Abstract
Background
Rho-associated coiled-coil containing protein kinases 2 (ROCK2) is one of the best characterized targets for the small GTPase Rho. It has been reported that ROCK2 is critical for cancer cell migration and invasion. The objective of this study was to investigate the association of ROCK2 expression with clinicopathological features and overall survival of breast cancer patients.
Material/Methods
The expression of ROCK2 in breast cancer and paired adjacent normal tissues was detected and compared by immunohistochemical staining of tissue array. ROCK2 mRNA expression and clinicopathological information was extracted from the TCGA breast cancer dataset. The association of ROCK2 expression with the clinicopathological characteristics of patients with breast cancer was evaluated using univariate and multivariate Cox proportional hazards models. Overall survival was analyzed using the Kaplan-Meier method.
Results
Immunohistochemistry showed that ROCK2 expression was significantly higher in tumor tissues than in paired adjacent normal tissues [immunoreactivity score (IRS): tumor, 5.25±2.10, n=40 vs. adjacent normal 3.83±1.06, n=40, P<0.01]. The IRS was correlated to breast cancer staging. Similarly, the mRNA level of ROCK2 was correlated to tumor stage. Notably, ROCK2 mRNA expression (hazard ratio [HR] 1.665 and 95% confidence interval [CI] 1.115–2.488, P=0.013) were also associated with overall survival in a multivariate analysis.
Conclusions
Upregulation of ROCK2 was associated with the progression of breast cancer. High expression of ROCK2 may predict poor overall survival rates for breast cancer patients.
MeSH Keywords: Breast Neoplasms, Prognosis, rho-Associated Kinases
Background
Breast cancer is the most common female malignancy in both developing and developed countries [1]. According to a 2012 report of World Health Organization (WHO), breast cancer is the leading cause of death among women worldwide, accounting for 23% of all cancer deaths [2]. There were 1.7 million newly diagnosed breast cancer cases in 2012 (the second most common cancer overall). Nearly 60% of deaths due to breast cancer occur in developing countries [3]. Although the mortality rate of breast cancer has decreased over the past 2 decades, the risk of breast cancer recurrence is still high. About one-third of patients with early-stage breast cancer experienced disease recurrence after initial diagnosis [4]. Considering the large affected population worldwide, it is important to find biomarkers to identify patients with high risk of recurrence.
ROCK2 is one of the most important targets for the Rho subfamily of small GTPases [5,6]. It is activated by interaction with Rho GTPases and modulates a series of processes related to cytoskeletal rearrangement, such as focal adhesion formation, cell motility, and tumor cell invasion [5,7]. Accumulating evidence links ROCK2 to the progression and pathogenesis of several human tumors [8,9]. The expression of ROCK2 in colorectal tumor tissues was significantly higher than in non-cancerous tissues, and elevated ROCK2 level was closely associated with tumor invasion [10]. It has been reported that the upregulation of ROCK2 promoted metastasis, proliferation, and invasion of gastric cancer cells [9]. Previous studies showed that ROCK2 genetic polymorphisms were associated with disease development. Of particular interest is that a study reported that Thr431Asn polymorphism in ROCK2 protein could be a risk factor for breast cancer metastases [11], indicating that ROCK2 may be of great value in predicting poor prognosis in patients with breast cancer.
In this study, we examined the association of ROCK2 expression level with clinicopathological features by using the TCGA breast cancer dataset and tissue array.
Material and Methods
Patients and tissue samples
The study was approved by the Research Ethics Committee of Tongxu County People’s Hospital, Henan Province, China. Informed consent was obtained from all of the patients. For immunohistochemical analysis, a tissue array including 40 adjacent non-cancerous breast tissues and 40 breast cancer tissues was obtained from Xi’an Alenabio Co, LTD (Cat No. BR804a). Patients who had received radiotherapy or chemotherapy before the surgery were excluded from the study. Clinical information was collected from the TCGA dataset, including 999 breast cancer tissues.
Immunohistochemical staining and analysis
The paraffin-embedded tissues in the tissue array were deparaffinized with xylene and rehydrated, and then were stained using the DAKO EnVision System (Dako Diagnostics, Switzerland). The tissue slides were incubated using a 1: 50 dilution of the primary antibody against ROCK2 overnight (rabbit polyclonal antibody, ab71598, Abcam Co., Ltd., UK) at 4°C. After washing with PBS, peroxidase-labeled polymer and substrate-chromogen were used to visualize the staining of the protein of interest.
The immunolabeling of cancer cells was evaluated. Cytoplasmic staining was regarded as positive signals. The semi-quantitative scoring of the expression intensity in each sample was calculated by multiplying the staining intensity (0 points=no staining, 1 point=mild staining, 2 points=moderate staining, and 3 points=strong staining) and the percentage of stained cells (0 points=<5%), 1 point=6–25%), 2 points=(26–50%), 3 points=51–75%, and 4 points=>75%).
Statistical analysis
SPSS 22.0 software (SPSS, Inc, IL, USA) was used for statistical analyses. The association of ROCK2 expression with clinicopathological characteristics was analyzed using Pearson chi-squared tests or Fisher exact test. The Kaplan-Meier method was used to analyze overall survivals. Cox proportional hazard regression models were used to perform univariate and multivariate analysis comparisons. When the P-value was <0.05, differences were considered statistically significant.
Results
ROCK2 expression was associated with clinicopathological features of breast cancer patients
We used tissue array to investigate whether the protein level of ROCK2 was correlated with the clinical features of breast invasive cancer specimens. The expression and localization of ROCK2 in 40 breast cancer specimens and paired non-cancerous breast specimens were analyzed by immunohistochemistry. Many cancer specimens were strongly stained in the cytoplasm (Figure 1A), whereas weak or negative staining was observed in paired adjacent normal tissues (Figure 1B). The average immunoreactivity scores were significantly higher in breast cancer tissues (n=40) when compared with non-cancerous breast tissues (n=40) (Figure 1C) (IRS: Cancer=5.25±2.10 vs. Normal=3.83±1.06, P<0.01, t test).
Figure 1.
Immunohistochemical staining for ROCK2 in breast invasive cancer tissue and normal breast tissues (original magnifications ×100 and ×400). (A) Strong ROCK2 staining was observed in the cytoplasm of tumor cells. Image at high magnification is shown in the right. The red arrow indicates positively-stained tumor cells. (B) Negative staining of ROCK2 expression was observed in the normal breast tissues. (C) Immunoreactivity score in cancer tissues was significantly higher than in normal tissues (Cancer=5.25±2.10 vs. Normal=3.83±1.06). ** Indicates P<0.01.
We analyzed immunohistochemical results in combination with the information of tumor node metastasis (TNM) classification from tissue array. Subgroup analyses were performed according to the ROCK2 staining scores. We categorized the samples into low- (IRS ≤4) and high- (IRS >4) expression subgroups. We found no relationship between ROCK2 expression and patient age or pathological grade (Table 1). However, a positive relationship between the occurrence of breast cancer and ROCK2 expression was found. Of 40 breast cancer tissues, 20 (50%) were defined as high-expression, while only 4 out of 40 (10%) were considered as high-expression in the non-cancerous specimens group (P<0.001, Table 1). Similar associations were also observed between ROCK2 expression and several tumor characteristics (Table 1), including clinical stage (I–II vs. III–IV, P<0.001), tumor stage (T1–T3 vs. T4, P=0.047), and lymph node metastasis (N0–N1 vs. N2, P=0.020). Furthermore, using ROCK2 mRNA data extracted from the TCGA 999 primary breast cancer dataset, we found that ROCK2 transcription level was significantly correlated with tumor staging (T1–T3 vs. T4, P=0.030).
Table 1.
Association of ROCK2 expression with clinicopathologic characteristics in patients with breast invasive cancer.
| Clinical feature | IRS of ROCK2 in our cohort | ROCK2 expression in TCGA datebase | |||||
|---|---|---|---|---|---|---|---|
| Case | Low (n %) | High (n %) | P-value | Case | x±s | P-value | |
| Tissue | |||||||
| Cancer | 40 | 20 (50.0) | 20 (50.0) | <0.001 | 999 | 934.27±568.90 | – |
| Adjacent normal | 40 | 36 (90.0) | 4 (10.0) | – | – | ||
| Age (years) | |||||||
| <50 | 30 | 15 (50.0) | 15 (50.0) | 1.000 | 268 | 987.76±555.61 | 0.072 |
| ≥50 | 10 | 5 (50.0) | 5 (50.0) | 731 | 914.60±572.84 | ||
| Pathological grade | |||||||
| ≤2 | 35 | 17 (48.6) | 18 (51.4) | 1.000 | – | – | – |
| >2 | 2 | 1 (50.0) | 1 (50.0) | – | – | ||
| Clinical stage | |||||||
| I–II | 28 | 20 (71.4) | 8 (28.6) | <0.001 | 742 | 930.09±583.73 | 0.931 |
| III–IV | 12 | 0 (0.0) | 12 (100.0) | 238 | 926.40±505.17 | ||
| Tumor stage | |||||||
| T1–T3 | 35 | 20 (57.1) | 15 (42.9) | 0.047 | 960 | 929.83±572.44 | 0.030 |
| T4 | 5 | 0 (0.0) | 5 (100.0) | 36 | 1111.45±425.10 | ||
| Lymph node metastasis stage | |||||||
| N0–N1 | 34 | 20 (58.8) | 14 (41.2) | 0.020 | 804 | 926.89±571.04 | 0.619 |
| N2 | 6 | 0 (0.0) | 6 (100.0) | 178 | 950.44±546.25 | ||
| pM stage | |||||||
| M0 | 40 | 20 (50.0) | 20 (50.0) | – | 859 | 966.35±572.05 | 0.621 |
| M1 | – | – | – | 15 | 890.28±420.17 | ||
“−“ – means a lack of relative information of patients in our cohort.
Prognostic implications of ROCK2 expression in breast cancer
Kaplan-Meier survival analyses together with univariate and multivariate analysis were performed using the TCGA dataset (n=999) to assess the prognostic implications of ROCK2. Patients were divided into a ROCK2 high-expression group and a low-expression group using median expression of ROCK2 as the cut-off value.
Kaplan-Meier method was used to analyze the relationship between overall survivals and ROCK2 expression in breast cancer patients. Patients with higher ROCK2 expression levels in tumors showed shorter overall survival as compared to those with lower expression (P=0.037, Figure 2).
Figure 2.
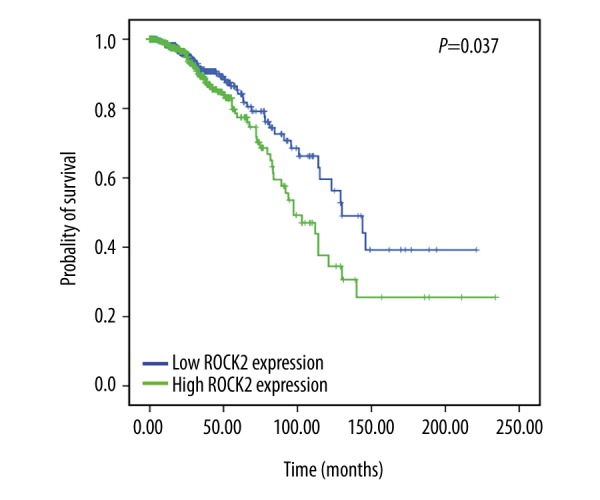
Kaplan-Meier survival curves of overall survival for ROCK2 expression in breast invasive cancer.
Univariate analysis showed that clinical stage (P<0.001) and lymph node metastasis stage (P=0.005) were potential clinical factors affecting overall survival rates in patients (Table 2). Furthermore, patients were assigned into low- or high-ROCK2 expression group based on ROCK2 mRNA expression level. Receiver operating characteristic (ROC) curve analysis was performed. The optimal cut-off value was set as 876.62. Notably, the overall survival rate was significantly different between the high- and low-ROCK2 expression groups. Patients with high ROCK2 expression in tumor specimen appeared to have a worse prognosis (HR 1.564; 95%; CI; 1.079–2.269; P<0.018, Table 2). Further multivariate analysis suggested that ROCK2 expression level (HR, 1.706; 95%CI: 1.144–2.544; P=0.009) and clinical stage (HR, 1.923;95%CI, 1.102–3.356; P=0.001) were factors correlated with overall survival (Table 2), indicating that ROCK2 and clinical stage might be prognostic biomarkers for breast cancer.
Table 2.
Prognostic value of ROCK2 expression for the overall survival by Cox proportional hazards model.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (<50 vs. ≥50) | 0.100 | |||
| Clinical stage (I–II vs. III–IV) | 2.038 (1.374–3.023) | <0.001 | 1.923 (1.102–3.356) | 0.021 |
| pT stage (T1–T3 vs. T4) | 1.877 (0.994–3.547) | 0.052 | ||
| pN stage (N0–N1 vs. N2) | 1.941 (1.228–3.068) | 0.005 | 0.810 | |
| ROCK2 expression (low vs. high)* | 1.564 (1.079–2.269) | 0.018 | 1.706 (1.144–2.544) | 0.009 |
Low and high ROCK2 expression was determined based on the ROC curve analysis using the mRNA expression level of TCGA breast cancer dataset.
The cut-off value was set as 876.62.
Discussion
It has been shown that ROCK2 is associated with cancer development in several reports. Functional studies showed that ROCK2 promoted invasion and metastasis of colon, liver, and lung cancer cells [12–14]. ROCK2 mediated the invasion of colon cancer through the regulation of MMP-13 and MMP-2 [10]. The activation of ROCK-2/moesin cascade mediated VEGF-C to promote endometrial cancer cell invasion [15]. Li et al. reported that ROCK2 functions as a key factor in the regulation of breast cancer cell invasion and motility [16]. In contrast, ROCK 2 knockdown decreased the invasion and motility capacity of breast cancer cells [17]. Even though the molecular function of ROCK2 has been intensively studied, the clinical relevance of ROCK2 expression is unclear.
The present study explored the difference in ROCK2 level between breast cancer tissues and adjacent non-cancerous breast tissues. Analysis of the TCGA database and tissue array showed that ROCK2 expression level in cancer tissues was significantly higher than in paired adjacent normal tissues. Moreover, both ROCK2 mRNA and protein level were associated with pT stage of breast cancer. Patients with advanced tumor stage have higher ROCK2 level. Our study demonstrated that only 28.6% of stage I and II cancer patients had high ROCK2 expression in breast cancer tissues. In contrast, the ratio of high ROCK2 level in stage III and IV phases was 100% in our cohort. Furthermore, high ROCK2 immunoreactivity was more common in tumor samples from pT4- (vs. pT1–pT3) and pN2- (vs. pN0–pN1) stage patients. Our study shows that ROCK2 might act as an oncogene in the development of breast cancer, which is consistent with the findings that ROCK2 may favor cancer cell proliferation or metastasis.
Breast cancer is a highly heterogeneous disease [18]. Individuals with breast cancer may have different responses to treatment. At present, due to the development of surgical techniques, adjuvant chemotherapy, radiotherapy, and targeted therapy, the survival rate of breast cancer patients has greatly improved. Although the incidence of metastasis and recurrence is still negligible after standard treatment, the current prognostic biomarkers are not satisfactory. Therefore, efforts to identify biomarkers for breast cancer patient prognosis and clinical therapy recommendation are still ongoing. The present study suggests that ROCK2 is such a factor. Using Cox proportional hazards model and Kaplan-Meier plots, we found that ROCK2 expression levels were significantly associated with overall survival. Patients with higher ROCK2 expression in tumor tissues had shorter overall survival. More importantly, ROCK2 expression and clinical stage appeared to be independent risk factors for overall survival as indicated by multivariate analysis. Our study suggests that ROCK2 and clinical stage can be used as biomarkers for prognosis of breast cancer patients. These preliminary findings encourage us to pursue further studies with larger sample sizes.
Conclusions
We showed that expression level of ROCK2 was higher in breast cancer tissue compared with paired adjacent breast specimens. ROCK2 level in the breast cancer tissue was associated with overall survival. In the univariate and multivariate Cox regression analysis, high ROCK2 expression and clinical stage predicted poor prognosis after treatment.
Footnotes
Conflicts of interest
None.
Source of support: This work was supported by the National Natural Science Foundation of China [grant number 81403202]; the Natural Science Foundation of Guangdong Province, China [grant number 2014A030313416]; and the Province Youth Elite Project of GUCM QNYC [grant number 20140103]
References
- 1.Bomalaski JJ, Tabano M, Hooper L, Fiorica J. Mammography. Curr Opin Obstet Gynecol. 2001;13(1):15–23. doi: 10.1097/00001703-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Donepudi MS, Kondapalli K, Amos SJ, Venkanteshan P. Breast cancer statistics and markers. J Cancer Res Ther. 2014;10(3):506–11. doi: 10.4103/0973-1482.137927. [DOI] [PubMed] [Google Scholar]
- 3.Millot P, Casadebaig L. Ultra wide X-band microwave imaging of concealed weapons and explosives using 3D-SAR technique. International Journal of Antennas and Propagation. 2015;2015:528103. [Google Scholar]
- 4.Metzger-Filho O, Sun Z, Viale G, et al. Patterns of recurrence and outcome according to breast cancer subtypes in lymph node–negative disease: Results from International Breast Cancer Study Group Trials VIII and IX. J Clin Oncol. 2013;31(25):3083–90. doi: 10.1200/JCO.2012.46.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kümper S, Mardakheh FK, McCarthy A, et al. Rho-associated kinase (ROCK) function is essential for cell cycle progression, senescence and tumorigenesis. Elife. 2016;5:e12203. doi: 10.7554/eLife.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka T, Nishimura D, Wu R-C, et al. Nuclear Rho kinase, ROCK2, targets p300 acetyltransferase. J Biol Chem. 2006;281(22):15320–29. doi: 10.1074/jbc.M510954200. [DOI] [PubMed] [Google Scholar]
- 7.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton. 2010;67(9):545–54. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vigil D, Kim TY, Plachco A, et al. ROCK1 and ROCK2 are required for non-small cell lung cancer anchorage-independent growth and invasion. Cancer Res. 2012;72(20):5338–47. doi: 10.1158/0008-5472.CAN-11-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li M, Ke J, Wang Q, et al. Upregulation of ROCK2 in gastric cancer cell promotes tumor cell proliferation, metastasis and invasion. Clin Exp Med. 2017;17(4):519–29. doi: 10.1007/s10238-016-0444-z. [DOI] [PubMed] [Google Scholar]
- 10.Vishnubhotla R, Sun S, Huq J, et al. ROCK-II mediates colon cancer invasion via regulation of MMP-2 and MMP-13 at the site of invadopodia as revealed by multiphoton imaging. Lab Invest. 2007;87(11):1149–58. doi: 10.1038/labinvest.3700674. [DOI] [PubMed] [Google Scholar]
- 11.Kalender ME, Demiryürek S, Oztuzcu S, et al. Association between the Thr431Asn polymorphism of the ROCK2 gene and risk of developing metastases of breast cancer. Oncol Res. 2009;18(11–12):583–91. doi: 10.3727/096504010x12767359113767. [DOI] [PubMed] [Google Scholar]
- 12.Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int J Cancer. 1999;81(5):682–87. doi: 10.1002/(sici)1097-0215(19990531)81:5<682::aid-ijc2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 13.Qiu Y, Yuan R, Zhang S, et al. Rock2 stabilizes β-catenin to promote tumor invasion and metastasis in colorectal cancer. Biochem Biophys Res Commun. 2015;467(4):629–37. doi: 10.1016/j.bbrc.2015.10.103. [DOI] [PubMed] [Google Scholar]
- 14.Huang D, Du X, Yuan R, et al. Rock2 promotes the invasion and metastasis of hepatocellular carcinoma by modifying MMP2 ubiquitination and degradation. Biochem Biophys Res Commun. 2014;453(1):49–56. doi: 10.1016/j.bbrc.2014.09.061. [DOI] [PubMed] [Google Scholar]
- 15.Cheng Y, Zheng-Tian GU. [VEGF-C promotes endometrial cancer cell invasion via activation of ROCK-2/Moesin cascade]. Journal of Hubei University for Nationalities. 2014 [in Chinese] [Google Scholar]
- 16.Li M, Zhou W, Yuan R, et al. ROCK2 promotes HCC proliferation by CEBPD inhibition through phospho-GSK3β/β-catenin signaling. FEBS Lett. 2015;589(9):1018–25. doi: 10.1016/j.febslet.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Lane J, Martin TA, Watkins G, et al. The expression and prognostic value of ROCK I and ROCK II and their role in human breast cancer. Int J Oncol. 2008;33(3):585–93. [PubMed] [Google Scholar]
- 18.Hsiao Y-H, Chou M-C, Fowler C, et al. Breast cancer heterogeneity: Mechanisms, proofs, and implications. J Cancer. 2010;1:6–13. doi: 10.7150/jca.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]



