Abstract
Background
The purpose of this study was to investigate whether DEX exerts protective mechanisms in rats with acute lung injury (ALI) induced by the endotoxin lipopolysaccharide (LPS). The mortality rate of ALI is extremely high. DEX, an α2 adrenergic receptor agonist, has potent anti-inflammatory and organ-protective effects in addition to its sedative and analgesic properties. We sought to elucidate whether DEX can attenuate acute lung injury.
Material/Methods
Forty-eight Wister rats were randomly divided into 4 groups (n=12, per group): the normal saline control (NS) group, receiving tail-vein injection of 0.9% normal saline (5 mL/kg); the LPS (L) group, receiving tail-vein injection of LPS (8 mg/kg); the LPS+DEX (L+D) group, receiving tail-vein injection of LPS (8 mg/kg), 0.5h before treated with DEX (50 ug/kg); and the DEX+LPS (D+L) group, receiving tail-vein injection of LPS (8 mg/kg) 0.5 h after being treated with DEX (50 ug/kg). Then, we measured the wet-to-dry weight ratio of lung tissue, the ALI pathology score, and HE staining of lung tissue, and assessed the Oxygen Tension index.
Results
The present study revealed that LPS-induced rats exhibited significant lung injury, characterized by the deterioration of histopathology, ALI Pathology Score, wet-to-dry weight ratio, and Oxygen Tension index (MBP, PaO2, PaCO2, PH, HCO3-, and Lac), which were attenuated by DEX treatment.
Conclusions
Collectively, the present results demonstrate elucidate the molecular mechanisms by which DEX ameliorates LPS-induced ALI.
MeSH Keywords: Acute Lung Injury, Dexmedetomidine, Endotoxins, Lipopolysaccharides
Background
Acute lung injury/acute respiratory distress syndrome (ALI/ARDS), a common clinically critical disease, is usually caused by severe infection, shock, trauma, and other factors. The comprehensive damage of pulmonary capillaries, severe hypoxemia, and pulmonary dysfunction, are the most prominent features of ALI/ARDS. ALI is one of the most common complications in critically ill patients, and lipopolysaccharide (LPS) is considered as the most important pathogen for ALI progression via the inhibition of neutrophil accumulation, pro-inflammatory mediator release, platelet aggregation, myeloperoxidase activity, and neutrophil extracellular trap (NET) release [1]. In recent years, although many international and domestic scholars have published many studies of ALI/ARDS, the mortality rate of ALI/ARDS remains as high as 30–60% [2]. ALI/ARDS remains key in critical care medicine.
Onset sepsis episodes induced by endotoxin involve a large number of vasoactive substances, such as cytokines, elastase, and oxygen-derived free radicals [3]. In addition, the onset of sepsis is closely related to systemic inflammatory states. These inflammatory mediators can lead to severe physical dysfunctions such as the destruction and continuity of capillary endothelial cells and damage to capillary barrier, leading to increased vascular permeability, both of which can eventually contribute to single or multiple organ dysfunctions [4]. More attention should be focussed on the lungs, which are the most sensitive organ suffering from these dysfunctions. Previous studies have shown that pathogenic factors may invade the body during the processes of systemic inflammation, and then activate various inflammatory cells, such as neutrophils, alveolar epithelial cells, monocytes, macrophages, and lymphocytes, followed by release of multiple inflammatory factors [5,6].
These important pathological processes contributing to the development of ALI/ARDS have led us to recognize that the excessive inflammatory reaction of the lungs, the disruptions of the normal alveolar epithelial cells, and the structure and function of pulmonary microvascular endothelial cells all play key roles in the pathogenesis of ALI/ARDS [7]. As a new type of highly selective alpha-2 adrenergic agonist, dexmedetomidine (DEX) has been widely used clinically for its sedative, painless, hypnotic, and antianxiety effects. DEX can reduce sympathetic activity and does not depress respiration, and it protects multiple organs, including the heart, brain, liver, lungs, and kidneys [8]. Studies have shown that DEX can attenuate lung injury induced by LPS, ischemia-reperfusion, and ventilation in animal models [9]. It has been reported that the pretreatment with DEX can ease pulmonary edema and hypertension in rabbits. Additionally, DEX can also reduce pulmonary tissue fibrosis in rats after acute lung injury, and reduce the amount of elastase, which can hydrolyze the composition of the lungs [10]. In the lungs, DEX can increase cGMP, preventing the proteins from leaking from pulmonary vascular endothelial cells, and pulmonary edema.
Recent studies have found that DEX has a significant anti-inflammatory effect [11,12], but it is unclear whether it has a protective effect against ALI/ARDS via anti-inflammatory responses. The mechanism of mouse ALI caused by LPS treatment is similar to that of human acute lung injury. Therefore, to investigate the protective effect of DEX on ALI, our study established a rat model of acute lung injury induced by endotoxin (LPS). The experimental results of this study may provide a basis for clinical treatment of ALI/ARDS.
Material and Methods
Animals
We obtained 48 adult male Sprague-Dawley rats (weight 200–250 g, age 7 weeks) from the Experimental Animal Center of Zhejiang Academy of Medical Sciences (license number: SCXK [Zhe] 2014–0001). The animals were housed in a quiet and comfortable room with temperature controlled between 22°C and 25°C, and the humidity at 40% to 60%. They were under a 12-h light-dark cycle (lights on from 08: 00 to 20: 00), with food and water available ad libitum. The Institutional Animal Care and Use Committee of Huzhou Hospital of Zhejiang University (Huzhou, Zhejiang Province, China) approved the experimental protocol of this study.
Models of rats with acute lung injury induced by LPS
As described from previous articles, the model was established by tail-vein injection of LPS 8 mg/kg.
Experimental groups and surgical procedures
Rats were randomly divided into 4 groups (n=12, per group) (Table 1): the normal saline (NS) control group, receiving tail-vein injection of 0.9% normal saline (8 mL/kg), followed by with 0.8 ml/kg/h NS continuous infusion and intraperitoneal injection of 50 ug/kg NS; the LPS (L) group, receiving tail-vein injection of LPS (8 mg/kg), followed by 0.8 ml/kg/h NS continuous infusion and intraperitoneal injection of 50 ug/kg NS; the LPS+DEX (L+D) group, receiving tail-vein injection of LPS (8 mg/kg) 0.5 h before being treated with intraperitoneal injection of DEX (50 ug/kg), followed by 0.8 ml/kg/h DEX continuous infusion; and the DEX +LPS (D+L) group, receiving tail-vein injection of LPS (8 mg/kg) 0.5 h after being treated with intraperitoneal injection of DEX (50 ug/kg), followed by 0.8 ml/kg/h DEX continuous infusion. Animals were sacrificed at 7 h by carotid artery bloodletting.
Table 1.
Groups of animals and drugs administration.
| Groups | 0 h | 0.5 h | 1 h | 1.5 h | 3 h | 5 h | 7 h |
|---|---|---|---|---|---|---|---|
| NS | NS | NS | NS | NS | NS | NS | Sacrifice |
| LPS | NS | NS | LPS | NS | NS | NS | Sacrifice |
| L+D | NS | NS | LPS | DEX | NS | NS | Sacrifice |
| D+L | NS | DEX | LPS | NS | NS | NS | Sacrifice |
Before the operation started, the weights of rats were measured and recorded. Then, rats were anesthetized by intraperitoneal injection of chloral hydrate in a supine position and placed on a thermostatically controlled heating pad.
Arterial oxygen tension
At the end of each experiment, blood (0.5 mL) was collected from the right common carotid artery. MBP, PaO2, PaCO2, PH, HCO3−, and Lac were immediately measured with a blood gas analyzer (Stat Profile PHOx, Nova Biomedical Corporation, USA) at 0 h, 0.5 h, 1 h, 3 h, 5 h, and 7 h.
Measurements of the ratio of W/D in lung tissue
To quantify the extent of pulmonary edema, we evaluated the W/D ratio of the lung. At 6 h after LPS or NS were injected, all rats were sacrificed, and then the W/D ratio in lung tissue of dead rats was measured. After blotting the blood on filter paper, the wet weight of the upper lobe of the left lung was determined, then the lung tissues were dried overnight in an oven at 60°C and reweighed when dry. Finally, we calculated the W/D ratio of lung tissue.
Staining of lung tissue with hematoxylin and eosin (HE)
Specimens from the right upper lobe were fixed in 10% (pH 7.2) formaldehyde for 48 h, soaked in alcohol, dipped in paraffin, and cut into 5-um–thick slices. After conventional HE staining, morphological characteristics were evaluated by light microscopy and recorded to evaluate lung injury.
Pathology scores of lung tissues
The lung histopathology score was based on reference to previous articles [13]. We evaluated the lungs using 4 scoring criteria: 1) Alveolar hemorrhage and congestion; 2) Alveolar hemorrhage; 3) Alveolar or vascular wall neutrophil infiltration or aggregation; and 4). Alveolar wall thickening and/or transparent membrane formation were assessed using 4 indicators, according to lesion severity using a 0–4 score: (0=no lesions or very mild lesions, 1=mild lesions, 2=moderate lesions, 3=severe lesions, and 4=very severe lesions). The sum of all scores was considered as the total score on pathology of lung tissues.
Statistical analysis
SPSS 16.0 statistical software was used for statistical analysis. All data are expressed as means ± standard deviation. The differences among the different groups were analyzed by one-way analysis of variance (ANOVA) and differences between the groups were evaluated using the t test for normally distributed data. Hematological and biochemical measurements and hemodynamics were compared using repeated measures analysis of variance followed by the Bonferroni post hoc test. Statistica 7.0 (Stat Soft, Inc., Tulsa, OK) statistical software was used for statistical analysis. A value of P<0.05 was considered to indicate a statistically significant difference.
Results
Comparison of histopathological changes of lung injury in rats in the 4 groups
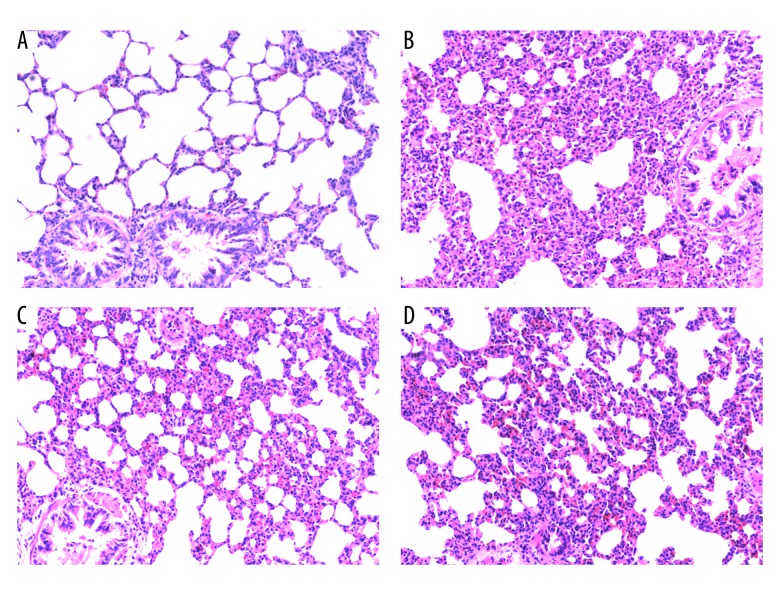
The NS group had no significant change under seen under light microscopy (Figure 1A). In the LPS group, there was severe destruction of alveolar structure, structural disorder, exudative edema, partial alveolar collapse, and atelectasis. In addition, the alveolar septum was significantly widened, with many inflammatory cells infiltrating the pulmonary interstitium, accompanied by obvious hemorrhage (Figure 1B). Rats in the L+D group and the D+L group all had some lung injury, but it was less severe than in the LPS group. Alveolar structure destruction, structural disorder, exudative edema, partial alveolar collapse, atelectasis, widened alveolar space, and interstitial inflammatory cell infiltration were also less severe in the L+D group and the D+L group when compared with the LPS group (Figure 1C, 1D).
Figure 1.
The histopathological changes of the lungs in different groups (HE staining, 200×). (A) No histological alteration was observed in the NS group. (B) Inflammatory process with marked infiltration of leukocytes into interstitial and alveolar space, edema, alveolar distortion, and thickening of alveolar wall was observed in the LPS group. (C) As compared with the LPS group, histological change of lungs was largely attenuated in L+D group. (D) As compared with the LPS group, histological change of lungs was attenuated in the D+L group.
Comparison of ALI pathology score
Compared with the NS group (score 0 (0–2)), histopathological changes of lung injury scores were obviously elevated in the LPS group (8 (6–10)), L+D group (5 (3–7)), and D+L group (4 (2–7)). Compared with the LPS group, histopathological changes in lung injury scores were significantly decreased in the L+D group and D+L group (P<0.05) (Table 2).
Table 2.
Comparison of ALI pathology score in the 4 groups.
| Grouping | ALI pathology score (Median (range)) |
|---|---|
| NS group | 0 (0–2) |
| LPS group | 8 (6–10)a |
| L+D group | 5 (3–7)ab |
| D+L group | 4 (2–7)abc |
Compared with NS group (P<0.05);
Compared with LPS group (P<0.05);
Compared with L+D group (P<0.05).
Effects of DEX on lung W/D ratio
The W/D ratio significantly increased in the LPS group (5.27±0.48) compared with the NS group (4.31±0.13) (** P<0.01). Compared with the LPS group, the W/D ratio was significantly decreased in the D+L group (4.49±0.17) (* P<0.05), but there was no significant difference between the L+D group and LPS group (4.98±0.34) (P>0.05). Interestingly, the W/D ratio in the D+L group was markedly reduced compared with the L+D group (* P<0.05) (Table 3).
Table 3.
Comparison of Lung W/D ratio in the 4 groups.
| Grouping | W/D ratio (mean) |
|---|---|
| NS group | 4.31±0.13 |
| LPS group | 5.27±0.48a |
| L+D group | 4.98±0.34a |
| D+L group | 4.49±0.17bc |
Compared with NS group (P<0.05);
Compared with LPS group (P<0.05);
Compared with L+D group (P<0.05).
Comparison of mean blood pressure (MBP) in arterial blood
Figure 2 shows there was no significant change in MBP within 7 h in the NS group. Compared with the NS group, MBP exhibited a marked reduction at 3–7 h in the LPS group and L+D group (* P<0.05) and at 1–7 h in the D+L group (* P<0.05). Compared with the LPS group, MBP exhibited a significant increase at 5–7 h in the L+D group (* P<0.05), and a significant decrease at 1 h, but an increase at 5–7 h in the D+L group. Compared with the L+D group, MBP decreased at h in the D+L group (all, P<0.05).
Figure 2.
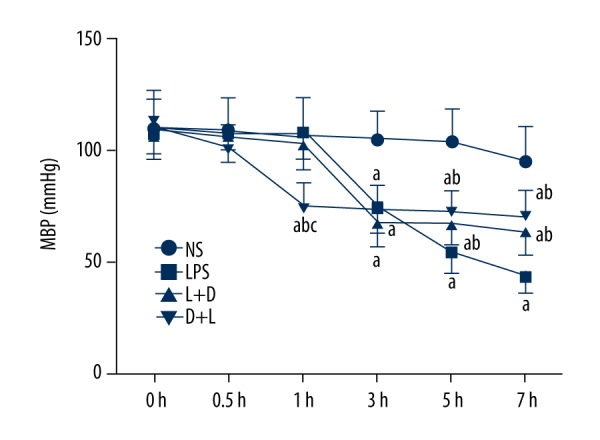
The level of MBP in rats at different time points. a. Compared with NS group (P<0.05); b. Compared with LPS group (P<0.05); c. Compared with L+D group (P<0.05).
Comparison of [HCO3−] in arterial blood in the 4 groups
The NS group had no significant change within 7 h. Compared with the NS group, [HCO3−] decreased in the 3–7 hours in the LPS group and L+D group while it also decreased in the 5–7 hours in the D +L group. Compared with the LPS group, [HCO3−] raised in the 3–7 hours in the L+D group and D+L group. Compared with the L+D group, [HCO3−] was increased at 3 h in the D+L group. Statistically significant difference was accepted at P<0.05 (Figure 3).
Figure 3.
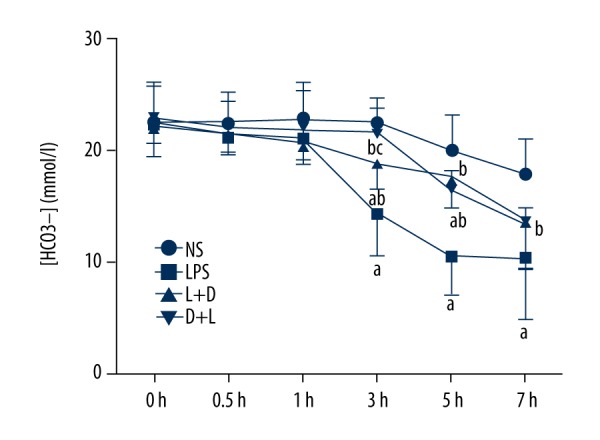
Comparison of [HCO3−] in rats at different time points. a. Compared with NS group (P<0.05); b. Compared with LPS group (P<0.05); c. Compared with L+D group (P<0.05).
Comparison of Blood oxygen partial pressure (PaO2) in arterial blood in the 4 groups
The NS group had no significant change within 7 h. Compared with the NS group, PaO2 had significantly increased at 7 h in the LPS group (P<0.05), but there was no significant difference among the L+D group, D+L group, and LPS group (P>0.05) (Figure 4).
Figure 4.
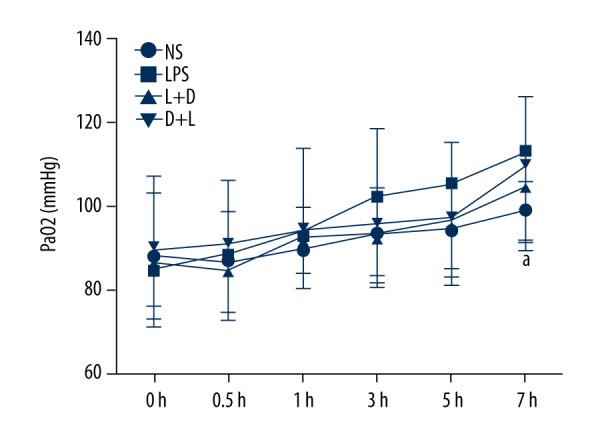
Comparison of PaO2 in rats at different time points. a. Compared with NS group (P<0.05).
Comparison of PaCO2 in arterial blood in the 4 groups
The NS group had no significant change at 7 h. Compared with the NS group, PaCO2 significantly decreased at 3–7 h in the LPS group. Compared with the LPS group, PaCO2 increased at 3–7 h in the L+D group and increased at 3–5 h in the D+L group. Compared with the L+D group, PaCO2 increased at 3 h in the D+L group (all, P<0.05) (Figure 5).
Figure 5.
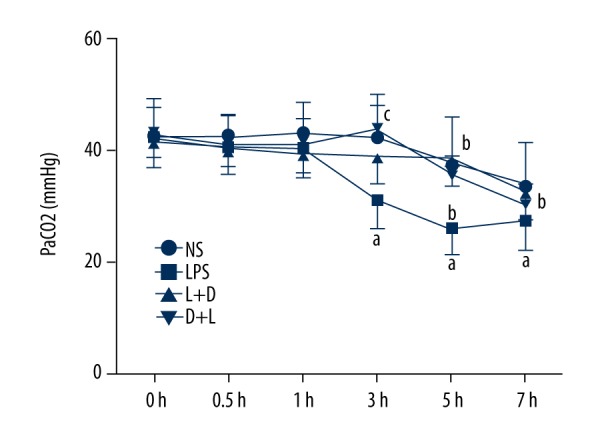
Comparison of PaCO2 in rats at different time points. a. Compared with NS group (P<0.05); b. Compared with LPS group (P<0.05); Compared with L+D group (P<0.05).
Comparison of pH in arterial blood in the 4 groups
The NS group had no significant change at 7 h. Compared with the NS group, pH significantly decreased at 3–7 hours in the LPS group and L+D group, and pH decreased at 5–7 h in the L+D group. Compared with the LPS group, pH increased at 5–7 h in the L+D group and D+L group (all, P<0.05). There was no significant difference between the L+D group and the D+L group (Figure 6).
Figure 6.
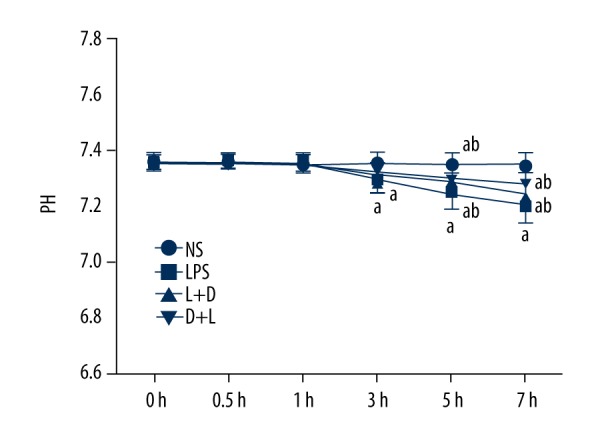
Comparison of pH in rats at different time points. a. Compared with NS group (P<0.05); b. Compared with LPS group (P<0.05); c. Compared with L+D group (P<0.05).
Comparison of Lac in arterial blood in the 4 groups
The NS group had no significant change within 7 h. Compared with the NS group, Lac significantly increased at 3–7 h in the LPS group, and Lac decreased at 0.5–1 h and increased at 7 h in the L+D group. Compared with the LPS group, Lac decreased at 1–7 h in the L+D group and D+L group. Compared with the L+D group, Lac was decreased at 7 h in the D+L group (all, P<0.05) (Figure 7).
Figure 7.
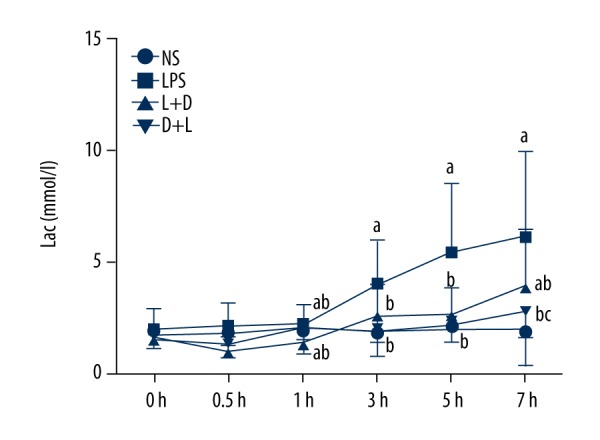
Comparison of Lac in rats at different time points. a. Compared with NS group (P<0.05); b. Compared with LPS group (P<0.05); c. Compared with L+D group (P<0.05).
Discussions
Excessive lung inflammations induced by endotoxin (LPS) and the destroyed structure and function of alveolar epithelial cells and lung microvascular endothelial cells are important pathological processes of ALI/ARDS [7]. Studies have shown that the basic pathological changes of ALI/ARDS are alveolar epithelial barrier damage (i.e., diffuse injury of alveolar epithelial cells), reduction of pulmonary surfactant, increased permeability of the basolateral membrane, accumulation of polymorphic nuclear leukocytes, injury to pulmonary parenchymal cells, and interstitial edema. Hence, the alveolar epithelial barrier damage caused by inflammation plays an important role in the pathogenesis of acute lung injury, and the degree of alveolar epithelial barrier damage determines the condition and prognosis of ALI/ARDS patients [14]. Therefore, it is important to explore the inflammatory mechanism of alveolar epithelial cell disintegration, which helps to identify the initial causes of ALI/ARDS and illustrates the importance of intervention.
DEX is a highly selective alpha 2- adrenergic receptor agonist, with sedative, anxiolytic, analgesic, and hypnotic abilities; it can also inhibit sympathetic nerve activity and hemodynamics stability. A recent study found that the systemic inflammatory response induced by endotoxins could be eased by DEX. This drug inhibits the expression of TNF-α, IL-6, MIP-2, and other inflammatory factors, and alleviates acute tissue injuries from sepsis in rats and humans, thereby protecting the brain, heart, kidneys, liver, and other organs [10,15–21]. Our data show that preconditioning and treatment can improve arterial oxygen tension and injuries to lung tissue. However, there is a difference between the preconditioning and treatment of DEX in the final treatment of lung injury.
Blood pressure fluctuation, lactic acid concentration, and lung injury scores in pretreatment group rats were significantly lower than in the treatment group. Hyperventilation and acid-base imbalance were obviously improved in treatment group rats. Whether the improved hyperventilation and acid-base imbalance and organ protection were related to anti-inflammatory responses requires further study. In the present study, we used DEX to reduce the early capillary leakage induced by endotoxin in a relatively short time (within 6 h), but for critical treatment, this period is not sufficient for active treatment. Therefore, to obtain the desired curative effects, it may be necessary to prolong the time of treatment after sepsis induced by endotoxin.
Compared with other studies, there may be a lack of early therapeutic effect and serum concentration of DEX, which led us to be unable to determine if the drug concentration is sufficient to obtain the pharmacological effects. To investigate whether the postoperative acid-base imbalance improved after applying DEX, all the work of in this study was done by the researchers under the guidance of the leader. However, further prospective randomized studies should be appropriately controlled due to the lack of randomized and double-blind subgroups. Low doses of DEX may not have the ability to treat endotoxin-induced sepsis. In addition, for intravenous injection of DEX, it must be kept in mind that humans and guinea pigs are more sensitive than abdominally-anesthetized rats.
Conclusions
The acid-base imbalance caused by endotoxin-induced sepsis can be released by pretreatment and treatment with DEX. DEX can ameliorate lung injury, but the mechanism remains unclear.
Footnotes
Source of support: This study was supported by the Public Welfare Technology Research Project of the Huzhou Science and Technology Bureau (2015GZ17)
References
- 1.Chang Y-W, Tseng C-P, Lee C-H, et al. β-Nitrostyrene derivatives attenuate LPS-mediated acute lung injury via the inhibition of neutrophil-platelet interactions and NET release. Am J Physiol Lung Cell Mol Physiol. 2018 doi: 10.1152/ajplung.00501.2016. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Frutos-Vivar F, Nin N, Esteban A. Epidemiology of acute lung injury and acute respiratory distress syndrome. Curr Opin Crit Care. 2004;10:1–6. doi: 10.1097/00075198-200402000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro L, Gelfand JA. Cytokines and sepsis: Pathophysiology and therapy. New Horizons. 1993;1(1):13–22. [PubMed] [Google Scholar]
- 4.Bone RC, Grodzin CJ, Balk RA. Sepsis: A new hypothesis for pathogenesis of the disease process. Chest. 1997;112(1):235–43. doi: 10.1378/chest.112.1.235. [DOI] [PubMed] [Google Scholar]
- 5.Suratt BT, Parsons PE. Mechanisms of acute lung injury/acute respiratory distress syndrome. Clin Chest Med. 2006;27(4):579–89. doi: 10.1016/j.ccm.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27(4):337–49. doi: 10.1055/s-2006-948288. [DOI] [PubMed] [Google Scholar]
- 7.Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31(4):S195–99. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 8.Venn RM, Bryant A, Hall GM, Grounds RM. Effects of dexmedetomidine on adrenocortical function, and the cardiovascular, endocrine and inflammatory responses in post-operative patients needing sedation in the intensive care unit. Br J Anaesth. 2001;86(5):650–56. doi: 10.1093/bja/86.5.650. [DOI] [PubMed] [Google Scholar]
- 9.Fu C, Dai X, Yang Y, et al. Dexmedetomidine attenuates lipopolysaccharide-induced acute lung injury by inhibiting oxidative stress, mitochondrial dysfunction and apoptosis in rats. Mol Med Rep. 2017;15(1):131–38. doi: 10.3892/mmr.2016.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Memiş D, Hekimoğlu S, Vatan İ, et al. Effects of midazolam and dexmedetomidine on inflammatory responses and gastric intramucosal pH to sepsis, in critically ill patients. Br J Anaesth. 2007;98(4):550–52. doi: 10.1093/bja/aem017. [DOI] [PubMed] [Google Scholar]
- 11.Taniguchi T, Kidani Y, Kanakura H, et al. Effects of dexmedetomidine on mortality rate and inflammatory responses to endotoxin-induced shock in rats. Crit Care Med. 2004;32(6):1322–26. doi: 10.1097/01.ccm.0000128579.84228.2a. [DOI] [PubMed] [Google Scholar]
- 12.Suliburk JW, Helmer KS, Gonzalez EA, et al. Ketamine attenuates liver injury attributed to endotoxemia: role of cyclooxygenase-2. Surgery. 2005;138(2):134–40. doi: 10.1016/j.surg.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Yang CH, Tsai PS, Wang TY, Huang CJ. Dexmedetomidine-ketamine combination mitigates acute lung injury in haemorrhagic shock rats. Resuscitation. 2009;80(10):1204–10. doi: 10.1016/j.resuscitation.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Matthay MA, Fukuda N, Frank J, et al. Alveolar epithelial barrier. Role in lung fluid balance in clinical lung injury. Clin Chest Med. 2000;21(3):477–90. doi: 10.1016/s0272-5231(05)70160-x. [DOI] [PubMed] [Google Scholar]
- 15.Gonzales JN, Gorshkov B, Varn MN, et al. Protective effect of adenosine receptors against lipopolysaccharide-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;306:L497–507. doi: 10.1152/ajplung.00086.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ueki M, Kawasaki T, Habe K, et al. The effects of dexmedetomidine on inflammatory mediators after cardiopulmonary bypass. Anaesthesia. 2014;69(7):693–700. doi: 10.1111/anae.12636. [DOI] [PubMed] [Google Scholar]
- 17.Xu L, Bao H, Si Y, Wang X. Effects of dexmedetomidine on early and late cytokines during polymicrobial sepsis in mice. Inflamm Res. 2013;62(5):507–14. doi: 10.1007/s00011-013-0604-5. [DOI] [PubMed] [Google Scholar]
- 18.Tasdogan M, Memis D, Sut N, Yuksel M. Results of a pilot study on the effects of propofol and dexmedetomidine on inflammatory responses and intraabdominal pressure in severe sepsis. J Clin Anesth. 2009;21(6):394–400. doi: 10.1016/j.jclinane.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Shen J, Fu G, Jiang L, et al. Effect of dexmedetomidine pretreatment on lung injury following intestinal ischemia-reperfusion. Exp Ther Med. 2013;6(6):1359–64. doi: 10.3892/etm.2013.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang L, Li L, Shen J, et al. Effect of dexmedetomidine on lung ischemia-reperfusion injury. Mol Med Rep. 2014;9(2):419–26. doi: 10.3892/mmr.2013.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu J, Chen J, Xia P, et al. Dexmedetomidine attenuates remote lung injury induced by renal ischemia-reperfusion in mice. Acta Anaesthesiol Scand. 2011;55(10):1272–78. doi: 10.1111/j.1399-6576.2011.02526.x. [DOI] [PubMed] [Google Scholar]