Abstract
Background
Alteration of DNA methylation of tumor suppressor genes (TSGs) is one of the most consistent epigenetic changes in human cancers. DNMTs play several important roles in DNA methylation and development of cancers. Regarding DNMTs protein expressions, little is known about the clinical significance and correlation with promoter methylation status of TSGs in human pituitary adenomas.
Material/Methods
We analyzed the protein expression of 3 DNMTs using immunohistochemistry and assessed DNA hypermethylation of RASSF1A, CDH13, CDH1, and CDKN2A (p16) in 63 pituitary adenomas. We examined associations between DNMTs expression and clinicopathological features or promoter methylation status of TSGs.
Results
Overexpression of DNMTs was detected in pituitary adenomas. Frequencies of DNMT1 overexpression were significantly higher in macroadenomas, invasive tumors, and grade III and IV tumors. DNMT3A was frequently detected in invasive tumors and grade IV tumors. In addition, DNMT1 and DNMT3A were frequently detected in high-methylation tumors. Furthermore, in multivariate logistic regression, the significant association between DNMT1 or DNMT3A and high-methylation status persisted after adjusting for clinicopathological features.
Conclusions
Our findings suggested that tumor overexpression of DNMT1 and DNMT3A is associated with tumor aggressive behavior and high-methylation status in pituitary adenomas. Our data support a possible role of DNMT1 and DNMT3A in TSG promoter methylation leading to pituitary adenoma invasion and suggest that inhibition of DNMTs has the potential to become a new therapeutic approach for invasive pituitary adenoma.
MeSH Keywords: Genes, Tumor Suppressor; Methylation; Methyltransferases; Pituitary Neoplasms
Background
Pituitary adenoma is a common tumor of the skull base and is also known as pituitary adenoma. It is a common neuroendocrine tumor and accounts for about 10–15% of central nervous system tumors [1]. They can cause mood disorders, sexual dysfunction, infertility, obesity, visual disturbances, hypertension, diabetes mellitus, and accelerated heart disease [2–4]. However, the treatment of pituitary adenoma has been a challenge in neurosurgery. Only by deeply studying its molecular mechanism and developing targeted drugs can the tumor be completely cured. Abnormal regulation of epigenetics is an important mechanism leading to the occurrence of cancer and DNA methylation is the most common phenomenon in epigenetics. About 80% of CpG sites in the human genome undergo methylation changes and 70% of sites are methylated at certain times, indicating that methylation regulation of the whole genome is common [2]. While genetic events are rarely involved in pituitary tumorigenesis, inactivation of several tumor suppressor genes (TSGs) and DNA repair genes by DNA hypermethylation has been reported in pituitary adenomas [5–14]. These finding suggest that the down-regulated expression of TSGs by DNA hypermethylation is an important mechanism contributing to pituitary tumorigenesis [2,15].
Among the CpG methylation enzymes associated with gene silencing, 3 functional DNA methyltransferases (DNMTs) – DNMT1, DNMT2, and DNMT3 – have distinctive roles [16–19]. DNMT1 is known to maintain methylation [20], no transmethylase activity has been found with DNMT2, and the DNMT3 family consists of 2 related gene product – DNMT3A and DNMT3B – which function as de novo methylation activity (based upon plasmid methylation) [5]. Studies have found that DNMT1, DNMT3A, and DNMT3B are overexpressed in a variety of cancers and result in poor histological differentiation and poor prognosis [21–27]. In addition, drugs that inhibit the reversal of abnormal DNA methylation patterns by DNMTs have been widely studied in cancer treatment, and the specificity and efficacy of these drugs have been confirmed in experimental and clinical trials [28–31]. The function of DNMTs, which mediate epigenetic control, has been identified in human and mouse pituitary tumors [19,32,33]. However, DNMT1, DNMT3A, and DNMT3B protein expression has not been comprehensively studied in sporadic human pituitary adenomas. Their clinical relevance and association with promoter methylation of TSGs in pituitary adenomas remain unknown.
In the present study, we used immunohistochemical methods to detect the expression of DNMT1, DNMT3A, and DNMT3B proteins and promoter methylation of 4 TSGs by methylation-specific PCR (MSP) in 63 pituitary adenomas. We analyzed the associations between the expression of DNMT1, DNMT3A, or DNMT3B proteins and clinicopathologic features and promoter methylation status of TSGs.
Material and Methods
Human normal and pituitary adenoma samples
Informed consent was obtained from all subjects involved in the study, and the study was approved by the Ethics Committees of The University of Tokushima and Toranomon Hospital.
Five normal human adenohypophyses were obtained at autopsy from patients with no evidence of endocrine abnormality; they were examined histologically and immunocytochemically to exclude the possibility of incidental pathology. We obtained 63 tissue specimens from patients with pituitary adenoma who underwent surgery at Tokushima University Hospital (Tokushima, Japan) or Toranomon Hospital (Tokyo, Japan). Pituitary tumor tissue was fresh-frozen with liquid nitrogen and stored at −80°C for DNA and RNA extraction. Tumors enrolled in this study were characterized according to clinical, radiological, histological, and immunohistochemical features (Table 1). They were divided into functional and non-functional categories according to the secretion hormone of pituitary adenoma. Clinically functional tumors comprised 24 somatotroph adenomas, 2 mammosomatotroph adenomas, 10 lactotroph adenomas, and 4 corticotroph adenomas associated with Cushing’s disease. Clinically non-functioning adenomas comprised 5 silent corticotroph adenomas, 12 gonadotroph adenomas, 3 silent subtype 3 adenomas, and 1 null cell adenoma characterized by immunoreactivity for all anterior pituitary hormones. The size and invasiveness of tumors were defined based on preoperative radiological examination, surgical results, and modified Hardy classification. Grade I (diameter of microadenoma <1 cm) and grade II (diameter of enclosed microadenomas with or without suprasellar extension ≥1 cm) tumors were defined as non-invasive. Grade III (local invasion of sphenoid and/or cavernous sinus) and grade IV (central nervous system/extracranial spread with or without metastasis) tumors were considered to be invasive. Thus, the 63 included tumors (30 non-invasive and 33 invasive adenomas) comprised 9 grade I tumors, 21 grade II tumors, 25 grade III tumors, and 8 grade IV tumors (Table 1). There was no evidence of recurrence of any tumor in this study. Hematoxylin and eosin-stained tissue sections from all 63 pituitary adenomas were reviewed and confirmed by 2 pathologists (Toshiaki Sano and ZRQ).
Table 1.
Clinicopathologic characteristics in pituitary adenomas.
| Variables | No. | DNMT1 | DNMT3A | DNMT3B | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| + | − | P | + | − | P | + | − | P | ||
| Total | 63 | 21 | 42 | 23 | 40 | 20 | 43 | |||
| Gender | 0.72 | 0.41 | 0.33 | |||||||
| Female | 34 | 12 | 22 | 14 | 20 | 9 | 25 | |||
| Male | 29 | 9 | 20 | 9 | 20 | 11 | 18 | |||
| Mean age (yrs) ±SD | 47±16 | 47±16 | 47±16 | 0.95 | 45±15 | 48±17 | 0.55 | 49±14 | 46±17 | 0.52 |
| Tumor type | 0.22 | 0.68 | 0.59 | |||||||
| GH (acromegaly) | 24 | 6 | 18 | 6 | 18 | 9 | 15 | |||
| GH/PRL (acromegaly) | 2 | 0 | 2 | 1 | 1 | 0 | 2 | |||
| PRL (prolactinoma) | 10 | 2 | 8 | 5 | 5 | 5 | 5 | |||
| TSH | 2 | 2 | 0 | 1 | 1 | 0 | 2 | |||
| ACTH (Cushing) | 4 | 1 | 3 | 1 | 3 | 0 | 4 | |||
| ACTH (silent) | 5 | 3 | 2 | 3 | 2 | 2 | 3 | |||
| FSH/LH (NF) | 12 | 5 | 7 | 4 | 8 | 3 | 9 | |||
| Null cell (NF) | 1 | 1 | 0 | 1 | 0 | 0 | 1 | |||
| Silent subtype 3 | 3 | 1 | 2 | 1 | 2 | 1 | 2 | |||
| Tumor size | 0.02 | 0.08 | 0.38 | |||||||
| Micro-adenoma | 9 | 0 | 9 | 1 | 8 | 4 | 5 | |||
| Macro-adenoma | 54 | 21 | 33 | 22 | 32 | 16 | 38 | |||
| Invasion | 0.03 | 0.03 | 0.42 | |||||||
| Non-invasive | 30 | 6 | 24 | 7 | 23 | 8 | 22 | |||
| Invasive | 33 | 15 | 18 | 16 | 17 | 12 | 21 | |||
| Grade | ||||||||||
| I | 9 | 0 | 9 | 1 | 8 | 4 | 5 | |||
| II | 21 | 6 | 15 | 0.07 | 6 | 15 | 0.30 | 4 | 17 | 0.14 |
| III | 25 | 9 | 16 | 0.03 | 11 | 14 | 0.07 | 10 | 15 | 0.81 |
| IV | 8 | 6 | 2 | 0.001 | 5 | 3 | 0.02 | 2 | 6 | 0.40 |
ACTH – corticotroph adenoma; ACTHs – silent corticotroph adenoma; FSH/LH – gonadotroph adenoma; GH – somatotroph adenoma; GH/PRL – mammosomatotroph adenoma; PRL – lactotroph adenoma; Null cell – null cell adenoma; TSH – TSH cell adenoma; Silent subtype 3 – silent subtype 3 adenoma; M – methylated; UM – unmethylated; SD – standard deviation. The chi-square test was used to analyze the differences in frequencies of DNMTs immunoreaction and promoter methylation of TSGs among each group of pituitary adenomas.
Immunohistochemistry
Immunolocalization of DNMT1, DNMT3A, DNMT3B, and MKI67 (Ki-67) antigen based on the streptavidin-biotin labeling method was performed on sections from representative blocks of paraffin-embedded tissues used for pathology diagnosis. After deparaffinization and antigen retrieval using an autoclave oven technique, the sections were incubated at 4°C overnight with antibodies: goat polyclonal anti-DNMT1 (1: 100; Santa Cruz Biotech, Santa Cruz, CA), goat polyclonal anti-DNMT3a (1: 150; Santa Cruz Biotech), goat polyclonal anti-DNMT3b (1: 100; Santa Cruz Biotech), or with MIB-1 mouse monoclonal antibody (1: 100, DakoCytomatin, Glostrup, Denmark). Antigen-antibody complexes were detected using the 3-amino-9- ethylcarbazole reaction. After immunohistochemical staining, the slides were observed and analyzed by an electron microscope. Colon and breast cancer samples known to be positive for DNMT1, DNMT3A, and DNMT3B were used as positive controls [21,24]. Nuclear immunoreactivity in the proliferative zones of noncancerous foveolar epithelia was used as a positive control for some sections. The sections were incubated with phosphate buffer solution without the primary antibody as a negative control. Furthermore, the specificity of all reactions for DNMT1, DNMT3A, and DNMT3B was verified by replacing the primary antibody with normal serum and pre-absorbing each primary antibody with blocking peptide (Santa Cruz Biotech). Each section was examined independently by 2 investigators in a blinded manner. Nuclear staining was considered to represent a positive stain for DNMT1, DNMT3A, DNMT3B, and MKI67. A total of 1000 cells were counted at several high-power fields (×200) selected from different staining density regions, including high-, moderate-, low-, and negative-staining areas. In a few samples with small size, there were fewer than 1000 counted cells. DNMT1, DNMT3A, and DNMT3B positivity (i.e., overexpression) was defined as ≥10% of tumor cells with nuclear staining. DNMTs 2 was defined as the presence of ≥2/3 DNMTs, DNMTs 1 as 1/3, and DNMTs 0 as 0/3 DNMTs.
Methylation analyses for TSG CpG islands
Genomic DNA was extracted from fresh-frozen tissue samples using the Qiagen DNeasy Tissue Kit (Qiagen, Stanford, CA) according to the manufacturer’s instructions. Genomic DNA was modified with sodium bisulfite and the modified genome DNA was purified by CpGenome DNA Modification Kit (Intergen, Purchase, NY),
Subsequently, the DNA promoter methylation status of the RASSF1A, CDH13, CDH1, and CDKN2A (P16) genes were investigated by MSP assay, as described previously [6]. The application of specific primers and annealing temperatures for methylated and unmethylated promoters were described in previous reports [6,10,11]. CpG universally methylated human DNA (Intergen, Purchase, NY) was used as positive control, while distilled water served as the negative control, accompanied by every amplification reaction. PCR products were separated by electrophoresis in 2% agarose gel or nondenaturing 6% polyacrylamide gel and were stained with ethidium bromide. To confirm the methylation state, the bisulfite reaction and MSP of all samples were repeated. Methylated and unmethylated PCR products randomly selected and purified from the gels were processed to the direct sequencing analysis using the NucleoSpin® Extract Kit (Macherey-Nagel, Düren, Germany). Cycle sequencing was performed using the BigDye Terminator V1.1 Cycle sequencing kit (Applied Biosystems) and was subsequently analyzed with the ABI PRISM 310 Genetic Analyzer (Applied Biosystems).
High-methylation status was defined as the presence of ≥2/4 methylated promoters of TSGs, and low-methylation/0 as 0/4–1/4 methylated promoters of TSGs, according to previously established criteria [34].
Statistical analysis
SAS software (version 9.1; SAS Institute, Inc, Cary, NC) was used for all statistical analyses. For categorical data, the chi-square test was performed. Probability (P) values were calculated by ANOVA (analysis of variance) for age (Tables 1, 2). To assess independent effects of tumoral DNMT1, DNMT3A, and DNMT3B expression on tumoral methylation status, multivariate logistic regression analysis was done (with DNMT1, DNMT3A, and DNMT3B as an exposure of interest and high-methylation status as an outcome variable) and odds ratio (OR) was adjusted for age (as a continuous variable), sex, and tumor grade (I–II vs. III–IV) (Table 3). P<0.05 were considered to be statistically significant.
Table 2.
Clinicopathologic characteristics in pituitary adenomas.
| Variables | No. | RASSF1A | CDH13 | CDH1 | CDKN2A (P16) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | UM | P | M | UM | P | M | UM | P | M | UM | P | ||
| Total | 63 | 23 | 40 | 20 | 43 | 24 | 39 | 32 | 31 | ||||
| Gender | 0.17 | 0.23 | 0.30 | 0.52 | |||||||||
| Female | 34 | 15 | 19 | 13 | 21 | 11 | 23 | 16 | 18 | ||||
| Male | 29 | 8 | 21 | 7 | 22 | 13 | 16 | 16 | 13 | ||||
| Mean age (yrs) ±SD | 47±16 | 49±18 | 46±15 | 0.48 | 45±16 | 48±16 | 0.54 | 48±18 | 46±15 | 0.73 | 48±16 | 46±17 | 0.68 |
| Tumor type | 0.49 | 0.85 | 0.08 | 0.23 | |||||||||
| GH (acromegaly) | 24 | 11 | 13 | 6 | 18 | 5 | 19 | 16 | 8 | ||||
| GH/PRL (acromegaly) | 2 | 0 | 2 | 1 | 1 | 0 | 2 | 1 | 1 | ||||
| PRL (prolactinoma) | 10 | 4 | 6 | 4 | 6 | 5 | 5 | 3 | 7 | ||||
| TSH | 2 | 0 | 2 | 1 | 1 | 2 | 0 | 2 | 0 | ||||
| ACTH (Cushing) | 4 | 2 | 2 | 1 | 3 | 1 | 3 | 1 | 3 | ||||
| ACTH (silent) | 5 | 2 | 3 | 2 | 3 | 1 | 4 | 1 | 4 | ||||
| FSH/LH (NF) | 12 | 2 | 10 | 3 | 9 | 7 | 5 | 6 | 6 | ||||
| Null cell (NF) | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | ||||
| Silent subtype 3 | 3 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | ||||
| Tumor size | 0.59 | 0.15 | 0.07 | 0.3 | |||||||||
| Micro-adenoma | 9 | 4 | 5 | 1 | 8 | 1 | 8 | 6 | 3 | ||||
| Macro-adenoma | 54 | 19 | 35 | 19 | 35 | 23 | 31 | 26 | 28 | ||||
| Invasion | 0.90 | 0.01 | 0.45 | 0.16 | |||||||||
| Non-invasive | 30 | 11 | 19 | 5 | 25 | 10 | 20 | 18 | 12 | ||||
| Invasive | 33 | 12 | 21 | 15 | 18 | 14 | 19 | 14 | 19 | ||||
| Grade | |||||||||||||
| I | 9 | 4 | 5 | 1 | 8 | 1 | 8 | 6 | 3 | ||||
| II | 21 | 7 | 14 | 0.56 | 4 | 17 | 0.59 | 9 | 12 | 0.09 | 12 | 9 | 0.62 |
| III | 25 | 6 | 19 | 0.24 | 10 | 15 | 0.11 | 9 | 16 | 0.16 | 11 | 14 | 0.24 |
| IV | 8 | 6 | 2 | 0.20 | 5 | 3 | 0.02 | 5 | 3 | 0.02 | 3 | 5 | 0.22 |
ACTH – corticotroph adenoma; ACTHs – silent corticotroph adenoma; FSH/LH – gonadotroph adenoma; GH – somatotroph adenoma; GH/PRL – mammosomatotroph adenoma; PRL – lactotroph adenoma; Null cell – null cell adenoma; TSH – TSH cell adenoma; Silent subtype 3 – silent subtype 3 adenoma; M – methylated; UM – unmethylated; SD – standard deviation. The chi-square test was used to analyze the differences in frequencies of DNMTs immunoreaction and promoter methylation of TSGs among each group of pituitary adenomas.
Table 3.
Multivariate logistic regression analysis to assess relationship between expressions of DNMT1, DNMT3A and methylation-high status in pituitary adenomas.
| Variable independently associated with methylation-high status* | Multivariate OR (95% CI) | P |
|---|---|---|
| DNMT1 + | 3.63 (1.12–11.7) | 0.031 |
| DNMT3A + | 3.38 (1.09–10.5) | 0.035 |
Multivariate logistic regression analysis assessing the relationship of DNMT1 and DNMT3A expression with methylation-high status in pituitary adenomas initially included age, gender, tumor grade.
Tumor showed ≥2 TSGs methylation.
CI – confidence interval; OR – odds ratio.
Results
Expression analysis of DNMT1, DNMT3A, and DNMT3B in pituitary adenomas
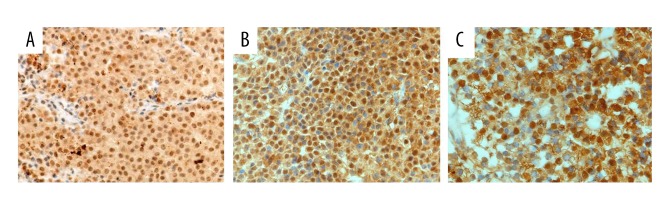
In adenoma specimens, immunopositivity for DNMT1, DNMT3A, and DNMT3B showed a clear nuclear pattern. When present, this reactivity was typically very intense throughout the nuclei of tumor cells of various types (Figure 1A–1C). In addition, no or weak immunoreactivity for DNMT1, DNMT3A, and DNMT3B was observed in normal pituitary cells or non-neoplastic cells contained in surgical specimens.
Figure 1.
Detection of immunostaining of DNMT1, DNMT3A, and DNMT3B in pituitary adenomas. Nuclear immunopositivities of DNMT1, DNMT3A, and DNMT3B were observed in tumor cells. The immunoreactivities of DNMT1, DNMT3A, and DNMT3B were variable but always very intense throughout the nuclei (positive cells are stained brown). (A) Strong nuclear DNMT1 positivity, Original magnification, ×200. (B) Tumor with strong nuclear DNMT3A positivity, original magnification ×200. (C) tumor with strong nuclear DNMT3B positivity, original magnification ×400.
Overexpression of DNMT1, DNMT3A, and DNMT3B was detected in 21 of 63 (33%), 20 of 63 (32%), and 23 of 63 (37%) adenomas, respectively (Table 1). DNMT1, DNMT3A, and DNMT3B overexpression did not show any significant differences among subtypes of pituitary adenomas (Table 1). The overexpression of DNMT1 was more frequent (P<0.05, Table 1) in macroadenomas (21/54, 39%) than in microadenomas (0/9, 0%). In addition, the overexpression of DNMT1 and DNMT3A was significantly more frequent in invasive adenomas (15/33, 45% and 16/33, 48%) than in non-invasive adenomas (6/30, 20% and 7/30, 23%) (P=0.03 and P=0.03, respectively; Table 1). Furthermore, the overexpression of DNMT1 was more frequent in aggressive grade IV (6/8, 75%) and grade III (9/16, 56%) cases compared with grade I (0/9, 0%) cases (P=0.001 and P=0.03, respectively; Table 1). The overexpression of DNMT3A was also more frequent in aggressive grade IV (5/8, 63%) cases compared with grade I (1/8, 13%) cases (P=0.02; Table 1).
The overexpression of DNMT1, DNMT3A, and DNMT3B was not related to patient age, sex (Table 1), or proliferation marker MKI67 labeling index (data not shown).
Promoter hypermethylation in RASSF1A, CDH13, CDH1, and CDKN2A (p16)
The promoter methylation of RASSF1A, CDH13, CDH1, and CDKN2A (p16) in 5 normal pituitary tissues and 63 pituitary adenomas was investigated by MSP. The results are summarized in Table 2. Hypermethylation of the promoter region of RASSF1A, CDH13, CDH1, and CDKN2A (p16) was detected in 23 (37%), 20 (32%), 24 (38%), and 32 (51%) pituitary adenomas, respectively. However, there was no methylation of either promoter in 5 normal pituitary tissues. The results suggest that promoter hypermethylation of RASSF1A, CDH13, CDH1, and CDKN2A (p16) is tumor-specific. Methylated patterns of RASSF1A, CDH13, CDH1, and CDKN2A (p16) did not show any significant differences among subtypes of pituitary adenomas (Table 2). The level of genomic DNA methylation was significantly different between invasive and non-invasive pituitary adenomas. We found that the methylation of CDH13 gene is more frequent in invasive pituitary adenomas than in non-invasive pituitary adenomas (5 of 30, 17%, P<0.05, Table 1). The methylation of CDH13 and CDH1 was more frequent in aggressive grade IV cases compared with grade I cases (P=0.02 and P=0.02, respectively, Table 2).
In addition, the specificity of MSP was confirmed by direct sequencing. In unmethylated MSP products, all cytosine nucleotides, including those in the CpG islands, changed to thymidines as a result of bisulfite modification. However, in methylated MSP products, cytosine nucleotides in most CpG islands were unchanged (data not shown).
Association between tumoral DNMTs overexpression and TSG hypermethylation
High-methylation status was detected in 51% (32 of 63) of tumors. DNMTs 2, DNMTs 1, and DNMTs 0 groups were found in 33% (21 of 63), 32% (20 of 63), and 35% (22 of 63) of tumors, respectively.
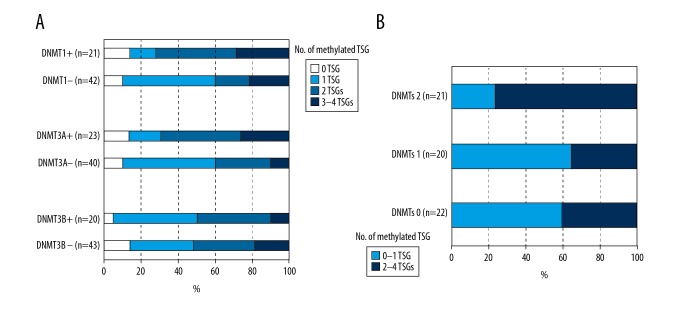
We examined the frequencies of TSG high-methylation status in adenomas with overexpression of DNMT1, DNMT3A, and DNMT3B and in adenomas with underexpression of DNMT1, DNMT3A, and DNMT3B. The groups with overexpression of DNMT1 and DNMT3A included more high-methylation tumors than the groups with underexpression of DNMT1 and DNMT3A (72% vs. 40% and 69% vs. 40%, respectively, Figure 2A). In addition, high-methylation tumors showed higher frequencies of overexpression of DNMT1 and DNMT3A (43%, 15 of 32 and 50%, 16 of 32, respectively) than in methylation-low tumors (19%, 6 of 32 and 22%, 7 of 32, respectively) (P=0.021 and P=0.023, respectively, Table 4). Furthermore, the DNMTs 2 group had more high-methylation tumors than DNMTs 1 and DNMTs 0 groups (76%, 35%, and 40%, respectively, Figure 2B).
Figure 2.
The frequencies of TSG methylation status in adenomas according to expression of DNMT1, DNMT3A, and DNMT3B. (A) The groups with overexpression of DNMT1 and DNMT3A had more high-methylation tumors than the groups with low-expression of DNMT1 and DNMT3A (72% vs. 40% and 69% vs. 40%, respectively). (B) The DNMTs 2 group had more high-methylation tumors than DNMTs 1 and DNMTs 0 groups (76%, 35%, and 40%, respectively).
Table 4.
Frequency of DNMT1, DNMT3A, and DNMT3B expression categorized by TSGs methylation status in pituitary adenomas.
| Variable | No. | DNMT1 | DNMT3A | DNMT3B | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| + | − | P | + | − | P | + | − | P | ||
| Total | 63 | 21 (33%) | 42 (67%) | 23 (38%) | 40 (62%) | 20 (32%) | 43 (68%) | |||
| RASSF1A | 0.45 | 0.82 | 0.86 | |||||||
| M | 23 (38%) | 9 (43%) | 14 (33%) | 8 (35%) | 15 (38%) | 7 (35%) | 16 (37%) | |||
| UM | 40 (87%) | 12 (57%) | 28 (67%) | 15 (65%) | 25 (62%) | 13 (65%) | 27 (63%) | |||
| CDH13 | 0.18 | 0.001 | 0.83 | |||||||
| M | 20 (32%) | 9 (43%) | 11 (26%) | 13 (57%) | 7 (18%) | 6 (30%) | 14 (33%) | |||
| UM | 43 (68%) | 12 (57%) | 31 (74%) | 10 (43%) | 33 (82%) | 14 (70%) | 29 (67%) | |||
| CDH1 | 0.09 | 0.08 | 0.83 | |||||||
| M | 24 (38%) | 11 (53%) | 13 (31%) | 12 (52%) | 12 (30%) | 8 (40%) | 16 (37%) | |||
| UM | 39 (62%) | 10 (47%) | 29 (69%) | 11 (48%) | 28 (70%) | 12 (60%) | 27 (63%) | |||
| CDKN2A (P16) | 0.85 | 0.16 | 0.93 | |||||||
| M | 32 (51%) | 11 (53%) | 21 (50%) | 9 (39%) | 23 (58%) | 10 (50%) | 22 (51%) | |||
| UM | 31 (49%) | 10 (47%) | 21 (50%) | 14 (61%) | 17 (43%) | 10 (50%) | 21 (49%) | |||
| Methylation-high* | 0.021 | 0.023 | 0.93 | |||||||
| + | 32 (51%) | 15 (71%) | 17 (40%) | 16 (70%) | 16 (40%) | 10 (50%) | 22 (51%) | |||
| − | 31 (49%) | 6 (29%) | 25 (60%) | 7 (30%) | 24 (60%) | 10 (50%) | 21 (49%) | |||
Tumor showed ≥2 TSGs methylation.
M, methylated; UM, unmethylated. The chi-square test was used to analyze the differences in frequencies of TSGs methylation status according to DNMTs immunoreaction.
To confirm an independent relation between expression of DNMT1 and DNMT3A and high-methylation status, we performed multivariate logistic regression analysis (Table 3). Overexpression of DNMT1 and DNMT3A was associated with high-methylation status (multivariate OR, 3.63, 95% CI, 1.12–11.7 and multivariate OR, 3.38, 95% CI, 1.09–10.5, respectively) after adjusting for age, sex, and tumor grade.
The CDH13-methylated group included more DNMT3A-overexpression tumors than the CDH13-unmethylated group (P=0.001, Table 4). However, there were no differential associations between overexpression of DNMT1, DNMT3A, and DNMT3B and individual TSG methylation status (Table 4).
Discussion
Overexpression of DNMT1, DNMT3A, and DNMT3B has been reported in various cancers and is significantly correlated with poor prognosis [3,21–26]. In addition, hypermethylation of CpG islands results from up-regulation of DNMTs in human tumors [2]. In this study, we detected overexpression of DNMT proteins in pituitary adenomas. DNMT1 overexpression was associated with macroadenomas, invasive tumors, and grade III and IV tumors. In addition, DNMT3A overexpression was associated with invasive tumors and grade IV tumors. Furthermore, overexpression of DNMT1 and DNMT3A was associated with high-methylation tumors. These findings indicate that DNMTs proteins have important roles as oncogenic factors and TSG methylation regulators in pituitary tumorigenesis and tumor progression.
It has been shown that activity of DNMT1, DNMT3A, and DNMT3B is increased in cancer cells and may be related to tumor aggressiveness and poor prognosis [21,22,24–26]. In addition, DNMT1 protein expression was higher in the advanced stages of hepatocellular cancer [7]. In the present study, the overexpression of DNMT1 and DNMT3A was significantly related to tumor invasion and tumor grade. Overexpression of DNMT1 and DNMT3A was more frequently detected in invasive adenomas than in non-invasive adenomas and in was more frequent in grade III and IV tumors than in grade I tumors. DNMTs may contribute to cancer progression by silencing invasion-related TSGs such as CDH1, CDH13, and RASSF1A. This study and our previous studies [10,11] suggest that promoter methylation of CDH1, CDH13, and RASSF1A is associated with tumor aggressiveness in pituitary adenomas. However, the exact mechanisms behind these associations remain to be elucidated.
Hypermethylation of gene promoter regions has been reported in several TSGs, including CDKN2A, RB1, DAPK, GADD45, RASSF1A, CDH1, CDH13, IKAROS, FGFR2, and GSTP1 in pituitary adenomas [5–14]. In addition, the association between expression of DNMT1 and DNMT3B and epigenetic control of genes has been identified in pituitary cells [19,32]. Studies of the relationship between the expression levels of DNMTs and aberrant methylation of CpG islands of genes have produced contradictory results in a variety of tumors. In the present study, DNMT1 and DNMT3A overexpression was associated with high-methylation status independent of other clinical variables, including age, sex, and tumor grade. Although expression of DNMT3B itself did not show an association with high-methylation status, tumors with multiple DNMTs overexpression were associated with high-methylation status. Our findings are the first to demonstrate the clinical significance of the DNMTs/methylation connection in human pituitary tumors.
Many previous studies have been designed to examine the association between individual DNMT expression and promoter methylation of individual TSG or multiple TSGs [21,35–40]. Although these studies produced contradictory results, it is clear that DNMTs overexpression was associated with multiple TSGs methylation status. DNMT3B was reported to be associated with the CpG island methylator high-phenotype status in colorectal cancers; however, in multivariate logistic regression analysis, DNMT3B expression remained significantly associated with just 3 TSGs promoter methylation out of 17 TSGs [37]. In the present study, although the number of TSGs analyzed was limited, DNMT1 and DNMT3A overexpression was associated with high-methylation status in multivariate analysis independently of clinicopathological features. Furthermore, high-methylation status was frequently detected in tumors with overexpression of multiple DNMTs. Therefore, the above findings demonstrate that DNMTs expression contributes to high-methylation status rather than individual TSG methylation.
Pituitary adenomas are usually benign tumors of the nervous system. In clinic practice, some pituitary adenomas are invasive and can invade surrounding structures, including the saddle area, the cavernous sinus, and even the brain [2,41–43]. The invasiveness of pituitary adenoma is regarded as the biggest obstacle to the control of long-term tumor disease because it can limit surgical resection and lead to tumor regrowth [2]. Thus, it is essential to develop new adjuvant treatment for patients with aggressive pituitary tumors. While genetic mutations are irreversible, epigenetic modifications are reversible. Therefore, epigenetic modifications are considered to be very attractive targets in developing new therapeutic approaches [29,44,45]. Hypermethylated gene promoters have the potential to be reactivated by nucleoside analogues such as 5-azacytidine and 5-aza-2-deoxycytidine (decitabine) [46]. Azacytidine and decitabine are currently in use to treat myelodysplastic syndrome and acute myeloid leukemia [28]. In addition, a number of non–nucleoside analogue DNMT inhibitors such as hydralazine have also been proposed as epigenetic–targeted drugs [47]. Furthermore, clinical trials for decitabine as treatment for solid tumors are already in the early stages [28]. Our data suggest that pituitary adenomas displaying overexpression of DNMT1 and DNMT3A would be good candidates for epigenetic therapy. In addition, as overexpression of DNMT1 and DNMT3A was significantly associated with invasiveness, clinical trials for decitabine in invasive pituitary adenomas are recommended.
Conclusions
Our study profiled the expression status of DNMT1, DNMT3A, and DNMT3B in pituitary adenomas and indicated that tumoral overexpression of DNMT1 and DNMT3A is associated with tumor aggressiveness and high-methylation status in pituitary adenomas. Our data demonstrated a possible role of DNMT1 and DNMT3A in TSG promoter methylation leading to pituitary adenoma invasion. Inhibition of DNMTs has the potential to become a new therapeutic approach for invasive pituitary adenoma.
Abbreviations
- ACTH
corticotroph adenoma
- ACTHs
silent corticotroph adenoma
- DNMT
DNA methyltransferase
- FSH/LH
gonadotroph adenoma
- GH
somatotroph adenoma
- GH/PRL
mammosomatotroph adenoma
- MSP
methylation-specific PCR
- PRL
lactotroph adenoma
- Null cell
null cell adenoma
- TSH
TSH cell adenoma
- Silent subtype 3
silent subtype 3 adenoma
- TSG
tumor suppressor gene
Footnotes
Source of support: This study was supported by the National Natural Science Foundation of China (No. 31500640 and No. 31770849), the Natural Science Foundation of Zhejiang Province (LY15C070002, No. LY16C050001), the Science Technology Department of Zhejiang Province (2015C33131, 2016F10005), and the Science and Technology Bureau of Jiaxing (No. 2015AY23007)
References
- 1.Osman M, Wild A. Spindle cell oncocytoma of the anterior pituitary presenting with an acute clinical course due to intraventricular hemorrhage. A case report and review of literature. Ame J Case Rep. 2017;18:894–901. doi: 10.12659/AJCR.903702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asa SL, Ezzat S. The pathogenesis of pituitary tumours. Nat Rev Cancer. 2002;2(11):836–49. doi: 10.1038/nrc926. [DOI] [PubMed] [Google Scholar]
- 3.Di Ieva A, Rotondo F, Syro LV, et al. Aggressive pituitary adenomas – diagnosis and emerging treatments. Nat Rev Endocrinol. 2014;10(7):423–35. doi: 10.1038/nrendo.2014.64. [DOI] [PubMed] [Google Scholar]
- 4.Wendel C, Campitiello M, Plastino F, et al. Pituitary metastasis from renal cell carcinoma: Description of a case report. Am J Case Rep. 2017;18:7–11. doi: 10.12659/AJCR.901032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simpson DJ, Hibberts NA, McNicol AM, et al. Loss of pRb expression in pituitary adenomas is associated with methylation of the RB1 CpG island. Cancer Res. 2000;60(5):1211–16. [PubMed] [Google Scholar]
- 6.Seemann N, Kuhn D, Wrocklage C, et al. CDKN2A/p16 inactivation is related to pituitary adenoma type and size. J Pathol. 2001;193(4):491–97. doi: 10.1002/path.833. [DOI] [PubMed] [Google Scholar]
- 7.Simpson DJ, Clayton RN, Farrell WE. Preferential loss of death associated protein kinase expression in invasive pituitary tumours is associated with either CpG island methylation or homozygous deletion. Oncogene. 2002;21(8):1217–24. doi: 10.1038/sj.onc.1205195. [DOI] [PubMed] [Google Scholar]
- 8.Bahar A, Bicknell JE, Simpson DJ, et al. Loss of expression of the growth inhibitory gene GADD45gamma, in human pituitary adenomas, is associated with CpG island methylation. Oncogene. 2004;23(4):936–44. doi: 10.1038/sj.onc.1207193. [DOI] [PubMed] [Google Scholar]
- 9.Simpson DJ, McNicol AM, Murray DC, et al. Molecular pathology shows p16 methylation in nonadenomatous pituitaries from patients with Cushing’s disease. Clin Cancer Res. 2004;10(5):1780–88. doi: 10.1158/1078-0432.ccr-1127-3. [DOI] [PubMed] [Google Scholar]
- 10.Qian ZR, Sano T, Yoshimoto K, et al. Inactivation of RASSF1A tumor suppressor gene by aberrant promoter hypermethylation in human pituitary adenomas. Lab Invest. 2005;85(4):464–73. doi: 10.1038/labinvest.3700248. [DOI] [PubMed] [Google Scholar]
- 11.Qian ZR, Sano T, Yoshimoto K, et al. Tumor-specific downregulation and methylation of the CDH13 (H-cadherin) and CDH1 (E-cadherin) genes correlate with aggressiveness of human pituitary adenomas. Mod Pathol. 2007;20(12):1269–77. doi: 10.1038/modpathol.3800965. [DOI] [PubMed] [Google Scholar]
- 12.Zhu X, Asa SL, Ezzat S. Ikaros is regulated through multiple histone modifications and deoxyribonucleic acid methylation in the pituitary. Mol Endocrinol. 2007;21(5):1205–15. doi: 10.1210/me.2007-0053. [DOI] [PubMed] [Google Scholar]
- 13.Zhu X, Lee K, Asa SL, Ezzat S. Epigenetic silencing through DNA and histone methylation of fibroblast growth factor receptor 2 in neoplastic pituitary cells. Am J Pathol. 2007;170(5):1618–28. doi: 10.2353/ajpath.2007.061111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan Y, Qian ZR, Sano T, et al. Reduction of GSTP1 expression by DNA methylation correlates with clinicopathological features in pituitary adenomas. Mod Pathol. 2008;21(7):856–65. doi: 10.1038/modpathol.2008.60. [DOI] [PubMed] [Google Scholar]
- 15.Farrell WE, Clayton RN. Epigenetic change in pituitary tumorigenesis. Endocr Relat Cancer. 2003;10(2):323–30. doi: 10.1677/erc.0.0100323. [DOI] [PubMed] [Google Scholar]
- 16.Okano M, Xie S, Li E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat Genet. 1998;19(3):219–20. doi: 10.1038/890. [DOI] [PubMed] [Google Scholar]
- 17.Bestor TH. Activation of mammalian DNA methyltransferase by cleavage of a Zn binding regulatory domain. EMBO J. 1992;11(7):2611–17. doi: 10.1002/j.1460-2075.1992.tb05326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99(3):247–57. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 19.Dudley KJ, Revill K, Whitby P, et al. Genome-wide analysis in a murine Dnmt1 knockdown model identifies epigenetically silenced genes in primary human pituitary tumors. Mol Cancer Res. 2008;6(10):1567–74. doi: 10.1158/1541-7786.MCR-08-0234. [DOI] [PubMed] [Google Scholar]
- 20.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3(6):415–28. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 21.Eads CA, Danenberg KD, Kawakami K, et al. CpG island hypermethylation in human colorectal tumors is not associated with DNA methyltransferase overexpression. Cancer Res. 1999;59(10):2302–6. [PubMed] [Google Scholar]
- 22.Patra SK, Patra A, Zhao H, Dahiya R. DNA methyltransferase and demethylase in human prostate cancer. Mol Carcinog. 2002;33(3):163–71. doi: 10.1002/mc.10033. [DOI] [PubMed] [Google Scholar]
- 23.Saito Y, Kanai Y, Nakagawa T, et al. Increased protein expression of DNA methyltransferase (DNMT) 1 is significantly correlated with the malignant potential and poor prognosis of human hepatocellular carcinomas. Int J Cancer. 2003;105(4):527–32. doi: 10.1002/ijc.11127. [DOI] [PubMed] [Google Scholar]
- 24.Girault I, Tozlu S, Lidereau R, Bieche I. Expression analysis of DNA methyltransferases 1, 3A, and 3B in sporadic breast carcinomas. Clin Cancer Res. 2003;9(12):4415–22. [PubMed] [Google Scholar]
- 25.Etoh T, Kanai Y, Ushijima S, et al. Increased DNA methyltransferase 1 (DNMT1) protein expression correlates significantly with poorer tumor differentiation and frequent DNA hypermethylation of multiple CpG islands in gastric cancers. Am J Pathol. 2004;164(2):689–99. doi: 10.1016/S0002-9440(10)63156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin RK, Hsu HS, Chang JW, et al. Alteration of DNA methyltransferases contributes to 5′CpG methylation and poor prognosis in lung cancer. Lung Cancer. 2007;55(2):205–13. doi: 10.1016/j.lungcan.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 27.Im AP, Sehgal AR, Carroll MP, et al. DNMT3A and IDH mutations in acute myeloid leukemia and other myeloid malignancies: Associations with prognosis and potential treatment strategies. Leukemia. 2014;28(9):1774–83. doi: 10.1038/leu.2014.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sigalotti L, Fratta E, Coral S, et al. Epigenetic drugs as pleiotropic agents in cancer treatment: Biomolecular aspects and clinical applications. J Cell Physiol. 2007;212(2):330–44. doi: 10.1002/jcp.21066. [DOI] [PubMed] [Google Scholar]
- 29.Song SH, Han SW, Bang YJ. Epigenetic-based therapies in cancer: Progress to date. Drugs. 2011;71(18):2391–403. doi: 10.2165/11596690-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Xiang Y, Ma N, Wang D, et al. MiR-152 and miR-185 co-contribute to ovarian cancer cells cisplatin sensitivity by targeting DNMT1 directly: A novel epigenetic therapy independent of decitabine. Oncogene. 2014;33(3):378–86. doi: 10.1038/onc.2012.575. [DOI] [PubMed] [Google Scholar]
- 31.Subramaniam D, Thombre R, Dhar A, Anant S. DNA methyltransferases: A novel target for prevention and therapy. Front Oncol. 2014;4:80. doi: 10.3389/fonc.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu X, Mao X, Hurren R, et al. Deoxyribonucleic acid methyltransferase 3B promotes epigenetic silencing through histone 3 chromatin modifications in pituitary cells. J Clin Endocrinol Metab. 2008;93(9):3610–17. doi: 10.1210/jc.2008-0578. [DOI] [PubMed] [Google Scholar]
- 33.Ho V, Ashbury JE, Taylor S, et al. Gene-specific DNA methylation of DNMT3B and MTHFR and colorectal adenoma risk. Mutat Res. 2015;782:1–6. doi: 10.1016/j.mrfmmm.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Ogino S, Nosho K, Kirkner GJ, et al. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58(1):90–96. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato M, Horio Y, Sekido Y, et al. The expression of DNA methyltransferases and methyl-CpG-binding proteins is not associated with the methylation status of p14(ARF), p16(INK4a) and RASSF1A in human lung cancer cell lines. Oncogene. 2002;21(31):4822–29. doi: 10.1038/sj.onc.1205581. [DOI] [PubMed] [Google Scholar]
- 36.Park HJ, Yu E, Shim YH. DNA methyltransferase expression and DNA hypermethylation in human hepatocellular carcinoma. Cancer Lett. 2006;233(2):271–78. doi: 10.1016/j.canlet.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 37.Nosho K, Shima K, Irahara N, et al. DNMT3B expression might contribute to CpG island methylator phenotype in colorectal cancer. Clin Cancer Res. 2009;15(11):3663–71. doi: 10.1158/1078-0432.CCR-08-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li B, Wang B, Niu LJ, et al. Hypermethylation of multiple tumor-related genes associated with DNMT3b up-regulation served as a biomarker for early diagnosis of esophageal squamous cell carcinoma. Epigenetics. 2011;6(3):307–16. doi: 10.4161/epi.6.3.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ben Gacem R, Hachana M, Ziadi S, et al. Clinicopathologic significance of DNA methyltransferase 1, 3a, and 3b overexpression in Tunisian breast cancers. Hum Pathol. 2012;43(10):1731–38. doi: 10.1016/j.humpath.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 40.Samaei NM, Yazdani Y, Alizadeh-Navaei R, et al. Promoter methylation analysis of WNT/beta-catenin pathway regulators and its association with expression of DNMT1 enzyme in colorectal cancer. J Biomed Sci. 2014;21:73. doi: 10.1186/s12929-014-0073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naguib MM, Mendoza PR, Jariyakosol S, Grossniklaus HE. Atypical pituitary adenoma with orbital invasion: Case report and review of the literature. Surv Ophthalmol. 2017;62(6):867–74. doi: 10.1016/j.survophthal.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim YH, Kim JH, Yang HK, Hwang JM. Preserved visual function with an orbital invasion of pituitary adenoma. B J Neurosurg. 2017;31(4):492–94. doi: 10.3109/02688697.2016.1139051. [DOI] [PubMed] [Google Scholar]
- 43.Abid FB, Abukhattab M, Karim H, et al. Primary pituitary tuberculosis revisited. Am J Case Rep. 2017;18:391–94. doi: 10.12659/AJCR.903233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan J, Yin WJ, Lu JS, et al. ER alpha negative breast cancer cells restore response to endocrine therapy by combination treatment with both HDAC inhibitor and DNMT inhibitor. J Cancer Res Clin Oncol. 2008;134(8):883–90. doi: 10.1007/s00432-008-0354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graca I, Sousa EJ, Baptista T, et al. Anti-tumoral effect of the non-nucleoside DNMT inhibitor RG108 in human prostate cancer cells. Curr Pharm Des. 2014;20(11):1803–11. doi: 10.2174/13816128113199990516. [DOI] [PubMed] [Google Scholar]
- 46.Gabbara S, Bhagwat AS. The mechanism of inhibition of DNA (cytosine-5-)-methyltransferases by 5-azacytosine is likely to involve methyl transfer to the inhibitor. Biochem J. 1995;307(Pt 1):87–92. doi: 10.1042/bj3070087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mai A, Altucci L. Epi-drugs to fight cancer: From chemistry to cancer treatment, the road ahead. Int J Biochem Cell Biol. 2009;41(1):199–213. doi: 10.1016/j.biocel.2008.08.020. [DOI] [PubMed] [Google Scholar]