Abstract
Endometriosis is a condition wherein an ectopic layer of endometrial tissue arises in an extra-uterine location, often effecting significant pelvic pain and infertility. While very uncommon, there have been reported cases of endometriosis undergoing malignant transformation, frequently involving the ovaries and seldom in extra-gonadal regions. We recount a case depicting a 63 year-old woman who presented with an apparent inguinal hernia in 2017; she was ultimately diagnosed with a pelvic side wall clear cell carcinoma and attendant metastatic disease to the medial groin, which emanated from endometriosis. Malignant transformation of endometriosis identified in the pelvic side wall is a very rare finding. Nevertheless, oncologists should maintain a high index of suspicion in patients with a history of endometriosis or pelvic surgery.
Keywords: Endometriosis, Malignant transformation, Pelvic side wall
Highlights
-
•
Malignant endometriosis periodically invades the ovaries and extragonadal regions.
-
•
We describe a pelvic side wall, clear cell carcinoma arising from endometriosis.
-
•
The disease may develop in patients with a history of endometriosis or pelvic surgery.
1. Introduction
Endometriosis is characterized by the presence of ectopic endometrial tissue. The condition, albeit benign, is often painful and a source of infertility in nearly 10–15% of reproductive-aged women (Kavoussi et al., 2016). Endometriosis originates from metaplasia of the coelomic epithelium and has the potential to infiltrate distant areas via hematogenous and lymphatic dissemination (Su et al., 2004).
Endometriosis has been identified in the ovaries, pelvis, cervix, labia and vagina (Agarwal & Subramanian, 2010); alternatively, uncommon implantation sites include the lung, bladder and within abdominal surgery scars (Acién & Velasco, 2013; Bodner et al., 2003). In very rare cases, the disease undergoes malignant transformation, with documented rates approximating 0.3% to 1.0% (Matter et al., 2003; Debus & Schuhmacher, 2001). When this neoplastic process arises, the disease typically is comprised of endometrioid or clear cell histology (Ruiz et al., 2015; Bats et al., 2008).
Endometriosis has been described in patients with a history of gynecologic surgery, namely following a caesarean section (Horton et al., 2008). Nevertheless, it is quite unusual to encounter a carcinoma originating from endometriosis in the abdominal wall (Marques et al., 2017; Taburiaux et al., 2015), let alone the pelvic side wall. Herein, we review a patient with clear cell carcinoma of the pelvic side wall, stemming from endometriosis.
2. Case report
A 63 year-old (gravida 1, para 1) woman initially presented with a suspected left inguinal hernia in May 2017; The patient's medical history was significant for hypertension and hypercholesterolemia. The initial herniorrhaphy was performed by a general surgeon who aborted the procedure when he ascertained that the condition was not an inguinal hernia but instead, a malignant condition. During the attempt at intraoperative repair, a necrotic, cancerous mass was encountered. Following a review of histologic and imaging data, the lesion was presumed to be ovarian cancer. The patient's preoperative CA-125 was 470 U/mL; she then commenced with neoadjuvant intravenous chemotherapy encompassing paclitaxel (80 mg/m2) on days 1, 8, and 15 of a 21-day cycle and carboplatin (AUC 6) on day 1.
After the completion of two cycles of chemotherapy, the patient was taken to the Operating Room and underwent an exploratory laparotomy, total abdominal hysterectomy and bilateral salpingo-oophorectomy, total omentectomy, bilateral pelvic lymph node dissection, bilateral inguinal lymph node dissection, radical dissection of the left inguinal ligament with radical tumor debulking and mesh reconstruction.
During an examination of the abdomen and pelvis, a 12 × 10 cm cystic and solid left inguinal mass was identified, which extended through the femoral canal into the abdominal cavity (Fig. 1). The entire neoplasm was retroperitoneal, with visible, gross tumor at the distal iliac vessels and in the medial left inguinal space. There was evidence of both left pelvic and inguinal lymphadenopathy; furthermore, a pathologically, enlarged right inguinal lymph node, measuring approximately 4 cm, was noted with an absence of pathologic right pelvic and peri-aortic adenopathy.
Fig. 1.

Large complex solid and cystic mass that is arising from the left hemipelvic sidewall, and extending to the inguinal region and skin surface.
The patient's appendix, small and large bowel were all palpably normal. There was no visible evidence of endometriosis on the uterus or ovaries. The left ovary was normal and adjacent to the large retroperitoneal pelvic and inguinal mass. The disease was noted to be densely adherent to the external iliac vessels and fixed to the surrounding tissue, with minimal mobility. An incision was made over the left groin and approximately 300 mL of dark bloody fluid was aspirated; thereafter, a large, solid and cystic mass in the medial groin incision was visualized.
The lesion was dissected, mobilized externally and ultimately delivered through the femoral canal into the abdomen. The remaining tumor and enlarged lymph nodes within the medial inguinal space were also excised. A left pelvic lymph node dissection, resection of proximal lymphatic tissue and removal of obturator nodes were also performed; this procedure was repeated on the contralateral side. Subsequently, a biologic mesh was inserted into the left pelvis, which was secured with 2–0 Vicryl and Tisseel. The right groin mass was completely excised; The patient tolerated the procedure well and was admitted to the Recovery Room in stable condition.
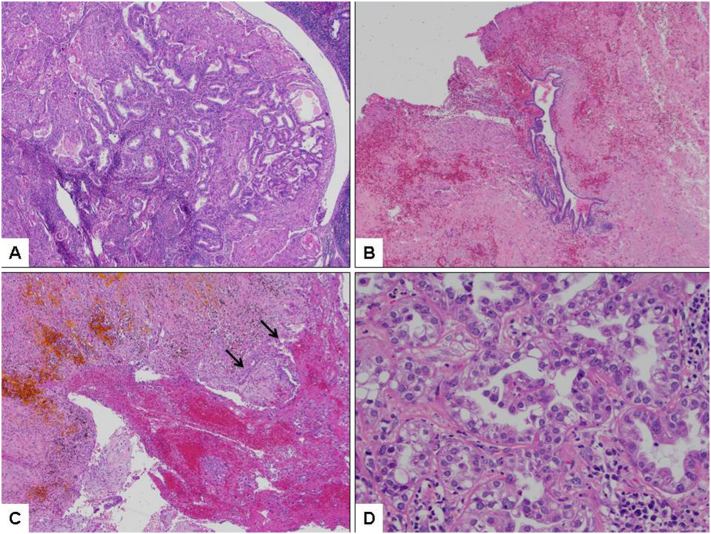
The final pathology revealed Stage IVB clear cell carcinoma arising from endometriosis with a synchronous but apparently unrelated stage IA endometrial adenocarcinoma (Fig. 2a–d). There were also 5 pelvic lymph nodes positive for malignancy. The disease was confined to the retroperitoneal/inguinal mass, bilateral inguinal nodes, and left pelvic lymph nodes. The uterus, cervix, tubes, ovaries, and right pelvic nodes were normal. Following a molecular biomarker evaluation, her tumor was determined to be MSI stable, effectively precluding Lynch syndrome. Postoperatively, the patient was slated for radiotherapy and completion of her chemotherapy regimen.
Fig. 2.
a) Representative histologic section of endometrial lesion showing FIGO grade 1 endometrioid-type adenocarcinoma with abundant squamous morules (H&E; 40×); b) Focus of endometriosis associated with pelvic side wall lesion (H&E; 40×); c) Area of presumed endometriosis with hemosiderin deposition in association with clear cell carcinoma (arrows; H&E; 40×); d) High-power view of clear cell carcinoma (pelvic side wall lesion) with variable eosinophilic cytoplasm (eosinophilic variant of clear cell carcinoma) (H&E; 400×).
3. Conclusions
Endometriosis undergoing malignant transformation is an extremely unusual finding. Sampson attributed this process to the disease's proximity to the neoplasm, exclusion of an additional primary site of malignancy, and the histologic appearance of endometrial stroma contiguous with epithelial glands (Debus & Schuhmacher, 2001; Sampson, 1925). Additionally, prolonged exposure to unopposed estrogen has been implicated (Agarwal & Subramanian, 2010; Debus & Schuhmacher, 2001).
When endometriosis transitions to a malignant pathology, the ovary is commonly involved (Agarwal & Subramanian, 2010). Conversely, abdominal wall carcinoma arising from endometriosis is exceedingly uncommon, with only 27 cases documented since 2014 (Taburiaux et al., 2015). Carcinoma of the pelvic side wall originating from endometriosis is seemingly more atypical, as we were only able to identify two patients in the literature with this condition (Leiserowitz et al., 2003).
In the current study, we review the history of a woman diagnosed with clear cell carcinoma of the pelvic side wall with metastatic involvement of the medial groin, stemming from endometriosis. Endometriosis has been identified as a precursor of clear cell carcinoma, which presumably accounts for the higher incidence of this histologic subtype (Taburiaux et al., 2015). The precipitant of the neoplastic event in the current study remains idiopathic, although one may conjecture that genetics, immunological or hormonal factors were contributing factors (Marques et al., 2017). We can ultimately preclude the influence of estrogen therapy since the patient did not have a history of taking this medication.
The clinical management of clear cell carcinoma of the pelvic side wall is indeterminate. Surgical treatment has reportedly been comprised of local resection, total abdominal hysterectomy or salpingo-oophorectomy (Ijichi et al., 2014). In addition to definitive surgery, a regimen of carboplatin and paclitaxel has been considered effective at treating this malignancy (Marques et al., 2017). Radiotherapy has also been reportedly beneficial in combination with platinum-based therapy for the treatment of endometriosis related cases of malignant transformation (Mihailovici et al., 2017); moreover, radiotherapy is potentially indicated when addressing metastatic disease (Macrie et al., 2014). Unfortunately, once a patient exhibits disease recurrence, secondary treatment is ostensibly ineffective (Ijichi et al., 2014).
When oncology physicians encounter primary and metastatic lesions in very unusual locations, they should consider the potential for malignant endometriosis in the differential diagnosis, especially in patients who have a history of endometriosis, underwent gynecologic surgery or were previously treated with hormone replacement therapy. Despite the remote incidence of pelvic side wall carcinoma arising from endometriosis, we recommend further study evaluating the optimal method in which to diagnose and manage this complicated disease.
Conflict of interest
All authors deny any conflict of interest associated with this manuscript.
Footnotes
This study was supported by the Women's Cancer Research Foundation.
References
- Acién P., Velasco I. Endometriosis: a disease that remains enigmatic. ISRN Obstet. Gynecol. 2013;2013 doi: 10.1155/2013/242149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal N., Subramanian A. Endometriosis - morphology, clinical presentations and molecular pathology. J. Lab. Phys. 2010;2:1–9. doi: 10.4103/0974-2727.66699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bats A.S., Zafrani Y., Pautier P., Duvillard P., Morice P. Malignant transformation of abdominal wall endometriosis to clear cell carcinoma: case report and review of the literature. Fertil. Steril. 2008;90 doi: 10.1016/j.fertnstert.2007.08.080. (1197.e13-6) [DOI] [PubMed] [Google Scholar]
- Bodner K., Zauner M., Bodner-Adler B., Spangler B., Grunberger W., Wierrani F. Parenchymatous pulmonary endometriosis - metastases of a low-grade endometrial stromal sarcoma? Med. Hypotheses. 2003;61:651–653. doi: 10.1016/s0306-9877(03)00268-8. [DOI] [PubMed] [Google Scholar]
- Debus G., Schuhmacher I. Endometrial adenocarcinoma arising during estrogenic treatment 17 years after total abdominal hysterectomy and bilateral salpingo-oophorectomy: a case report. Acta Obstet. Gynecol. Scand. 2001;80:589–590. [PubMed] [Google Scholar]
- Horton J.D., Dezee K.J., Ahnfeldt E.P., Wagner M. Bdominal wall endometriosis: a surgeon's perspective and review of 445 cases. Am. J. Surg. 2008;196:207–212. doi: 10.1016/j.amjsurg.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Ijichi S., Mori T., Suganuma I., Yamamoto T., Matsushima H., Ito F. Clear cell carcinoma arising from cesarean section scar endometriosis: case report and review of the literature. Case Rep. Obstet. Gynecol. 2014;2014 doi: 10.1155/2014/642483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavoussi S.K., Lim C.S., Skinner B.D., Lebovic D.I., As-Sanie S. New paradigms in the diagnosis and management of endometriosis. Curr. Opin. Obstet. Gynecol. 2016;28:267–276. doi: 10.1097/GCO.0000000000000288. [DOI] [PubMed] [Google Scholar]
- Leiserowitz G.S., Gumbs J.L., Oi R., Dalrymple J.L., Smith L.H., Ryu J. Endometriosis-related malignancies. Int. J. Gynecol. Cancer. 2003;13:466–471. doi: 10.1046/j.1525-1438.2003.13329.x. [DOI] [PubMed] [Google Scholar]
- Macrie B.D., Strauss J.B., Helenowski I.B., Rademaker A., Schink J.C., Lurain J.R. Patterns of recurrence and role of pelvic radiotherapy in ovarian clear cell adenocarcinoma. Int. J. Gynecol. Cancer. 2014;24:1597–1602. doi: 10.1097/IGC.0000000000000270. [DOI] [PubMed] [Google Scholar]
- Marques C., Silva T.S., Dias M.F. Clear cell carcinoma arising from abdominal wall endometriosis - brief report and review of the literature. Gynecol. Oncol. Rep. 2017;20:78–80. doi: 10.1016/j.gore.2017.03.008. (March 14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter M., Schneider N., McKee T. Cystadenocarcinoma of the abdominal wall following caesarean section: case report and review of the literature. Gynecol. Oncol. 2003;91:438–443. doi: 10.1016/j.ygyno.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Mihailovici A., Rottenstreich M., Kovel S., Wassermann I., Smorgick N., Vaknin Z. Endometriosis-associated malignant transformation in abdominal surgical scar: a PRISMA-compliant systematic review. Medicine. 2017;96 doi: 10.1097/MD.0000000000009136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz M.P., Wallace D.L., Connell M.T. Transformation of abdominal wall endometriosis to clear cell carcinoma. Case Rep. Obstet. Gynecol. 2015;2015:123740. doi: 10.1155/2015/123740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson J.A. Endometrial carcinoma of the ovary arising in endometrial tissue in that organ. Arch. Surg. 1925;10:1–72. [Google Scholar]
- Su H.Y., Chen W.H., Chen C.H. Extra-pelvic endometriosis presenting as a vulvar mass in a teenage girl. Int. J. Gynaecol. Obstet. 2004;87:252–253. doi: 10.1016/j.ijgo.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Taburiaux L., Pluchino N., Petignat P., Wenger J.M. Endometriosis-associated abdominal wall cancer: a poor prognosis? Int. J. Gynecol. Cancer. 2015;25:1633–1638. doi: 10.1097/IGC.0000000000000556. [DOI] [PubMed] [Google Scholar]