Roche, Basel, Switzerland) and PMSF (Roche). The supernatants were collected as the proteins after centrifugation of 12000 rpm for 15 min at 4°C, separating by 10% SDS-PAGE gels, and then being transferred to nitrocellulose membranes. Equal amounts of protein were loaded for each lane in gels. After blocking with 5% BSA in TBST buffer, the membranes were incubated with primary antibody of KRT17 (Cell Signaling Technology) overnight at 4°C. Subsequently, appropriate horseradish peroxidase (HRP)-conjugated secondary antibody was added and KRT17 protein bands were detected with ECL chromogenic substrate as described by others [28].
Cell proliferation assay
A549 (3000/well) cells were seeded in 96-well plates 24 h after KRT17 siRNA or plasmid transfection. Cell proliferation was determined by MTT assay (Roche Applied Science). Cell viability was monitored every 24 h for the next 5 days. Experiments were independently repeated at least 3 times.
Transwell migration/invasion assay
Cell migration and invasion assays were performed with Transwell filters (Corning Costar Corp., Cambridge, MA, USA). After 24-h transfection, the cells (3×104/well for migration, 105/well for invasion) suspended in RPMI1640 were seeded in the upper chamber coated with or without Matrigel (BD Bioscience, Mountain View, CA, USA), invading towards the lower chambers with 15% FBS for 24 h at 37°C. After fixing in methanol, the cells that had penetrated to the bottom side of the filter were stained by 0.1% crystal violet for 15 min [29]. Then, the cells were counted under the microscope.
Statistical analysis
Data are represented as mean ± standard deviation (SD). Statistical analyses of 2 independent groups were performed using a two-tailed t test. Correlations between KRT17 expression and clinicopathological features were assessed with the χ2 test. For survival curves, Kaplan-Meier analysis was used, and log-rank analysis was performed for comparison. All statistical analyses were performed with SPSS ver. 18.0 software, and P<0.05 at the 95% confidence level was considered to be statistically significant.
Results
Patients’ information
Our cohort included 23 females and 58 males, with a median age of 59 years old. Forty-seven patients were smokers, and 34 patients were non-smokers (less than 5 cigarettes per month during the past 5 years). Most patients (50/81, 61.7%) showed a tumor size larger than 3.0 cm diameter. There were 12 patients (12/81, 14.8%) with well-differentiated tumors according to histological examination, 37 patients (37/81, 45.8%) with moderate differentiation, and the other 32 cases (32/81, 39.5%) with poor differentiation. Among all the patients, 39 (39/81, 48.1%) showed positive lymph node metastasis, and 31 (31/81, 38.3%) were classified as TNM stage III–IV (Table 1).
Table 1.
Characteristics of the LAC patients and associations with KRT17 expression level.
| Variable | Cases (n=81) | KRT17 expression | P value | |
|---|---|---|---|---|
| Low (n=41) | High (n=40) | |||
| Gender | 0.860 | |||
| Female | 23 | 12 | 11 | |
| Male | 58 | 29 | 29 | |
| Age (years) | 0.579 | |||
| ≤59 yrs | 40 | 19 | 21 | |
| >59 yrs | 41 | 22 | 19 | |
| Smoker | 0.420 | |||
| No | 34 | 19 | 15 | |
| Yes | 47 | 22 | 25 | |
| Tumor size | 0.752 | |||
| ≤3.0 cm | 31 | 15 | 16 | |
| >3.0 cm | 50 | 26 | 24 | |
| Differentiation | 0.005* | |||
| Well/moderate | 49 | 31 | 18 | |
| Poor | 32 | 10 | 22 | |
| LN metastasis | 0.011* | |||
| Negative | 42 | 27 | 15 | |
| Positive | 39 | 14 | 25 | |
| TNM stage | 0.032* | |||
| I–II | 50 | 30 | 20 | |
| III–IV | 31 | 11 | 20 | |
KRT17 is highly expressed in LAC tissues and is correlated with tumor progression
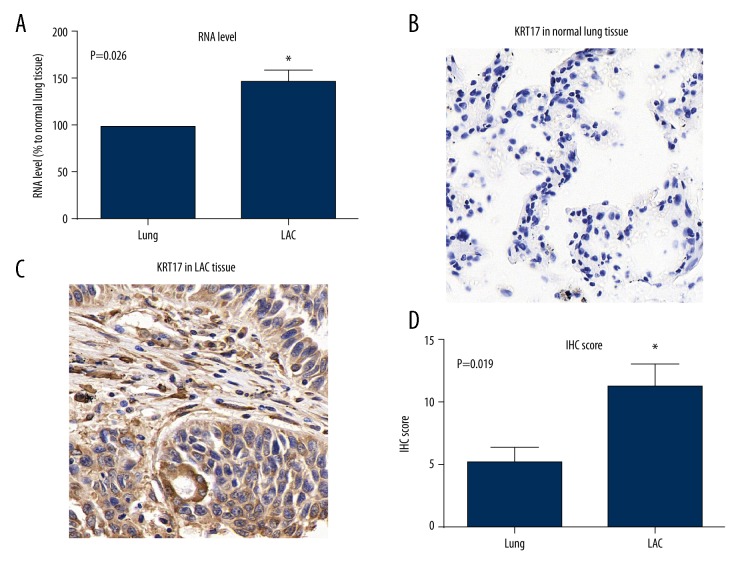
The RNA level of KRT17 was tested in 18 fresh-frozen LAC tissues and matched adjacent normal lung tissues by RT-qPCR. As shown in Figure 1A, the RNA level of KRT17 was significantly elevated in LAC tissues. Therefore, we further tested the protein expression and location of KRT17 in clinically collected tissues. Accordingly, KRT17 showed little or negative expression in normal lung tissues (Figure 1B), but showed remarkable staining intensity in the tumor tissues (Figure 1C). The KRT17 protein mainly existed in the cytoplasm of LAC cells, which was expressed at a significantly higher rate than that in normal lung tissues (Figure 1D).
Figure 1.
Expression of krt17 in normal lung and lac tissues. (A) The mRNA levels of KRT17 in LAC tissues and adjacent non-tumor tissues were analyzed by qPCR, and data are shown as mean ±SD (* P=0.026). (B) Representative immunohistochemical staining of KRT17 in normal lung tissue. (C) Representative protein expression of KRT17 in LAC tissue, showing the positive staining in the cytoplasm of tumor cells. (D) Statistical analysis of the average IHC scores in normal lung tissues and LAC tumor tissues (* P=0.019).
According to the IHC score as described before, we classified patients into a low-KRT17 group and s high-KRT17 group to explore its involvement in LAC progression by chi-square test. Patients with poor differentiation (P=0.005) or positive LN metastasis (P=0.011) or advanced TNM stages (P=0.032) were more likely to show elevated KRT17 expression (Table 1), suggesting that KRT17 may have tumor-promoting roles in LAC. No significant difference was found between USP33 expression levels and other clinicopathological variables such as sex, age, smoking, or tumor size.
High KRT17 indicates poor overall survival of LAC patients
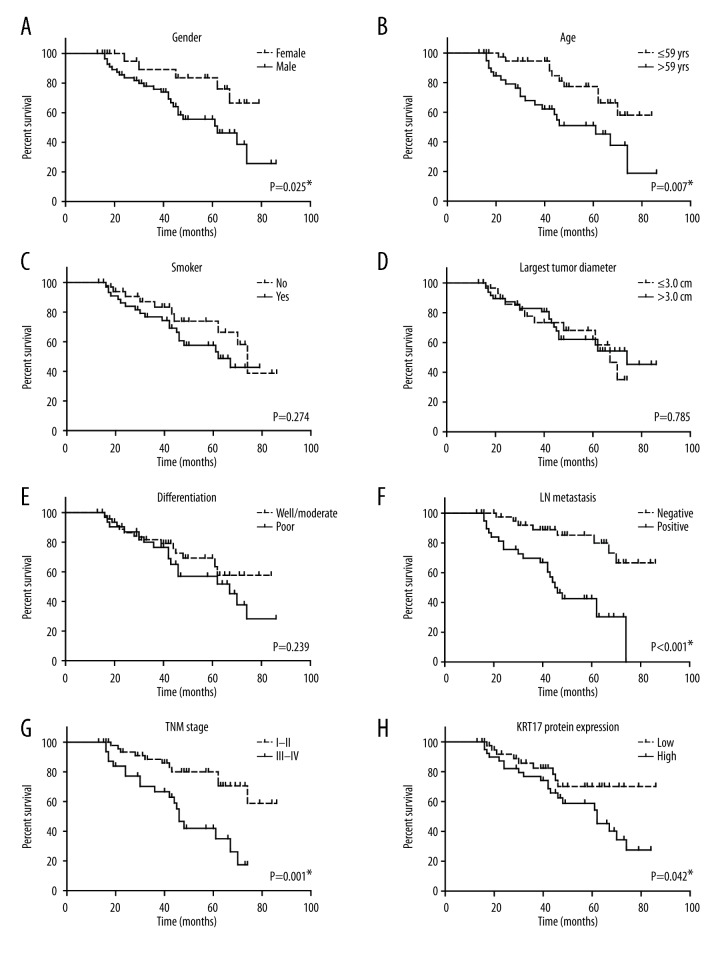
To further investigate whether KRT17 was correlated with patient survival, we performed univariate analysis and found that patients with high KRT17 expression showed shorter overall survival (OS) compared with those possessing lower KRT17 levels (5-year OS 54.3% and 70.0%, respectively; Table 2). Other prognostic parameters included sex (P=0.025), age (P=0.007), lymph node metastasis (P<0.001), and TNM stages (P=0.001, Figure 2).
Table 2.
Univariate analysis for the overall survival of LAC patients.
| Variable | Cases (n=81) | Overall survival | P value | |
|---|---|---|---|---|
| Mean ±SD (months) | 5-year (%) | |||
| Gender | 0.025* | |||
| Female | 23 | 69.0±4.1 | 83.6% | |
| Male | 58 | 57.2±3.8 | 55.6% | |
| Age (years) | 0.007* | |||
| ≤59 yrs | 40 | 70.2±3.6 | 77.4% | |
| >59 yrs | 41 | 53.2±4.6 | 51.0% | |
| Smoker | 0.274 | |||
| No | 34 | 66.5±4.7 | 73.8% | |
| Yes | 47 | 56.9±3.7 | 57.6% | |
| Tumor size | 0.785 | |||
| ≤3.0 cm | 31 | 57.4±4.1 | 68.1% | |
| >3.0 cm | 50 | 63.2±4.0 | 62.1% | |
| Differentiation | 0.239 | |||
| Well/moderate | 49 | 65.2±4.1 | 69.2% | |
| Poor | 32 | 58.6±4.7 | 56.9% | |
| LN metastasis | <0.001* | |||
| Negative | 42 | 74.5±3.5 | 85.2% | |
| Positive | 39 | 48.5±3.8 | 42.5% | |
| TNM stage | 0.001* | |||
| I–II | 50 | 71.4±3.7 | 80.0% | |
| III–IV | 31 | 48.1±3.9 | 41.9% | |
| KRT17 expression | 0.042* | |||
| Low | 61 | 70.3±4.5 | 70.0% | |
| High | 47 | 57.0±4.0 | 54.3% | |
Figure 2.
Kaplan-Meier analysis of overall survival. Kaplan-Meier curve analysis showed the correlations of overall survival of LAC patients with patients’ sex (A, P=0.025); age (B, P=0.007); smoking (C, P=0.274); tumor size (D, P=0.785); tumor differentiation (E, P=0.239); lymph node metastasis (F, P<0.001); TNM stage (G, P=0.001); and KRT17 protein level (H, P=0.042). * Indicates P<0.05 by log-rank test.
We then enrolled the parameters above into a multivariate analysis to identify the independence (Table 3). Statistical results revealed that high levels of KRT17 were independently correlated with worse OS (P=0.010, HR=2.882, 95% CI 1.287–6.453). The data also indicated that LN metastasis (P=0.001, HR=4.880, 95% CI 1.983–12.009) and advanced TNM stages (P=0.001, HR=4.168, 95% CI 1.780–9.759) were both independent prognostic factors for the OS of LAC patients.
Table 3.
Multivariate analysis for the overall survival of LAC patients.
| Variable | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Gender (vs. Female) | 2.345 | 0.827–6.652 | 0.109 |
| Age (vs. ≤59 yrs) | 1.906 | 0.850–4.275 | 0.117 |
| LN metastasis (vs. negative) | 4.880 | 1.983–12.009 | 0.001* |
| TNM stage (vs. I–II) | 4.168 | 1.780–9.759 | 0.001* |
| KRT17 expression (vs. low) | 2.882 | 1.287–6.453 | 0.010* |
Silencing KRT17 significantly inhibits LAC proliferation and invasion
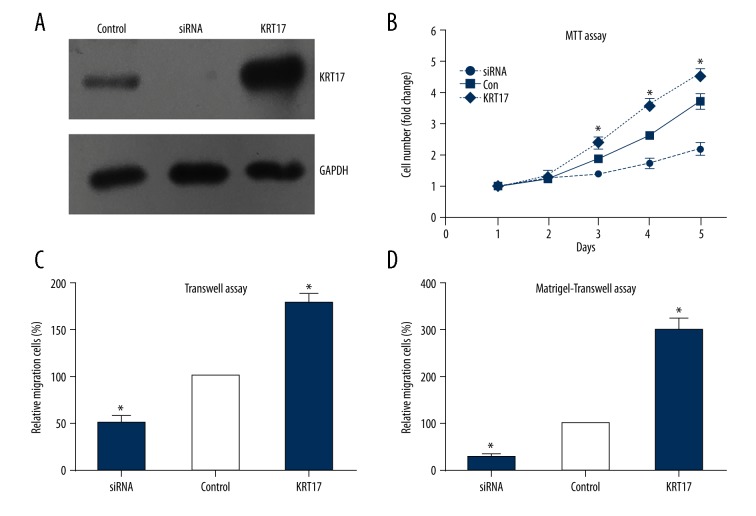
To investigate the effects of KRT17 on tumor biology, we chose the LAC cell line A549 for the in vitro experiments. Cells were transfected with KRT17 plasmids or siRNA, and the transfection efficiencies were validated by Western blot analysis (Figure 3A).
Figure 3.
KRT17 promotes the proliferation and invasion of LAC cells. (A) Transfection efficiency of KRT17 plasmids and siRNA in A549 cells were validated by Western blot. (B) The MTT assay showed that overexpression of KRT17 enhanced the proliferation of A549 cells. (C). Transwell assay showed that overexpression of KRT17 up-regulated the migration capacity of A549 cells. (D) Matrigel-transwell assay confirmed the oncogenic role of KRT17 in promoting the invasion process of LAC cells.
Cell proliferation assessed with MTT assay demonstrated that overexpression of KRT17 markedly enhanced cell viability of the A549 cells as compared with the control group from day 3 to day 5 (Figure 3B). In contrast, silencing KRT17 significantly inhibited cell proliferation. The effects of KRT17 on the migration and invasion of LAC cells were analyzed initially in vitro using Transwell assays. The results showed that both the migratory and invasive abilities of the LAC cells were suppressed by KRT17 knockdown when compared to the control cells (Figure 3C, 3D). On the other hand, KRT17 overexpression significantly enhanced the cell migration and invasion capacities. Collectively, cellular studies suggest the oncogenic effects of KRT17 on the progression of LAC.
Discussion
Keratin is widely expressed in skin cells, and its abnormal expression in other tissues may be correlated with pathological changes. For the first time, our study revealed high expression of KRT17 in some LAC patients. Exploring the role and function of KRT17 in lung cancer is needed to better classify LAC patients according to their molecular patterns. By grouping patients into low-KRT17 and high-KRT17 groups, we found that high expression of KRT17 was closely correlated with lymph node metastasis and TNM stage. The association between KRT17 and aggressive properties of LAC engaged us to further test its effect on patient survival. Univariate and multivariate analyses showed that KRT17 expression, as well as lymph node metastasis and TNM stage, are independent prognostic factors for the overall survival of LAC. Our data are consistent with a recent study reporting that KRT17 promotes tumor growth and is associated with poor prognosis in gastric cancer [23]. The inhibitory effect on tumor progression was achieved by silencing KRT17 in gastric cancer cells [30]. Progression of another digestive system tumor type, oral squamous cell carcinomas, is also regulated by KRT17 [24].
We also conducted in vitro cellular experiments to explore the underlying mechanisms of KRT17 in HCC. According to our results, KRT17 can directly enhance the cell proliferation, migration, and invasion processes. Besides our identification of KRT17 as an unfavorable prognostic factor, several other keratins were also involved in the pathogenesis of HCC. KRT19 was positively regulated by upstream HGF and promoted tumor progression of HCC [14]. KRT19 was also reported to be positively expressed in cancer stem cells of HCC [31]. The expression of KRT19 in the invasion front of HCC drives tumor metastasis perhaps by increasing yes-associated protein 1 signaling [32,33]. In addition, KRT18 was shown to participate in steatohepatitis-associated liver carcinogenesis [34]. Another example is KRT8, whose variants are infrequent in patients with alcohol-related liver cirrhosis, but shows no association with development of hepatocellular carcinoma [35]. Therefore, our study provides initial evidence of the role of KRT17 in HCC, serving as a supplement to the functions of KRT family members in HCC.
More and more documented evidence has identified novel biomarkers in tumors, but markers from limited studies were translated into clinical monitoring of HCC. KRT19 is a well-established and clinical-used biomarker, indicating the potential of other KRTs. Monitoring the alterations of these biomarkers would be helpful to evaluate disease progression and to direct targeted treatment therapy. Our study has some limitations. Firstly, this study is based on the conclusion from only 2 medical centers, and the sample size is not large enough. Secondly, although our cellular studies showed the direct role of KRT17 in tumor proliferation and invasion, we did not explore the signaling mechanisms. Further research is required to forecast the targets and the exact mechanism underlying KRT17 function.
Conclusions
We demonstrated that expression of KRT17 was significantly correlated with HCC progression. High expression of KRT17 was notably associated with a lower 5-year overall survival rate by directly promoting the proliferation and invasion of HCC cells. Our data have remarkable diagnostic, predictive, and therapeutic potential in clinical HCC treatment.
Footnotes
Conflict of interest
None
Source of support: Departmental sources
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Bilfinger T, Keresztes R, Albano D, Nemesure B. Five-year survival among stage IIIA lung cancer patients receiving two different treatment modalities. Med Sci Monit. 2016;22:2589–94. doi: 10.12659/MSM.898675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vokes E. Lung cancer, 1. Lancet. 2000;355:479–85. doi: 10.1016/S0140-6736(00)82038-3. [DOI] [PubMed] [Google Scholar]
- 4.Lu C, Onn A, Vaporciyan A. 78: Cancer of the lung, Holland-Frei Cancer Medicine. People’s Medical Publishing House; 2010. [Google Scholar]
- 5.Baltayiannis N, Chandrinos M, Anagnostopoulos D, et al. Lung cancer surgery: An up to date. J Thorac Dis. 2013;5(Suppl 4):S425–39. doi: 10.3978/j.issn.2072-1439.2013.09.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanukoglu I, Fuchs E. The cDNA sequence of a Type II cytoskeletal keratin reveals constant and variable structural domains among keratins. Cell. 1983;33(3):915–24. doi: 10.1016/0092-8674(83)90034-x. [DOI] [PubMed] [Google Scholar]
- 7.Coulombe PA, Hutton ME, Letal A, et al. Point mutations in human keratin 14 genes of epidermolysis bullosa simplex patients: Genetic and functional analyses. Cell. 1991;66(6):1301–11. doi: 10.1016/0092-8674(91)90051-y. [DOI] [PubMed] [Google Scholar]
- 8.OH SW, Kim MY, Lee JS, KIM SC. Keratin 17 mutation in pachyonychia congenita type 2 patient with early onset steatocystoma multiplex and Hutchinson-like tooth deformity. J Dermatol. 2006;33(3):161–64. doi: 10.1111/j.1346-8138.2006.00037.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Fang H, Wang R, et al. Effect of calcipotriol on IFN-gamma-induced keratin 17 expression in immortalized human epidermal keratinocyte cells. Med Sci Monit. 2017;23:6049–56. doi: 10.12659/MSM.904850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cabibi D, Fiorentino E, Pantuso G, et al. Keratin 7 expression as an early marker of reflux-related columnar mucosa without intestinal metaplasia in the esophagus. Med Sci Monit. 2009;15(5):CR203–10. [PubMed] [Google Scholar]
- 11.Giroux V, Lento AA, Islam M, et al. Long-lived keratin 15+ esophageal progenitor cells contribute to homeostasis and regeneration. J Clin Invest. 2017;127(6):2378–91. doi: 10.1172/JCI88941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mueller S, Nahon P, Rausch V, et al. Caspase-cleaved keratin-18 fragments increase during alcohol withdrawal and predict liver-related death in patients with alcoholic liver disease. Hepatology (Baltimore, Md) 2017;66(1):96–107. doi: 10.1002/hep.29099. [DOI] [PubMed] [Google Scholar]
- 13.Papafotiou G, Paraskevopoulou V, Vasilaki E, et al. KRT14 marks a subpopulation of bladder basal cells with pivotal role in regeneration and tumorigenesis. Nat Commun. 2016;7:11914. doi: 10.1038/ncomms11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhee H, Kim HY, Choi JH, Woo HG. Keratin 19 expression in hepatocellular carcinoma is regulated by fibroblast-derived HGF via a MET-ERK1/2-AP1 and SP1 axis. Cancer Res. 2018 doi: 10.1158/0008-5472.CAN-17-0988. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Liu C, Liu ED, Meng YX, et al. Keratin 8 reduces colonic permeability and maintains gut microbiota homeostasis, protecting against colitis and colitis-associated tumorigenesis. Oncotarget. 2017;8(57):96774–90. doi: 10.18632/oncotarget.18241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan HS, Jiang WH, He Y, et al. KRT8 upregulation promotes tumor metastasis and is predictive of a poor prognosis in clear cell renal cell carcinoma. Oncotarget. 2017;8(44):76189–203. doi: 10.18632/oncotarget.19198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Depianto D, Kerns ML, Dlugosz AA, Coulombe PA. Keratin 17 promotes epithelial proliferation and tumor growth by polarizing the immune response in skin. Nat Genet. 2010;42(10):910–14. doi: 10.1038/ng.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolokythas A, Schwartz JL, Pytynia KB, et al. Analysis of RNA from brush cytology detects changes in B2M, CYP1B1 and KRT17 levels with OSCC in tobacco users. Oral Oncol. 2011;47(6):532–36. doi: 10.1016/j.oraloncology.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 19.Kim CY, Jung WY, Lee HJ, et al. Proteomic analysis reveals overexpression of moesin and cytokeratin 17 proteins in colorectal carcinoma. Oncol Rep. 2012;27(3):608–20. doi: 10.3892/or.2011.1545. [DOI] [PubMed] [Google Scholar]
- 20.Chiang CH, Wu CC, Lee LY, et al. Proteomics analysis reveals involvement of Krt17 in areca nut-induced oral carcinogenesis. J Proteome Res. 2016;15(9):2981–97. doi: 10.1021/acs.jproteome.6b00138. [DOI] [PubMed] [Google Scholar]
- 21.Kiflemariam S, Andersson S, Asplund A, et al. Scalable in situ hybridization on tissue arrays for validation of novel cancer and tissue-specific biomarkers. PLoS One. 2012;7(3):e32927. doi: 10.1371/journal.pone.0032927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Escobar-Hoyos LF, Yang J, et al. Keratin 17 in premalignant and malignant squamous lesions of the cervix: Proteomic discovery and immunohistochemical validation as a diagnostic and prognostic biomarker. Mod Pathol. 2014;27(4):621–30. doi: 10.1038/modpathol.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu H, Xu DH, Huang XX, et al. Keratin17 promotes tumor growth and is associated with poor prognosis in gastric cancer. J Cancer. 2018;9(2):346–57. doi: 10.7150/jca.19838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khanom R, Nguyen CT, Kayamori K, et al. Keratin 17 is induced in oral cancer and facilitates tumor growth. PLoS One. 2016;11(8):e0161163. doi: 10.1371/journal.pone.0161163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mikami Y, Fujii S, Nagata K, et al. GLI-mediated Keratin 17 expression promotes tumor cell growth through the anti-apoptotic function in oral squamous cell carcinomas. J Cancer Res Clin Oncol. 2017;143(8):1381–93. doi: 10.1007/s00432-017-2398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu H, Zhang Q, Li K, et al. Prognostic significance of USP33 in advanced colorectal cancer patients: New insights into β-arrestin-dependent ERK signaling. Oncotarget. 2016;7(49):81223–40. doi: 10.18632/oncotarget.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Liu Z, Li K, et al. TBL1XR1 predicts isolated tumor cells and micrometastasis in patients with TNM stage I/II colorectal cancer. J Gastroenterol Hepatol. 2017;32(9):1570–80. doi: 10.1111/jgh.13749. [DOI] [PubMed] [Google Scholar]
- 28.Xu Y, Yang X, Li Z, et al. Sprouty2 correlates with favorable prognosis of gastric adenocarcinoma via suppressing FGFR2-induced ERK phosphorylation and cancer progression. Oncotarget. 2017;8(3):4888. doi: 10.18632/oncotarget.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H, Xu Y, Zhang Q, et al. Correlations between TBL1XR1 and recurrence of colorectal cancer. Sci Rep. 2017;7:44275. doi: 10.1038/srep44275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chivu-Economescu M, Dragu DL, Necula LG, et al. Knockdown of KRT17 by siRNA induces antitumoral effects on gastric cancer cells. Gastric Cancer. 2017;20(6):948–59. doi: 10.1007/s10120-017-0712-y. [DOI] [PubMed] [Google Scholar]
- 31.Kawai T, Yasuchika K, Ishii T, et al. Identification of keratin 19-positive cancer stem cells associating human hepatocellular carcinoma using CYFRA 21-1. Cancer Med. 2017;6(11):2531–40. doi: 10.1002/cam4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Govaere O, Petz M, Wouters J, et al. The PDGFRalpha-laminin B1-keratin 19 cascade drives tumor progression at the invasive front of human hepatocellular carcinoma. Oncogene. 2017;36(47):6605–16. doi: 10.1038/onc.2017.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee K, Lee KB. The correlation between poor prognosis and increased yes-associated protein 1 expression in keratin 19 expressing hepatocellular carcinomas and cholangiocarcinomas. BMC Cancer. 2017;17(1):441. doi: 10.1186/s12885-017-3431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bettermann K, Mehta AK, Hofer EM, et al. Keratin 18-deficiency results in steatohepatitis and liver tumors in old mice: A model of steatohepatitis-associated liver carcinogenesis. Oncotarget. 2016;7(45):73309–22. doi: 10.18632/oncotarget.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Usachov V, Nahon P, Lunova M, et al. Keratin 8 variants are infrequent in patients with alcohol-related liver cirrhosis and do not associate with development of hepatocellular carcinoma. BMC Gastroenterol. 2012;12:147. doi: 10.1186/1471-230X-12-147. [DOI] [PMC free article] [PubMed] [Google Scholar]