Abstract
Optical studies of ex vivo brain slices where blood is absent show that neural activity is accompanied by significant intrinsic optical signals (IOS) related to activity-dependent scattering changes in neural tissue. However, the neural scattering signals have been largely ignored in vivo in widely-used IOS methods where absorption contrast from hemoglobin was employed. Changes in scattering were observed on a time scale of seconds in previous brain slice IOS studies, similar to the time scale for the hemodynamic response. Therefore, potential crosstalk between the scattering and absorption changes may not be ignored if they have comparable contributions to IOS. In vivo, the IOS changes linked to neural scattering have been elusive. To isolate neural scattering signals in vivo, we employed 2 implantable optodes for small-separation (2mm) transmission measurements of local brain tissue in anesthetized rats. This unique geometry enables us to separate neuronal activity-related changes in neural tissue scattering from changes in blood absorption based upon the direction of the signal change. The changes in IOS scattering and absorption in response to up-states of spontaneous neuronal activity in cortical or subcortical structures have strong correlation to local field potentials, but significantly different response latencies. We conclude that activity-dependent neural tissue scattering in vivo may be an additional source of contrast for functional brain studies that provides complementary information to other optical or MR-based systems that are sensitive to hemodynamic contrast.
Keywords: Intrinsic optical signal, neural scattering activity, neurovascular coupling, optical transmission measurement, in vivo
Introduction
Intrinsic optical signals (IOS) carry rich information about neuronal activity, which manifests from two different processes that dictate light propagation through biological tissue: absorption and scattering. One is the well-established light absorption signal that reflects changes in blood oxygenation linked to neural activity via neurovascular coupling, and the other is the light scattering signal from alteration of the optical properties of neural tissue as a secondary consequence of neural excitation. The former oxygenation contrast has been widely utilized in vivo and arises from the same mechanism as the blood oxygenation level dependent (BOLD) signal that forms the basis of fMRI and functional connectivity studies (Howseman and Bowtell, 1999; Pan et al., 2015). The latter scattering contrast has been less investigated in in vivo studies and the neuroimaging community, due to the dominance of the absorption contrast in most commonly used experimental setups (Hillman, 2007; Scholkmann et al., 2014). Notably, from in vitro brain slice IOS studies where there is no interference from hemoglobin, we know that scattering reduction due to neural activity can be readily observed (Aitken et al., 1999; Lipton, 1973; Witte et al., 2001), appearing to arise from ionic redistribution and osmotically induced cellular volume increase (Andrew and MacVicar, 1994; MacVicar and Hochman, 1991; Pal et al., 2013). This intrinsic signal from activity-dependent scattering could be valuable for neuroimaging research, as it eliminates the dependence on consistent neurovascular coupling required by hemodynamic-based methods and is therefore less influenced by non-neuronal perturbations in the vasculature and by cardiac pulsation and respiration.
The neural tissue IOS scattering is also the likely source of changes observed with diffusion-weighted fMRI (dw- fMRI). Researchers introduced dw-fMRI in an attempt to circumvent limitations of fMRI that arise from its dependence on neurovascular coupling, as dw-fMRI is, in theory, sensitive to activity-dependent physiological events such as transient extracellular space shrinkage (Holthoff and Witte, 1996) and cell swelling (Holthoff and Witte, 1996; MacVicar and Hochman, 1991; Witte et al., 2001) that result in a reduction in the apparent diffusion coefficient of water (Darquie et al., 2001; Le Bihan et al., 2006; Tsurugizawa et al., 2013). While dw-fMRI is postulated to reflect changes in tissue swelling processes, the physiological underpinnings are not completely understood and remain somewhat controversial (Bai et al., 2016; Jin and Kim, 2008; Miller et al., 2007). Researchers have referred to the in vitro optical studies to support the hypothesized origins of the signal changes detected with dw-fMRI. However, the brain slice preparation is far simpler than the intact brain, where hemoglobin absorption is strong, and the relatively low sampling rate of dw-fMRI and the challenges involved in acquiring multimodal data in the scanner have made it difficult to directly resolve these issues. Ideally, an optical method that allows the in vivo study of both activity-induced scattering changes and absorption changes from neurovascular coupling would allow concurrent assessment of neuronal tissue swelling dynamics and the hemodynamic response to neuronal activity, effectively bridging the gap between tissue scattering signal studies in in vitro IOS, dw-fMRI, and the BOLD studies in widely-used functional brain imaging.
Current in vivo IOS imaging relies on sensitive measurement of hemoglobin-based signals that arise via neurovascular coupling (Hillman, 2007). The propagation of light in tissue is influenced by both the absorption at a given wavelength and the amount of scattering the tissue creates (Malonek and Grinvald, 1996; Strangman et al., 2002). The absorption changes related to the hemodynamic response to neural activity occur on a time scale of seconds. Despite the distinct origins of the activity-related changes in scattering and absorption, scattering signals may also occur on a time scale of seconds in response to a stimulation, as demonstrated by brain-slice IOS studies (Holthoff and Witte, 1996; Pal et al., 2013; Takagi et al., 2002). The slow scattering activity has not been reported in vivo although faster scattering activity has been observed (Rector et al., 2005). It would be certainly challenging to clearly separate the slow scattering and hemodynamic signals by using the frequency information alone, e.g. temporal filtering. Moreover, in local reflected light studies (e.g. short source-detector separation), swelling reduces reflectance, i.e. changes the intensity in the same direction as the absorption effects, because the decrease in scattering and reduced opacity that facilitates light transmission also reduces reflection output (Aitken et al., 1999). In contrast, in the in vitro optical studies, measurements were made in brain slices using mostly transmittance, which shows an increase in transmitted light intensity from reduced scattering due to the refractive index change in the tissue from the swelling process during neuronal activation (Witte et al., 2001). The transmission measurement is relatively straightforward and far less complicated than a reflection mode measurement. Notably, in transmittance in vivo, the scattering reduction in neural activation leads to increased light detection as indicated by previous brain slice studies; the absorption from augmented hemoglobin with intact blood circulation leads to decreased light detection. Therefore changes in decreased scattering and increased absorption in the intact brain during neural activity have opposite effects on the detected light signals when transmission measurement is used. We thus hypothesized that the effects of tissue scattering could be separated from absorption in vivo based on transmission measurements in a localized brain area of small tissue volume using multiple wavelengths with varying scattering/absorption ratios. The IOS from a relatively small volume of tissue may have a lower contribution from absorption but greater scattering contribution, thus facilitating the detection of the scattering signal (Mourant et al., 1997).
We thus propose a method to separate absorption from scattering with minimal crosstalk during spontaneous neural activity. We utilize implanted optodes spaced 2 mm apart to measure in vivo optical transmittance at 3 wavelengths spanning the visible to near-infrared within a localized brain area in the rodent. This miniature, minimally-invasive probe can be used for monitoring both cortical and deep brain structures. The extracted changes in tissue scattering and absorption reflect an average of bulk activity between the optodes, which is comparable to an fMRI voxel (~2mm slice thickness). Herein we demonstrate the capabilities of this setup in the rat cortex (primary somatosensory area, S1) and deep brain in the caudate putamen (CP).
Materials and Methods
Materials and simultaneous IOS/LFP recording
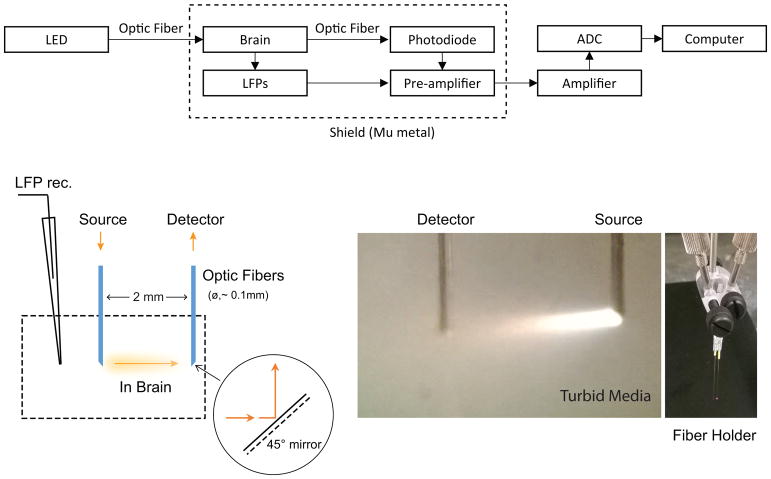
All animal experiments were performed in compliance with NIH guidelines and were approved by the Emory University Institutional Animal Care and Use Committee. Spontaneous local field potentials and IOS signals in S1 and/or CP were simultaneously recorded in Sprague-Dawley rats (male, 300–400g, S1: n=7, CP: n=6) under 1.8–2% isoflurane in oxygen. The animals were kept in normal physiological condition with ~37°C rectal temperature and ~98% SpO2 for the duration of the experiment. A micro-glass pipette (Ag/AgCl) electrode was used for local field potential (LFP) recording, implanted in the primary somatosensory area (S1, frontal limb area: 1-mm frontal to and 4-mm lateral to bregma, 1-mm depth under the dura) or deep brain (caudate putamen, CP, 4-mm in depth). Some details of the technique were described in our previous reports (Pan et al., 2010; Pan et al., 2011; Pan et al., 2013). Similarly, for IOS recording, a pair of mirrored optodes was aligned in transmission geometry with a separation of 2-mm in an adjustable holder (Figure 1) and positioned at the site of LFP recording in S1 or CP using a stereotactic micromanipulator (Narishige, SM-25A). The microelectrode was positioned ~1 mm beside of the optodes (illustrated in Figure 1). The animals were under 2% isoflurane for ~2 hours for surgery and implantation. After implantation of the micro optodes and electrodes, we waited >30 minutes for the brain signal to stabilize before a recording to minimize any effects from the invasive procedure. Recordings were performed in 7 minute intervals; multiple recording sessions were made per animal to enable multi-wavelength IOS data acquisition (see below). During recording, the area was shielded by a Faraday cage of Mu metal enclosure to avoid interference from outside light and broad band electromagnetic noise.
Fig. 1.
Simultaneous electrophysiology and IOS recordings with transmission measurement. The schematic illustration of simultaneous LFP and IOS recordings is shown in the top panel. The LED light is coupled into the optical fiber to targeted brain sites and transmitted through the sampled tissue. The detection fiber of the transmission probe collects the light to a photodiode. Both photodiode and LFP recording signals from the same site were ×10 pre-amplified respectively, then amplified and digitized. The animal and recording system were enclosed in a full-band electromagnetic shield during data collection. (Left bottom)Schematic representation of transmission measurement for the target area’s tissue-level neural activity using the minimally-invasive fiber pair. The mirror-coating of the 45° tips that allows light to turn 90° between source emission and detector collection with the parallel fiber-pair configuration is illustrated. (Middle bottom) Lighting (full-band wavelengths) path is demonstrated, captured in the turbid liquid of a tissue-simulating phantom. The fiber pair was assembled on a holder, shown in the right bottom panel, which was calibrated to ensure 2-mm separation and equal length.
Minimally-invasive IOS transmission measurements
A specialized IOS probe was developed to measure transmittance in vivo, illustrated in Figure 1. The probe consists of a source and detector fiber (105 μm core, Thorlabs FG105LCA), each constructed with a 45 degree slope tip surface that was coated with a full-band reflective mirror in an IEN clean room (Georgia Tech, 100-nm aluminum reflection layer and protection layers of 300-nm gold and 2-μm parylene C). Three collimated LED light sources were used (525nm, 660nm and 810nm, Thorlabs, LED528EHP; Marktech, MTE5066N5J-UR; Marktech, MTE2081-OH5, respectively; spectral response of each LED was examined, shown in supplementary Figure S1). Due to limitations in the acquisition hardware, simultaneous data acquisition from all three wavelengths was not possible. Thus, the source fiber delivered a single wavelength of light to the tissue for each 7 minute recording session; 3 recording sessions were performed per animal to obtain data from all wavelengths. The detector fiber was coupled to a photodiode (Thorlabs FDS02) in photovoltaic mode for high SNR detection, (Pan et al., 2018). For each recording session, an average baseline (i.e. offset voltage) for detected light intensity, I0(λ), was first obtained. The detected offset signal was amplified with a DC amplifier at ×50 gain (A-M System - model 3000). Then, continuous IOS data was collected concurrently with LFP recording. Given the small changes seen during LFP, the detected IOS signal during continuous recording, I(λ,t), was acquired at ×5k-10k gain. Concurrently LFP recording was performed in parallel channels on the DC amplifier, and then the IOS and LFP signals were digitized at 1.2 kHz or 2.4 kHz with an analog-to-digital device (PCI-6281, National Instruments). The simultaneous IOS and LFP recording is illustrated in Figure 1 top panel.
Data processing
Data were preprocessed before the response to neural activity was calculated. The physiological pulses of respiration and cardiac rates were identified in the IOS and removed with notch-filters. The envelop of 1–100 Hz LFP amplitudes were extracted using the Hilbert transform, as previous studies have shown that the hemodynamic response is coupled to broadband electrical activity during isoflurane anesthesia (Pan et al., 2011). Both IOS and LFP data were detrended (4th order) and down-sampled to 6.25 Hz. Optical density was calculated for each recording session as ΔOD(λ,t) = −log(I(λ,t)/I0(λ)). We estimated a nonparametric response function for ΔOD(λ,t) to LFP amplitude (Hilbert) across each individual 7-min recording session using a least-squares estimation procedure based on the first order differencing method (Seghouane and Shah, 2013). For this estimation, ΔOD(λ,t), denoted as y[n] was modeled as the convolution of LFP envelop x[n] with a non-causal impulse response plus zero mean noise i.e. . The time span of response function h′ was set to −5 to 25 sec (i.e. M1 = 5, M2 = 25), . Least squares approach for this model uses a closed form matrix formulation, h′ = (AHA)−1 AHy, where the optical signal y[n] was modeled as the convolution of the LFP envelop x[n].
For each animal, the overall ΔOD(λ,t) response functions for all three wavelengths (525nm, 660nm and 810nm) were approximated as a truncated Taylor series expansion, i.e.,
| (1) |
where Δμa(λ,t) and Δμs′(λ,t) are the changes in the absorption and reduced scattering coefficients during neuronal activation, and and are the wavelength-dependent absorption and scattering differential pathlength factors. We estimate da(λ) and ds(λ) as described in the Differential Pathlength Estimation Section below. Δμa(λ,t) in units of cm−1, can be expressed in terms of the concentrations of the main chromophores in the brain in the visible/NIR spectral region (oxy- and deoxygenated hemoglobin, HbO2 and Hb, respectively), i.e., Δμa(λ,t) = εHbO,2(λ)Δ[HbO2] (t) + εHb(λ)Δ[Hb] (t), where εHbO,2(λ) and εHbO,2(λ)are the molar extinction coefficients of HbO2 and Hb, and Δ[HbO2] (t) and Δ[Hb] (t) are the change in the molar concentration of HbO2 and Hb during neuronal activation. Further, we assume that the wavelength-dependent reduced scattering coefficient follows a power law such that Δμs′(λ,t) = Δ[a](t)(λ/500 (nm))−b. Here we are using a 500nm wavelength reference, we assume a scattering power (b) of 1.611 for brain tissue (Jacques, 2013), and we use Δ[a](t) to denote the change in the scattering amplitude during neuronal activation. Thus, Eq. 1 becomes:
| (2) |
Equation 2 was used to convert the wavelength dependent response functions to Δ[HbO2] (t), Δ[Hb] (t), and Δ[a](t). Change in total hemoglobin concentration, which is proportional to the change in blood volume, was also calculated as Δ[tHb] (t) = Δ[HbO2] (t) + Δ[Hb] (t).
Differential pathlength factor estimation
da(λ) and ds(λ) were computed using the embedded Monte Carlo simulation function in a commercial ray tracing software ZEMAX. One-hundred and seventy-six simulations were run across a wide range of physiologically-relevant optical properties (10 <μs′< 25 cm−1 and 0 <μa < 10 cm−1). For each simulation, the medium was approximated as semi-infinite and homogenous. Two virtual fibers (105 μm core, 0.22 numerical aperture, 45° slope mirror tip) were placed 2 mm apart in parallel with mirrors facing each other. The tips of the fibers were embedded 2 mm from the glass-tissue interface. Five hundred million photons were launched per each simulation, and the detected optical density, OD (μa,μs′) was defined as −log ([detected intensity]/ [input intensity]). Finally, we computed maps of ∂OD(μa,μs′)/∂μa and ∂OD(μa, μs′)/∂μs′ as a function of both μa and μs′. Using these maps, da(λ) and ds(λ) were determined using the wavelength-dependent tissue optical properties that corresponded to the following literature-derived values for a healthy rat brain: [HbO2]= 52.4uM, [Hb] = 34.9uM, (Abookasis et al., 2009), brain tissue water fraction of 0.8, scattering amplitude a = 24.2 cm−1, and scattering power b = 1.611 (Jacques, 2013).
RESULTS
Wavelength-dependence of transmittance in response to LFP activity
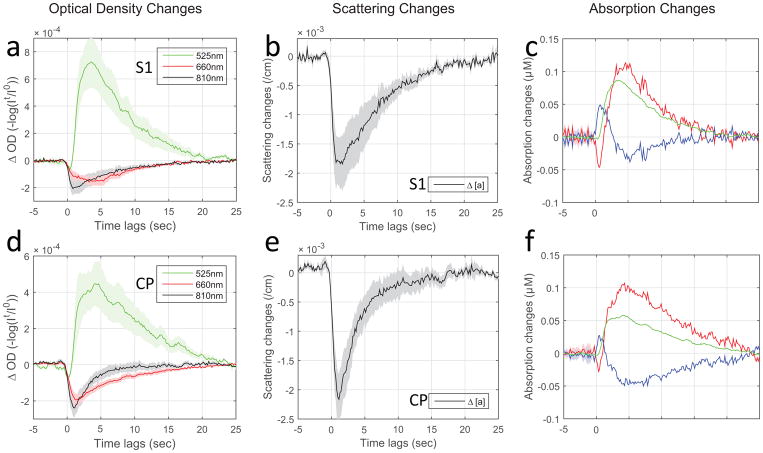
It is evident from Figure 2 (a, d from S1 and CP respectively; individual data plotted in supplementary Figure S2) that the light transmittance in response to neuronal bursting activity, i.e. ΔOD, is wavelength dependent. The transmittance at 525nm is consistently attenuated (reflected as an increase in OD) in response to LFP for both cortex and deep brain recording. In contrast, the transmittance at 810nm (and 660nm) showed a monophasic change in response to LFP activity in the opposite direction of 525nm.
Fig. 2.
Optical intrinsic signals of scattering vs. absorption in response to neuronal activation. The optical responses to local field potentials for different wavelengths, 810nm/525nm/660nm, are estimated respectively by least-squares fitting of simultaneous LFP/IOS recording data sets. The group results expressed as tissue optical density are plotted in first column (a and d, mean+/−SEM: S1, n=7 rats; CP, n=6 rats). Using the modified Beer-Lambert law and considering both tissue absorption and scattering changes that occur in response to neural activity, the concentration changes in HbO2, Hb (tHb=HbO2+Hb) and scattering per centimeter were calculated and plotted in mean+/−SEM (scattering: b and e; absorption: c and f from S1 and CP respectively). The results from the S1 group are shown in the first row and CP in the second row.
The wavelength-dependence of transmittance may provide the possibility of direct separation of the scattering and absorption components based on the direction of the IOS changes. For instance, we employed two wavelengths that have greatly different absorption coefficients for total hemoglobin, green light (525nm, μa = 6.55 cm−1, μs′ = 22.37 cm−1) and near-infrared light (810nm, μa = 0.18 cm−1, μs′ = 11.06 cm−1). We utilized the relative differences in weighting for scattering vs. absorption at the two wavelengths to determine if the dominant contributor could be identified by the direction of the signal change in the in vivo transmission mode. In our results, the optical signals at 525nm where absorption is stronger are consistently attenuated in output intensity for both cortex and deep brain recording, indicating that hemoglobin absorption is the dominant source of contrast (Figure 2a and d, plotted in green traces). In contrast, the 810nm signals showed a monophasic change in the opposite direction in response to LFP activity (Figure 2a and d, plotted in black), i.e. the opposite response to what would be expected from hemoglobin absorption.
Further separation of the scattering and absorption signals were evaluated in Modified Beer-Lambert Law as detailed in data processing. The scattering signals (Δ[a]) and absorption signals (Δ[HbO2], Δ[Hb] and Δ[tHb]) are shown in Figure 2b, c and e, f for comparison across cortical and subcortical regions, S1 and CP. The results provide the necessary correction for crosstalk between the IOS components (i.e. neural tissue scattering and effects as well as hemoglobin absorption) and confirm that scattering and absorption signals in response to neural activity are opposite in direction in transmittance
Short latency in neuro-scattering response relative to the vascular response
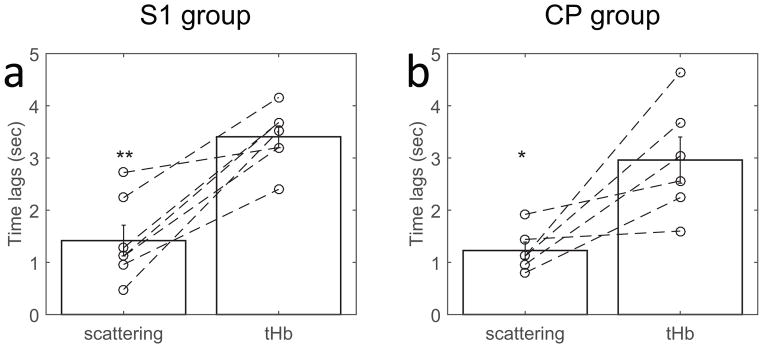
The derived changes in scattering (Δ[a])) and total hemoglobin absorption (Δ[tHb]) exhibited different peak times in response to LFP activation, Figure 2b and e, c and f for both cortical and subcortical regions, S1 and CP. The hemodynamic response of cerebral blood volume (CBV, indicated by tHb) exhibits about 4 sec lag time to peak (Figure 3, S1 and CP respectively). The scattering responses have significantly shorter latency, peaking about 1–2 sec on average sooner than the tHb signals for both cortical and subcortical structures.
Fig. 3.
Short latency of neuro-scattering responses relative to neurovascular coupling and their wavelength representatives. The peak times of scattering (neural tissue) and tHb (vascular volume) are plotted individually along with the group mean+/−SEM (a: S1, 1.41+/−0.30 vs 3.41+/−0.21 and b: CP, 1.22+/−0.16 vs 2.96+/−0.44). Paired t-tests between scattering and tHb for both groups, found a significantly faster response in scattering activity than vascular activity (*: p<0.05, **: p<0.001, two-tailed).
Strong correlation between neural scattering signal and LFPs
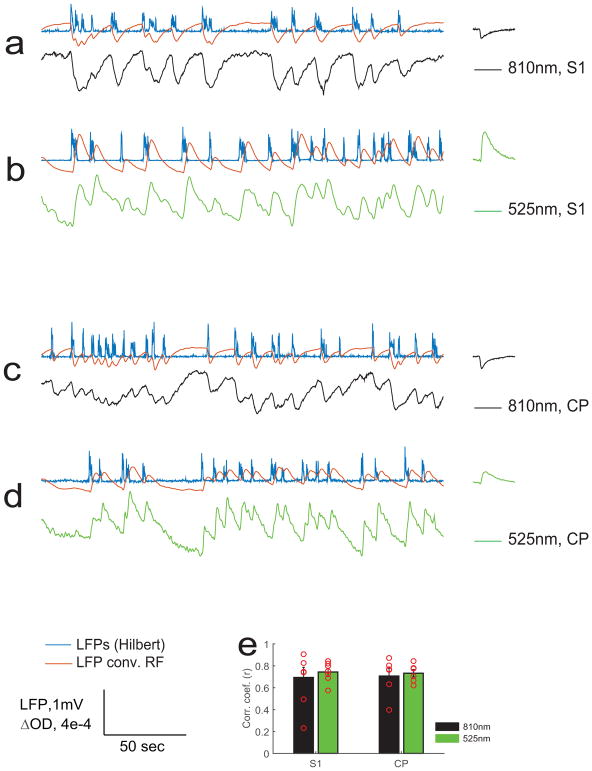
For the absorption-dominant light signals (525nm) and scattering-dominant light signals (810nm), we also conducted a correlation analysis with local field potentials. The LFPs were convolved with each group-mean wavelength’s response functions (RF) to predict light responses. The results show high correlations with neural activity for both 525nm and 810nm in both S1 and CP (Figure 4).
Fig. 4.
Strong correlation between local field potentials and neuro-scattering as well as neurovascular coupling. The time courses of neural scattering activity are represented by 810nm of NIR light, shown in black in the plotted time courses; the neurovascular activity are represented by 525nm of green light, shown in green in the plotted time courses. The simultaneous recorded LFPs were convolved with the response function (group mean, shown at the end of each row) to predict optical responses (red time courses). Samples are shown in a and b from S1, c and d from CP. The experimental measurements, whether made with 810nm or 525nm, exhibit high correlation coefficients with the predicted time courses respectively. The group results of correlation coefficients are shown in e, S1: 0.69+/−0.09 and 0.74+/−0.03; CP:0.71+/−0.07 and 0.74+/−0.03 for 810nm (scattering) and 525nm (tHb) respectively.
DISCUSSION
Our results show that neuronal LFP activity introduces two independent effects in the brain that can be detected by IOS in vivo: the hemodynamic response and the neural tissue scattering response. In localized transmission measurements in the rat brain, the detected signal is affected by both light absorption and scattering related transparency of the tissue that it travels through. The transmittance is reduced by increased absorption from the increased hemoglobin concentration in response to neural activity, but transmittance is enhanced by the reduction in overall tissue scattering related to neural activation. We demonstrated that the responses of the two IOS components are opposite in nature, and either can dominate the detected transmittance in our experiments, depending on the wavelength used. This study provides a unique demonstration of transmitted signals sensitive to IOS scattering activity that can be detected in the presence of intact hemodynamics in vivo. The observed in vivo scattering signal time courses are analogous to the observations in vitro obtained with transmitted light signals in blood-free brain slice studies of previous reports (Pal et al., 2013).
Origin of IOS scattering signals
Despite the potentially confounding presence of the hemodynamic response in the intact brain, activity-dependent changes in scattering were readily detectable with the miniaturized transmission probe. The increased transmittance cannot arise from increased hemoglobin absorption, which would cause a reduction in light output to be detected. As potential confounds, the scattering signal could also be attributed to the increased cellularity of the blood or to blood flow, in theory (Villringer and Chance, 1997). Neural activity results in a local increase of blood volume in brain, and the increased number of blood cells will increase overall tissue scattering. However, this effect is opposite in nature to the scattering contribution from neural cellular swelling for a transmission-measurement signal. The scattering effects of increased blood cellularity would influence the transmitted signal in the same way as increased absorption from hemoglobin, i.e. elevated blood cell numbers reduce IOS signals by either increased hemoglobin absorption or by increased scattering from the blood cells. Similarly, for a given tissue volume, if an increase in CBV leads to an increase in the blood fraction in brain tissue, it will induce a greater scattering effect because the scattering coefficient of blood is larger than brain tissue (Jacques, 2013). Also, the blood volume fraction (0.01) in the brain is much smaller than the neural cell volume fraction (0.8) and the blood scattering effects may be negligible. Thus neither effect can account for the increased transmittance we observed in response to neural activity, leaving neural tissue scattering reduction as the most likely source of the signal. This is in agreement with the present simultaneous recordings of optical and LFP signals, which show that the scattering reduction changes closely follows neural activity.
The neural origin of the scattering signals can include fast responses associated with membrane potential changes along with the slow response of cell swelling (Villringer and Chance, 1997). The transmitted light increases observed in response to LFP activity in our study occurred on the scale of seconds, with little evidence of changes at the sub-second time scale for each spontaneous neuronal event. Therefore the second-scale responses are likely to be predominant in our observed scattering signals. The faster neural scattered components have been observed primarily in averaged, repeated responses to stimulation. For example, Rector et. al., employed single electric (0.1ms) shock (Rector et al., 2001), or a brief stimulation of a single whisker twitch (Rector et al., 2005) in interleaved random events. By averaging hundreds to thousands of event-related responses, the small fast scattered light changes were discerned from background noise. In the present study of spontaneous neural activity, each LFP bursting event lasts for a few seconds, which could blur faster changes. The spontaneous bursting makes it difficult to apply an effective averaging strategy as in an event-related stimulation study. However the slow scattering signal examined in this study appears to have higher amplitude than the fast scattering signal, since the second-scale light scattering responses in both S1 and CP can be observed following individual neural bursting events without averaging. An example of raw data and attempted coherence analysis of fast IOS are shown in supplementary Figure S3.
In-vivo IOS scattering vs. absorption signals
Our results show substantial differences in the signal properties of neural scattering and hemoglobin absorption. The peak in the scattering signal occurs at 1–2 seconds, which is faster than the 4-sec CBV or BOLD responses. The relatively faster response of light scattering signals coincides with previous reports in the cat visual cortex (Malonek and Grinvald, 1996) and the mouse glomerulus (Vincis et al., 2015). It is comparable to the peak time of the initial dip of BOLD but arises from a different process, i.e. neural scattering vs. neurovascular coupling. The “initial dip” is interpreted as a transient increase in Hb concentration prior to the decrease in Hb upon the arrival of fresh, oxygenated blood (Hu and Yacoub, 2012). In BOLD fMRI, the initial dip is seldom observed; instead, the subsequent increase in oxygenation provides contrast. The peak of the second phase of the HbO2 or Hb signal occurs at ~ 4–5 seconds, which agrees well with fMRI findings in similar isoflurane anesthetized rats (22, 23). The initial dip that we observed could be attributed to Hb absorption; however, during the same initial seconds, increases were also observed in transmitted light intensity, which suggests that neural scattering activity may contribute as well. The results demonstrate that the potential crosstalk between hemo-absorption and neuro-scattering may be significant particularly at the initial 1–2 seconds. The increased transmission that we observed cannot arise from these early changes in blood oxygenation, as the elevated deoxygenated hemoglobin during the initial dip causes more absorption and reduces transmitted light intensity. Also, the transmission increase in response to neuronal activity is monotonic, unlike the biphasic pattern of the Hb response. Therefore the initial dip and activity-dependent scattering are parallel processes that arise via different mechanisms.
IOS scattering signals associated with neural activity
In vivo IOS signals provide important information about the physiological processes that they originate from. The underlying sources of the scattered IOS have been investigated mostly by in vitro imaging of brain slices in combination with pharmacological manipulations. The results suggest a complex interplay of neuron-to-astroglia signaling (Pal et al., 2013), which involves water redistribution away from extracellular space (Holthoff and Witte, 1996) and tissue swelling in neurons (Cesetti et al., 2011), and possibly in astrocytes (Larsen and MacAulay, 2017; Witte et al., 2001) and neuronal axons (Vincis et al., 2015) as well. We observed that the scattered signals began immediately with spontaneous LFP activation, and reached their peak relatively rapidly compared to the vascular response. The ~1–2 second time to peak that we observed is consistent with reports from in vitro brain slice studies where no blood is present (Pal et al., 2013). Similarly, dw-fMRI signals also show a more rapid response to neuronal activity compared to the BOLD response, reaching their peak quickly and slowly returning to baseline (Aso et al., 2009). The similar short latencies suggest that the optical scattering signals and dw-fMRI are likely to originate in a sensitivity to the same cellular processes.
In theory, the scattering amplitude (Δ[a])) can be influenced by (1) number of scattering particles (Corlu et al., 2005), (2) the ratio of the refractive index outside (extra-cellular) to inside (intra-cellular) the scattering particle (Abookasis et al., 2009), and (3) particle distribution. In our modeling for a normal physiological neuronal activity, 1 and 3 are assumed not to change, so the variations in scattering amplitudes most likely reflect 2, i.e. a dynamic of refractive-index homogeneity across extra- and intra-cellular spaces following ionic/water exchange during neuronal activity processes.
Comparison of cortical and subcortical IOSs
Very little is known about IOS activity in deep brain areas since common IOS techniques are limited to the brain surface. The development of a fine-fiber probe allowed us to measure local IOS in the brain parenchyma in a minimally invasive means, with virtually any target site made accessible by varying the location and depth of the probe. Effects from any possible tissue damage were minimized since the sampled tissue in the 2 mm region between fibers is much wider than the fiber size, ~0.1mm diameter. In addition, the use of spontaneous neural activity for the calculation of the hemodynamic response eliminates the difficulty of identifying the most appropriate stimulus to activate the area of interest. We compared IOSs across brain regions between S1 and CP. The peak times were largely similar for both. The findings suggest that a similar mechanism generates the initial IOS scattering response in both regions. The initiation of cellular swelling during neuronal activity appears to be related to K+ and Cl−, but the recovery process may employ a separate process through VRAC (volume-regulated anion channel) (Pal et al., 2013). The glutamate uptake by astrocytes may also contribute to IOS scattering activity (Pal et al., 2013), but the timing would be slow. Yet, the neurovascular coupling in regions outside of the cortex is relatively little studied, and previous work has suggested that there may be critical regional differences to be explored (Sloan et al., 2010). Further work needs to be done to verify the potential difference in neural scattering signals across regions.
Implications for neuroimaging methods
The majority of current neuroimaging methods, including IOS imaging and fMRI, rely on neurovascular coupling as a proxy for neural activity. The widely-used measurements of IOS with CCD cameras or near infrared spectroscopy (NIRS) from source-detector pairs are sensitive to hemoglobin absorption and exclusively detect reflectance (Hillman, 2007; Jones et al., 2001; Ma et al., 2016). In surface-based reflection detection, the detected depth and photon migration pathway are complicated and the reflected light is sensitive to large vessel surface profiles that add non-neural variability. In comparison, the signals sampled directly in transmission mode by using the miniature fiber-pair probe are sensitive to the tissue of bulk activity between them, whether implanted in shallow or deep brain areas. Since neural swelling and recovery processes occur on a similar time scale to the hemodynamic response, as demonstrated here, potential interference between the two processes should be considered in local IOS studies. In addition, we compared the calculated scattering and tHb with optical density in each wavelength. Remarkably we found the 810nm signal and the calculated scattering to be largely similar, as were 525nm and tHb (see supplementary Figure S4). Therefore, it may be possible to employ a single representative wavelength as a reasonable approximation for neural scattering or neural vascular activity studies to simplify an experiment of local IOS detection in practice.
The neural optical scattering signal that we observed in response to neural activity may also have significant implications for fMRI. Typical fMRI studies rely on contrast based on hemoglobin concentration (BOLD). As an alternative method, diffusion-based fMRI (dw-fMRI) was introduced and developed primarily by Le Bihan et. al. (Darquie et al., 2001; Le Bihan, 2014), as a promising approach to measure neuronal tissue activity-dependent water signal directly to eliminate the dependence on neurovascular coupling. Dw-fMRI appears to be a consequence of the same physiological events that are measured in IOS scattering activity. Our findings of the IOS scattering time scale and short latency are in good agreement with results from dw-fMRI (Le Bihan, 2007).
Technical limitation in scattering signal modeling
In our studies, we assumed the scattering changes were primarily due to changes in tissue refractive homogeneity during neural ionic activity and followed water redistribution, i.e. Δa in Δμs′ = Δa(λ)−b. The b is scattering power, related to overall scatterer size. In the case of ischemia (Abookasis et al., 2009), both a and b changed. For a normal physiological condition, neuronal activity may be accompanied by significant ionic exchanges across the membrane and resulting water redistribution in neural tissue, therefore altering the tissue refractive homogeneity. Meanwhile the cell size (scatter size) is likely to increase, as it was suggested by previous brain-slice studies (Witte et al., 2001), though the increase is probably smaller than those observed during strong experimental stimulation. However, whether there are measurable changes of scatterer size response to physiological activity in vivo remains an open question. For a full evaluation of contributions from both Δa and Δb, i.e. Δμs′ = Δa(λ)−Δb in the future, a minimum of 4 wavelengths will be required. Ideally, we would use more wavelengths, as there could be other chromophores not currently considered (e.g. cytochrome oxidase) or crosstalk between absorption and scattering that lead to errors in our estimations.
We conclude that the effects of neural activity on IOS scattering holds potential as an important intrinsic biomarker, parallel to but different from contrast obtained via neurovascular coupling.
Supplementary Material
Highlights.
Investigated intrinsic optical signals (IOS) linked to local field potentials in the rat brain in vivo.
First transmission measurement of IOS in localized brain areas.
Transmittance geometry enables separation of IOS scattering from absorption.
Time scales of seconds for both neuro-scattering and hemoglobin absorption signals.
Shorter latency of neuro-scattering response relative to neurovascular response.
Acknowledgments
We thank Dr. Dieter Jaeger for his technical support in electrophysiology, and thank Dr. Elizabeth Hillman for her insightful comments on our IOS measurement and Dr. Joseph Culver for his helpful comments on our findings. Funding sources are from NIH R01 NS078095 and NSF BCS INSPIRE 1533260. The authors declare no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abookasis D, Lay CC, Mathews MS, Linskey ME, Frostig RD, Tromberg BJ. Imaging cortical absorption, scattering, and hemodynamic response during ischemic stroke using spatially modulated near-infrared illumination. J Biomed Opt. 2009;14:024033. doi: 10.1117/1.3116709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken PG, Fayuk D, Somjen GG, Turner DA. Use of intrinsic optical signals to monitor physiological changes in brain tissue slices. Methods. 1999;18:91–103. doi: 10.1006/meth.1999.0762. [DOI] [PubMed] [Google Scholar]
- Andrew RD, MacVicar BA. Imaging cell volume changes and neuronal excitation in the hippocampal slice. Neuroscience. 1994;62:371–383. doi: 10.1016/0306-4522(94)90372-7. [DOI] [PubMed] [Google Scholar]
- Aso T, Urayama S, Poupon C, Sawamoto N, Fukuyama H, Le Bihan D. An intrinsic diffusion response function for analyzing diffusion functional MRI time series. Neuroimage. 2009;47:1487–1495. doi: 10.1016/j.neuroimage.2009.05.027. [DOI] [PubMed] [Google Scholar]
- Bai R, Stewart CV, Plenz D, Basser PJ. Assessing the sensitivity of diffusion MRI to detect neuronal activity directly. Proc Natl Acad Sci U S A. 2016;113:E1728–1737. doi: 10.1073/pnas.1519890113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesetti T, Ciccolini F, Li Y. GABA Not Only a Neurotransmitter: Osmotic Regulation by GABA(A)R Signaling. Front Cell Neurosci. 2011;6:3. doi: 10.3389/fncel.2012.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlu A, Choe R, Durduran T, Lee K, Schweiger M, Arridge SR, Hillman EMC, Yodh AG. Diffuse optical tomography with spectral constraints and wavelength optimization. Applied Optics. 2005;44:2082–2093. doi: 10.1364/ao.44.002082. [DOI] [PubMed] [Google Scholar]
- Darquie A, Poline JB, Poupon C, Saint-Jalmes H, Le Bihan D. Transient decrease in water diffusion observed in human occipital cortex during visual stimulation. Proc Natl Acad Sci U S A. 2001;98:9391–9395. doi: 10.1073/pnas.151125698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman EM. Optical brain imaging in vivo: techniques and applications from animal to man. J Biomed Opt. 2007;12:051402. doi: 10.1117/1.2789693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthoff K, Witte OW. Intrinsic optical signals in rat neocortical slices measured with near-infrared dark-field microscopy reveal changes in extracellular space. J Neurosci. 1996;16:2740–2749. doi: 10.1523/JNEUROSCI.16-08-02740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howseman AM, Bowtell RW. Functional magnetic resonance imaging: imaging techniques and contrast mechanisms. Philos Trans R Soc Lond B Biol Sci. 1999;354:1179–1194. doi: 10.1098/rstb.1999.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Yacoub E. The story of the initial dip in fMRI. Neuroimage. 2012;62:1103–1108. doi: 10.1016/j.neuroimage.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques SL. Optical properties of biological tissues: a review. Phys Med Biol. 2013;58:R37–61. doi: 10.1088/0031-9155/58/11/R37. [DOI] [PubMed] [Google Scholar]
- Jin T, Kim SG. Functional changes of apparent diffusion coefficient during visual stimulation investigated by diffusion-weighted gradient-echo fMRI. Neuroimage. 2008;41:801–812. doi: 10.1016/j.neuroimage.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M, Berwick J, Johnston D, Mayhew J. Concurrent optical imaging spectroscopy and laser-Doppler flowmetry: the relationship between blood flow, oxygenation, and volume in rodent barrel cortex. Neuroimage. 2001;13:1002–1015. doi: 10.1006/nimg.2001.0808. [DOI] [PubMed] [Google Scholar]
- Larsen BR, MacAulay N. Activity-dependent astrocyte swelling is mediated by pH-regulating mechanisms. Glia. 2017;65:1668–1681. doi: 10.1002/glia.23187. [DOI] [PubMed] [Google Scholar]
- Le Bihan D. The ‘wet mind’: water and functional neuroimaging. Phys Med Biol. 2007;52:R57–90. doi: 10.1088/0031-9155/52/7/R02. [DOI] [PubMed] [Google Scholar]
- Le Bihan D. Diffusion MRI: what water tells us about the brain. EMBO Mol Med. 2014;6:569–573. doi: 10.1002/emmm.201404055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D, Urayama S, Aso T, Hanakawa T, Fukuyama H. Direct and fast detection of neuronal activation in the human brain with diffusion MRI. Proc Natl Acad Sci U S A. 2006;103:8263–8268. doi: 10.1073/pnas.0600644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P. Effects of membrane depolarization on light scattering by cerebral cortical slices. J Physiol. 1973;231:365–383. doi: 10.1113/jphysiol.1973.sp010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Shaik MA, Kim SH, Kozberg MG, Thibodeaux DN, Zhao HT, Yu H, Hillman EM. Wide-field optical mapping of neural activity and brain haemodynamics: considerations and novel approaches. Philos Trans R Soc Lond B Biol Sci. 2016:371. doi: 10.1098/rstb.2015.0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVicar BA, Hochman D. Imaging of synaptically evoked intrinsic optical signals in hippocampal slices. J Neurosci. 1991;11:1458–1469. doi: 10.1523/JNEUROSCI.11-05-01458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malonek D, Grinvald A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: Implications for functional brain mapping. Science. 1996;272:551–554. doi: 10.1126/science.272.5261.551. [DOI] [PubMed] [Google Scholar]
- Miller KL, Bulte DP, Devlin H, Robson MD, Wise RG, Woolrich MW, Jezzard P, Behrens TE. Evidence for a vascular contribution to diffusion FMRI at high b value. Proc Natl Acad Sci U S A. 2007;104:20967–20972. doi: 10.1073/pnas.0707257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourant JR, Fuselier T, Boyer J, Johnson TM, Bigio IJ. Predictions and measurements of scattering and absorption over broadwavelength ranges in tissue phantoms. Applied Optics. 1997;36:949–957. doi: 10.1364/ao.36.000949. [DOI] [PubMed] [Google Scholar]
- Pal I, Nyitrai G, Kardos J, Heja L. Neuronal and astroglial correlates underlying spatiotemporal intrinsic optical signal in the rat hippocampal slice. PLoS ONE. 2013;8:e57694. doi: 10.1371/journal.pone.0057694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W-J, Majeed W, Billings J, Keilholz S. Detecting Ultra-low Light Level Signals with Optical Fiber Probe for Intrinsic Neural Signals in vivo. Biophotonics Congress: Biomedical Optics Congress 2018 (Microscopy/Translational/Brain/OTS); Hollywood, Florida: Optical Society of America; 2018. p. JW3A.59. [Google Scholar]
- Pan W-J, Thompson G, Magnuson M, Majeed W, Jaeger D, Keilholz S. Simultaneous FMRI and electrophysiology in the rodent brain. J Vis Exp. 2010 doi: 10.3791/1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WJ, Thompson G, Magnuson M, Majeed W, Jaeger D, Keilholz S. Broadband Local Field Potentials Correlate with Spontaneous Fluctuations in Functional Magnetic Resonance Imaging Signals in the Rat Somatosensory Cortex Under Isoflurane Anesthesia. Brain Connectivity. 2011;1:119–131. doi: 10.1089/brain.2011.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WJ, Billings JC, Grooms JK, Shakil S, Keilholz SD. Considerations for resting state functional MRI and functional connectivity studies in rodents. Front Neurosci. 2015;9:269. doi: 10.3389/fnins.2015.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WJ, Thompson GJ, Magnuson ME, Jaeger D, Keilholz S. Infraslow LFP correlates to resting-state fMRI BOLD signals. Neuroimage. 2013;74:288–297. doi: 10.1016/j.neuroimage.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector DM, Carter KM, Volegov PL, George JS. Spatio-temporal mapping of rat whisker barrels with fast scattered light signals. Neuroimage. 2005;26:619–627. doi: 10.1016/j.neuroimage.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Rector DM, Rogers RF, Schwaber JS, Harper RM, George JS. Scattered-light imaging in vivo tracks fast and slow processes of neurophysiological activation. Neuroimage. 2001;14:977–994. doi: 10.1006/nimg.2001.0897. [DOI] [PubMed] [Google Scholar]
- Scholkmann F, Kleiser S, Metz AJ, Zimmermann R, Mata Pavia J, Wolf U, Wolf M. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage. 2014;85(Pt 1):6–27. doi: 10.1016/j.neuroimage.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Seghouane AK, Shah A. Consistent hemodynamic response function estimation in functional MRI by first order differencing. 2013 IEEE 10th International Symposium on Biomedical Imaging; 2013. pp. 282–285. [Google Scholar]
- Sloan HL, Austin VC, Blamire AM, Schnupp JW, Lowe AS, Allers KA, Matthews PM, Sibson NR. Regional differences in neurovascular coupling in rat brain as determined by fMRI and electrophysiology. Neuroimage. 2010;53:399–411. doi: 10.1016/j.neuroimage.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Strangman G, Boas DA, Sutton JP. Non-invasive neuroimaging using near-infrared light. Biol Psychiatry. 2002;52:679–693. doi: 10.1016/s0006-3223(02)01550-0. [DOI] [PubMed] [Google Scholar]
- Takagi S, Obata K, Tsubokawa H. GABAergic input contributes to activity-dependent change in cell volume in the hippocampal CA1 region. Neurosci Res. 2002;44:315–324. doi: 10.1016/s0168-0102(02)00153-0. [DOI] [PubMed] [Google Scholar]
- Tsurugizawa T, Ciobanu L, Le Bihan D. Water diffusion in brain cortex closely tracks underlying neuronal activity. Proc Natl Acad Sci U S A. 2013;110:11636–11641. doi: 10.1073/pnas.1303178110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villringer A, Chance B. Non-invasive optical spectroscopy and imaging of human brain function. Trends Neurosci. 1997;20:435–442. doi: 10.1016/s0166-2236(97)01132-6. [DOI] [PubMed] [Google Scholar]
- Vincis R, Lagier S, Van De Ville D, Rodriguez I, Carleton A. Sensory-Evoked Intrinsic Imaging Signals in the Olfactory Bulb Are Independent of Neurovascular Coupling. Cell Rep. 2015;12:313–325. doi: 10.1016/j.celrep.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte OW, Niermann H, Holthoff K. Cell swelling and ion redistribution assessed with intrinsic optical signals. An Acad Bras Cienc. 2001;73:337–350. doi: 10.1590/s0001-37652001000300005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.