Abstract
ApoC-III is a critical cardiovascular risk factor, and humans expressing null mutations in apoC-III are robustly protected from cardiovascular disease. Because of its critical role in elevating plasma lipids and CVD risk, hepatic apoC-III regulation has been studied at length. Considerably less is known about the factors that regulate intestinal apoC-III. In this work, we use primary murine enteroids, Caco-2 cells, and dietary studies in wild-type mice to show that intestinal apoC-III expression does not change in response to fatty acids, glucose, or insulin administration, in contrast to hepatic apoC-III. Intestinal apoC-III is not sensitive to changes in FoxO1 expression (which is itself very low in the intestine, as is FoxO1 target IGFBP-1), nor is intestinal apoC-III responsive to western diet, a significant contrast to hepatic apoC-III stimulation during western diet. These data strongly suggest that intestinal apoC-III is not a FoxO1 target and support the idea that apoC-III is not regulated coordinately with hepatic apoC-III, and establishes another key aspect of apoC-III that is unique in the intestine from the liver.
Keywords: FoxO1, Intestine, Dietary fat, Chylomicrons, Dietary fat absorption, Lipoproteins, Apolipoprotein
1. Introduction
Apolipoprotein C-III (apoC-III) is expressed in both liver and the intestine, and is secreted from these tissues on triglyceride-rich lipoproteins (TRLs) [1–3]. ApoC-III was first established as an inhibitor of lipoprotein lipase (LPL), and it has been more recently established that the most robust role of apoC-III is likely its inhibition of hepatic low-density lipoprotein receptor (LDLR) [4–6]. These inhibitory actions of apoC-III increase the plasma residence time of TRLs and their remnants. In humans, plasma apoC-III levels are independently associated with both an increase in plasma triglycerides and CVD risk [7,8,25].
Elevated plasma triglycerides are an independent risk factor for cardiovascular disease (CVD), the leading cause of mortality in the United States. The importance of apoC-III in human CVD incidence has been well-established: in patients with the R19X null mutation in apoC-III, CVD incidence is significantly reduced, whereas in patients with elevations in plasma apoC-III, CVD incidence in robustly increased [9–14].
We have recently established an intestinal role for apoC-III. We find that overexpression of apoC-III results in a delay in dietary lipid absorption and causes the secretion of smaller chylomicrons [15,16]. This is paradoxical to both the triglyceride-raising role apoC-III plays in the plasma, and cell culture data suggesting that intracellular apoC-III promotes the synthesis and secretion of larger, more triglyceride-rich VLDL from the liver [17,18,27].
Research on the mechanisms that control apoC-III gene expression has focused on hepatic apoC-III. In primary rat hepatocytes, glucose upregulates apoC-III transcription by activating transcription factors HNF-4α and ChREBP, which bind E-boxes found in the proximal C3P footprint in the apoC-III promoter [19]. Conversely, insulin down-regulates hepatic apoC-III through phosphorylation and nuclear exclusion of the transcription factor Forkhead box O1 (FoxO1) [20]. Furthermore, hepatic apoC-III mRNA is significantly elevated in mice with streptozotocin-induced insulin deficiency [21]. In liver, polyunsaturated fatty acids inhibit FoxO1 expression and its target genes in liver, including apoC-III [22].
While the regulation of hepatic apoC-III gene expression has been well studied, the factors that regulate apoC-III in the intestine are still largely unknown. In Caco-2 cells overexpressing constitutively active FoxO1, apoC-III mRNA is significantly increased [20]. However, little is known about the role of physiological levels of FoxO1 in apoC-III regulation in the intestine. Unlike hepatic apoC-III, which is inhibited by long chain polyunsaturated fatty acids [22], it is unknown how intestinal apoC-III expression responds to dietary fat.
Since we have previously established that the role of apoC-III in the intestine differs significantly from that in the liver, the purpose of this study is to establish the in vivo regulation of intestinal apoC-III.
2. Methods
2.1. Animals
Male and female C57Bl/6J mice (Jackson Laboratories, Bar Harbor, ME), 8–12 weeks old, were housed 3–4 per cage in a temperature-controlled (23 ± 1 °C) vivarium on a 12-h light-dark cycle. Mice received free access to water, and were maintained on either standard rodent chow (Teklad global cat.#2918) or western diet (42% kcal from fat, 0.2% cholesterol by weight) from EnvigoTD.88137, for 12 weeks. Animals were sacrificed under isoflurane anesthesia following an overnight fast. For gavage studies, mice continued on chow diet and were gavaged at the same time daily for one week with saline, corn oil (60 μL), or corn oil (60 μL) with glucose (135 μL of 5 M glucose). Each gavage was made isovolumetric (195 μL) using saline. Mice were fasted overnight on the last day before they were sacrificed. All animal procedures were performed in accordance with the University of Connecticut Internal Animal Care and Use Committee and in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Caco-2 cells
Caco-2 cells were used for experiments at 17d post-confluence [23]; this protocol produces the most abundant apoB-lipoproteins. Cells were kept at 37 °C with 5% CO2 in Eagle's MEM (Corning Cellgro #10-010-CM), 10% FBS, 1% L-glutamine, penicillin/ streptomycin, sodium pyruvate, non-essential amino acids, and 1.5 g/L sodium bicarbonate. For experiments, cells were cultured in low-glucose (1 g/L glucose) or high-glucose DMEM (4.5 g/L) +/− human insulin (final concentration 80 nM, Sigma, St. Louis, MO. cat# I9278). Cells were serum starved overnight prior to treatments and then treated with 2 mL of treatment media in each well for 24 h. Fatty acid treatment: Cells were incubated with fatty acid growth medium for 4 h, washed, then incubated for 24h in fresh media prior to protein and RNA isolation.
2.3. Enteroid culture
We isolated intestinal stem cells (ISCs) from crypts as described previously [16,24]. Primary crypts were isolated from WT mice, age 8–12 weeks. Crypts were placed in Matrigel; following 30min polymerization, crypts were treated with 500 μl of enteroid medium (Advanced DMEM/F12 (12634- 010; Life Technologies, Carlsbad, CA, USA) with 2 mM L-Glutamine,10 mM HEPES, 100U/mL penicillin/100 μg/mL streptomycin and 1× N2 and 1× B27 supplements, plus 1 μL of R-spondin 1 (250 μg/mL), 1 μL of Noggin (50 μg/mL) and 0.25 μL of EGF (100 μg/mL). Media was replaced every 3 days. For treatment with lipid, mature enteroids were dissociated from Matrigel by washing with ice cold DPBS, followed by a 150 × g spin for 10 min. After removing the supernatant, the intact enteroids were then placed in 1 ml of treatment media containing 400 μM OA: BSA or BSA alone, or enteroid growth media containing glucose, or enteroid growth media containing insulin; all media contained Rho-kinase inhibitor. The enteroids were very gently opened by pipetting up and down with a p1000 pipette, followed by incubation with the lids open in a 37° 5% CO2 incubator for 2 h. After 2 h, the enteroids were centrifuged at 150 × g for 10 min and the supernatant collected. Following an additional wash and centrifuge with 1 ml of DPBS (which was added to the media samples), the enteroids were resuspended in 1 ml of enteroid growth media and placed back in the incubator for 6 h, with the lid to their tubes left open for gas exchange. The media and cell pellet were then collected via centrifugation at 150 × g for 10 min.
2.4. Preparation of BSA-bound FFA
We treated the enteroids and Caco-2 cells with BSA-bound FFA. Oleic acid (Nu-Check Prep) was prepared as 4 mM stock solutions in complex with fatty acid-free bovine serum albumin (BSA) at a 1:4 M ratio and the stock contained butylated-hydroxytoluene 0.1% [19]. Cells not receiving the 400 μM OA: BSA complex were treated with an equivalent amount of BSA.
2.5. Immunofluorescence
Enteroids were grown in chamberglass wells and fixed with 4% PFA in PBS for 30 min, followed by 50 mM NH4Cl in PBS for 30min to quench autofluorescence. Cells were washed and blocked in 5%BSA in PBST overnight at 4°C. Fixed enteroids were incubated with primary antibody (anti-apoB, 1:100, Abcam #ab20737) for 3 days at 4°C. Followed by incubation with secondary Alexa 488 (1:500, Abcam #ab150065) overnight at 4°C. Enteriods were stained with Hoechst 33342 (10 μg/ml in PBST). Confocal images were captured with NiKon A1R (20X and 60× water objective lens).
2.6. Gene expression
RNA was isolated from Caco-2 cells and enteroids, and isolated tissues with Trizol. RNA pellets were dissolved in nuclease-free dH2O before concentration was determined by BioTek Epoch spectrophotometer. cDNA was synthesized with iScript cDNA synthesis kit (BioRad, #1708890). Quantitative real-time polymerase chain reaction (PCR) used CFX Connect real time system (BioRad) and iTaq SYBR® Green Supermix (Bio-Rad). Human RPLPO was used as the reference gene in the Caco-2 studies. Mouse cyclophilin was used as the reference gene in dietary studies. Gene expression was calculated using the comparative threshold cycle method.
2.7. Plasma lipid and glucose analysis
For plasma lipid concentrations, Randox (catalog # TR210) triglyceride and total cholesterol assays (catalog # CH200) were used, in fasted mice (6pm–6am). Plasma glucose was measured in the fasted state, using liquid glucose oxidase assay (Pointe Scientific, Canton, MI G7521-120).
2.8. Statistics
All data are presented as the mean ± the SEM. Statistics were performed using GraphPad Prism (version 6.0). Differences were analyzed by Student's t-test. Analyses of more than 1 experimental group compared to control used one-way ANOVA. Differences were considered significant at P < 0.05.
3. Results
3.1. In murine intestinal enteroids, apoC-III is not regulated by glucose, insulin, or fatty acid
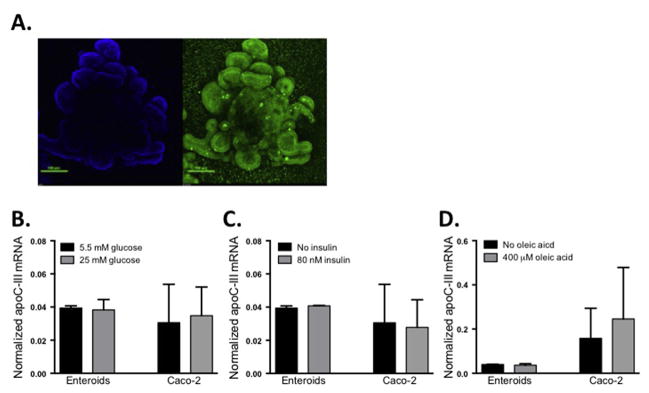
Although previous studies have determined that insulin [20], glucose [19], and fatty acids [22] regulate apoC-III expression in the liver, little is known about intestinal apoC-III regulation. To determine how intestinal apoC-III is regulated, we utilized a primary intestinal enteroid culture system. Primary enteroids are derived from WT mouse duodenal and jejunal crypt stem cells, and we have previously established that this tissue culture system significantly improves upon Caco-2 cells as a model for dietary fat absorption and chylomicron secretion [16]. In Fig. 1A we show the 3-dimensional architecture of mature primary enteroids, with apoB-positive cells surrounding a central lumen (reflecting the in vivo architecture of the intestine). Because hepatic apoC-III is regulated by both glucose and insulin, we tested the hypothesis that these conditions may also regulate intestinal apoC-III. Treatment of enteroids with glucose or insulin did not alter intestinal apoC-III expression in the enteroids (Fig. 1B and C). Since apoC-III is secreted from enterocytes on chylomicrons (a process that is stimulated by dietary fat), we hypothesized that intestinal apoC-III would also be responsive to dietary fatty acids4. After incubation of primary enteroids with oleic acid (Fig. 1D), we determined that intestinal apoC-III mRNA expression is not stimulated by this fatty acid. We confirmed these results in Caco-2 cells, since it has been previously shown that Caco-2 cells transfected with FoxO1, have decreased apoC-III expression in response to increasing insulin concentrations [20]. As in our enteroid culture studies, incubation with glucose, insulin, or oleic acid did not alter apoC-III expression in Caco-2 cells. Taken together, these results indicate in both primary intestinal enteroids and Caco-2 cells, that apoC-III mRNA is uniquely non-responsive to changes in glucose, insulin, or fatty acids. This contrasts with the well-established regulation of hepatic apoC-III by these factors.
Fig. 1. ApoC-III mRNA expression in primary murine enteroids or Caco-2 cells is not affected by treatment with glucose, insulin, or oleic acid.
(A) Primary duodenal murine enteroids were cultured for 10 days. Enteroids were fixed and stained with Hoechst stain (blue: nuclei) and anti-apoB (green) to visualize the 3D architecture of the enteroid culture. Enteroid and Caco-2 cell (cultured for 17d post-confluence) apoC-III mRNA expression in response to (B) glucose, (C) insulin, (D) BSA bound oleic acid. Bars represent mean apoC-III expression ±SEM, n = 3–7. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. FoxO1 expression is low in the intestine
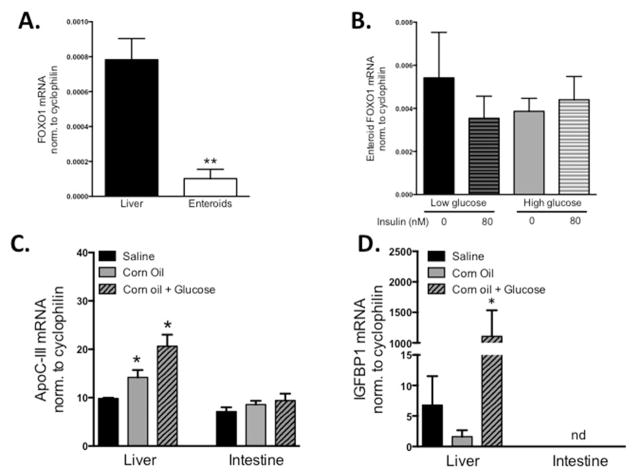
Since we saw no changes in intestinal apoC-III expression in response to glucose, insulin, and fatty acid, we asked whether intestinal apoC-III is a target of FoxO1 inhibition in the intestine. We find that in comparison to hepatic FoxO1 mRNA expression, its expression in duodenal enteroids is low to undetectable (Fig. 2A). We also find that treating primary enteroids with insulin, in the presence of either low or high glucose, does not change FoxO1 mRNA expression (Fig. 2B).
Fig. 2. FoxO1 mRNA expression.
A) FoxO1 mRNA expression in WT mouse liver (fasted) and mature murine duodenal enteroids FOXO-1, normalized to cyclophilin. (B) FoxO1 mRNA expression in mature murine duodenal enteroids when treated for 6-h with insulin, in both low glucose (5.5 mM) and high glucose (25 mM) media. n = 3–5. (C) ApoC-III mRNA expression in liver or intestine in response to 1 week of daily gavages of isovolumetric saline, corn oil (60 μL), or corn oil (60 μL) with glucose (135 μL of 5 M glucose). (D) IGFBP-1 mRNA expression in liver or intestine of gavaged mice. Bars represent ±SEM of normalized mRNA expression, *P < 0.05 versus saline, not detectable (nd), n = 3–4 WT mice per gavage group.
3.3. In vivo, intestinal apoC-III mRNA expression is not regulated in response to gavaged lipid and glucose
To further explore the differences in acute hepatic and intestinal apoC-III regulation in response to diet, we took an in vivo approach. We gavaged WT mice daily for one week with either saline, corn oil, or corn oil + plus glucose. After the treatment period, hepatic apoC-III expression increased with both the corn oil and corn oil + glucose treatment, confirming that hepatic apoC-III expression is regulated by these dietary nutrients (Fig. 2C). In contrast, intestinal apoC-III expression did not change under any treatment condition, further corroborating the cell culture findings (Fig. 2C). Since FoxO1 is regulated by both expression, phosphorylation, and nuclear exclusion, we measured the expression of canonical FoxO1 target gene, insulin-like growth factor binding protein 1 (IGFBP-1), in response to the gavaged nutrients (Fig. 2D). We find that in the liver, corn oil + glucose robustly stimulate IGFBP-1, in parallel with apoC-III expression (and suggesting that FoxO1 is active under these conditions). In contrast, IGFBP-1 is non-detectable in the intestine in response to gavage.
3.4. Western diet stimulates hepatic apoC-III expression but does not alter intestinal apoC-III expression
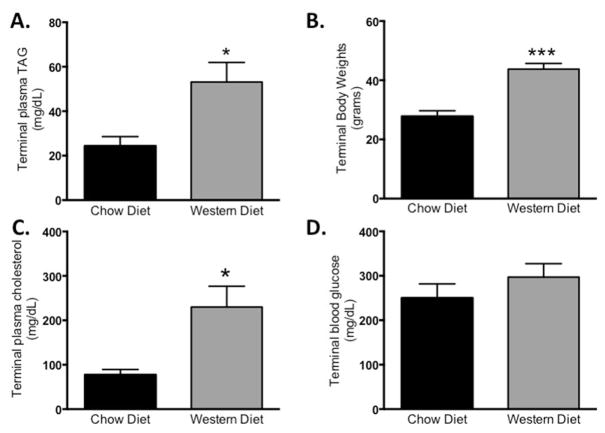
Western diet is known to increase plasma triglycerides, and the expression of genes in the liver that increase CVD risk. It is unknown to what extent the increase in plasma triglycerides are due to an increase in intestinal or hepatic apoC-III. We challenged WT mice for 12 weeks with western diet, consisting of 42% calories from butterfat and 0.2% total cholesterol, and compared these to mice provided standard chow diet. In response to western diet, mice have a significant increase in body weight compared to chow-fed controls (Fig. 3A). As expected, WT mice on the western diet had an approximately 2-fold increase in plasma TAG (53.15 mg/dL versus 24.37 mg/dL; p = 0.01. Fig. 4B) and an approximately 3-fold increase in plasma cholesterol (230 mg/dL versus 77 mg/dL; p = 0.01. Fig. 3C). Plasma glucose was not significantly increased in response to the western diet (Fig. 3D). Suggesting that the mice do not lose insulin-tolerance after the 12 week western diet.
Fig. 3. Western type diet alters plasma lipids and increases body weight compared to a chow diet.
Mice were provided free access to chow diet or western diet (42% kcal from butterfat, 0.2% cholesterol by weight) for 12 weeks. (A) Fasting plasma triglycerides, (B) terminal body weights, (C) fasting plasma cholesterol, and (D) fasting blood glucose in chow versus western diet-fed mice. Data represent mean ± SEM. *P < 0.05, ***P < 0.001, n = 5/diet.
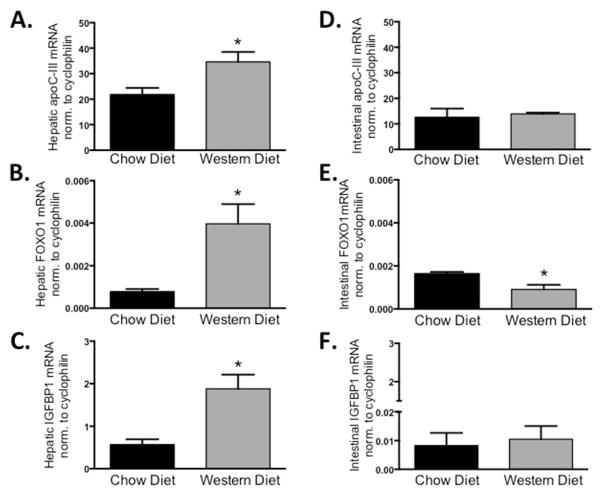
Fig. 4. Western diet increases hepatic but not intestinal apoC-III.
Hepatic mRNA expression of (A) apoC-III, (B) FoxO1 and (C) IGFBP-1 expression in chow-fed versus WTD-fed mice. Intestinal expression of (D) apoC-III, (E) FoxO1 and (F) IGFBP-1. Bars represent ±SEM of normalized mRNA expression, *P < 0.05 versus chow-fed, n = 5/diet.
In response to the western diet, hepatic apoC-III expression is significantly higher compared to chow-fed controls (Fig. 4A), and both hepatic FoxO1 mRNA and its canonical target gene, IGFBP1, are also increased in parallel to apoC-III (Fig. 4B and C). In contrast to these hepatic changes, western diet did not alter the expression of intestinal apoC-III (Fig. 4D). Interestingly, intestinal FoxO1 expression was decreased in the intestine and IGFBP1 is not changed (Fig. 4E and F). These data confirm that apoC-III is not regulated in the intestine, as it is in the liver, and strongly suggests that FoxO1 is not responsible for intestinal apoC-III mRNA regulation, in contrast to its importance in regulating apoC-III in the liver.
4. Discussion
In these studies, we have focused on dietary lipid and glucose, since these were previously identified as major drivers of apoC-III expression in liver [20,26,28]. Because we have identified a unique consequence of apoC-III overexpression in the intestine (the inhibition of dietary fat absorption coupled with altered chylomicron secretion) [15,16], and because regulation of apoC-III in Caco-2 cell culture is so far removed from in vivo conditions that might regulate apoC-III, we wanted to establish the critical dietary factors in the regulation of intestinal apoC-III.
Dong et al. previously established that hepatic apoC-III is a target of FoxO1, through the −498/−403 element in the APOC3 promoter [26]. They showed that deletion of this consensus IRE causes unrestrained apoC-III expression during insulin-resistance and diabetes, leading to hypertriglyceridemia. As part of their assessment of FoxO1 and apoC-III, Dong et al. also provide evidence from Caco-2 cells overexpressing constitutively active FoxO1 that apoC-III expression is also under the control of FoxO1 in the intestine. They suggest that apoC-III is coordinately regulated in both intestine and liver.
Instead of Caco-2 cells, here we have used primary murine enteroids, isolated and cultured from duodenal stem cells. We have established that these duodenal enteroids absorb fatty acid and form chylomicrons, which mirrors the small intestine. This primary culture system significantly improves upon Caco-2 cells because of their 3D architecture, with enterocytes arranged around a central lumen, and a basolateral surface facing the media. Therefore, the lack of apoC-III regulation in response to individual stimuli in this culture system strongly supports the idea that intestinal apoC-III, within the in vivo context, is not regulated by dietary factors in parallel with the liver.
We find that FoxO1 expression in mouse intestine is extremely low, and treatment with insulin changes this very slightly. This is in contrast to Dong et al. However, our data support recent work by Accili et al. [29], who have established a role of FoxO1 in the intestine in endocrine progenitor and serotonin-producing cells. Accili et al. use immunohistochemistry for a detailed analysis of FoxO1 expression in the human gut. They establish that FoxO1 is enriched in the crypt bottoms, and specifically co-localizes with serotonin (5HT)-positive endocrine (rather than absorptive enter-ocyte) cells. Our data in intact mouse intestine supports this finding because we see such low expression in whole tissue (endocrine cells are vastly outnumbered by enterocytes). Primary enteroids also maintain a small population of enteroendocrine cells, which is likely the source of FoxO1 expression in our cultures. The restriction of FoxO1 to endocrine cells in the intestine would preclude it from directly regulating apoC-III expression in the absorptive epithelium, though this does not necessarily mean that endocrine cells don't play an important role in modulating the enterocyte function.
Why does it matter that intestinal apoC-III in not regulated by western diet, or through the action of FoxO1? Our findings are additional support for the notion that while the liver and intestine share the role of triglyceride-rich lipoprotein synthesis and secretion, they do not share identical regulatory pathways for proteins that are involved. Our data also support the notion that apoC-III secretion from the intestine is under a unique set of pressures. Whereas the liver can quite significantly change VLDL secretion rates in response to the presence or absence of substrate and hormones, the intestine is less labile in the face of dietary lipid, which is quickly and efficiently secreted in chylomicrons. Our data support the idea that apoC-III is not regulated coordinately with hepatic apoC-III, and establishes another key aspect of apoC-III that is unique in the intestine from the liver.
Acknowledgments
This work was funded by grants to ABK: NIH (DK101663), USDA NIFA (11874590), and USDA Hatch Formula Funds (2015-31200-06009).
Abbreviations
- apo
apolipoprotein
- FFA
free fatty acids
- TAG
triacylglycerol
- VLDL
very-low-density lipoproteins
- TRLs
triglyceride-rich lipoproteins
- HDL
high-density lipoproteins
- CM
chylomicron
Footnotes
Conflicts of interest
The authors have no conflicts of interest to report.
Transparency document
Transparency document related to this article can be found online at http://dx.doi.org/10.1016/j.bbrc.2017.07.116.
References
- 1.Haddad IA, Ordovas JM, Fitzpatrick T, Karathanasis SK. Linkage, evolution, and expression of the rat apolipoprotein A-I, C-III, and A-IV genes. J Biol Chem. 1986 Oct 05;261:13268–13277. [PubMed] [Google Scholar]
- 2.Wu AL, Windmueller HG. Identification of circulating apolipoproteins synthesized by rat small intestine in vivo. J Biol Chem. 1978 Apr 25;253:2525–2528. [PubMed] [Google Scholar]
- 3.Zheng C, Khoo C, Ikewaki K, Sacks FM. Rapid turnover of apolipoprotein C-III-containing triglyceride-rich lipoproteins contributing to the formation of LDL subfractions. J Lipid Res. 2007;48:1190–1203. doi: 10.1194/jlr.P600011-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Aalto-Setala K, Fisher EA, Chen X, Chajek-Shaul T, Hayek T, Zechner R, Walsh A, Ramakrishnan R, Ginsberg HN, Breslow JL. Mechanism of hypertriglyceridemia in human apolipoprotein (apo) CIII transgenic mice. Diminished very low density lipoprotein fractional catabolic rate associated with increased apo CIII and reduced apo E on the particles. J Clin Invest. 1992 Nov 01;90:1889–1900. doi: 10.1172/JCI116066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aalto-Setälä K, Weinstock PH, Bisgaier CL, Wu L, Smith JD, Breslow JL. Further characterization of the metabolic properties of triglyceride-rich lipoproteins from human and mouse apoC-III transgenic mice. J Lipid Res. 1996;37:1802–1811. [PubMed] [Google Scholar]
- 6.Gordts PLSM, Nock R, Son NH, Ramms B, Lew I, Gonzales JC, Thacker BE, Basu D, Lee RG, Mullick AE, et al. ApoC-III inhibits clearance of triglyceride-rich lipoproteins through LDL family receptors. J Clin Invest. 2016;126:2855–2866. doi: 10.1172/JCI86610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohn JJSJ, Tremblay M, Batal R, Jacques H, Rodriguez C, Steiner G, Mamer O, Davignon J. Increased apoC-III production is a characteristic feature of patients with hypertriglyceridemia. Atherosclerosis. 2004;177:137–145. doi: 10.1016/j.atherosclerosis.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Olivieri O, Stranieri C, Bassi A, Zaia B, Girelli D, Pizzolo F, Trabetti E, Cheng S, Grow MA, Pignatti PF, et al. ApoC-III gene polymorphisms and risk of coronary artery disease. J Lipid Res. 2002;43:1450–1457. doi: 10.1194/jlr.m200145-jlr200. [DOI] [PubMed] [Google Scholar]
- 9.Sacks FM, Alaupovic P, Moye La, Cole TG, Sussex B, Stampfer MJ, Pfeffer Ma, Braunwald E. VLDL, apolipoproteins B, CIII, and E, and risk of recurrent coronary events in the cholesterol and recurrent events (CARE) trial. Circulation. 2000;102:1886–1892. doi: 10.1161/01.cir.102.16.1886. [DOI] [PubMed] [Google Scholar]
- 10.Jensen MK, Rimm EB, Furtado JD, Sacks FM. Apolipoprotein C-III as a potential modulator of the association between HDL-cholesterol and incident coronary heart disease. J Am Heart Assoc. 2012;1 doi: 10.1161/JAHA.111.000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onat A, Hergenç G, Sansoy V, Fobker M, Ceyhan K, Toprak S, Assmann G. Apolipoprotein C-III, a strong discriminant of coronary risk in men and a determinant of the metabolic syndrome in both genders. Atherosclerosis. 2003;168:81–89. doi: 10.1016/s0021-9150(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 12.Pollin TIT, Damcott CCMCCM, Shen H, Ott SSH, Shelton J, Horenstein RB, Post W, McLenithan JCJJCJ, Bielak LF, Peyser PA, et al. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Sci (80- ) 2008;322:1702–1705. doi: 10.1126/science.1161524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crosby J, Peloso GM, Auer PL, Crosslin DR, Stitziel NO, Lange LA, Lu Y, Tang Z, Zhang H, Hindy G, et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371:22–31. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. Loss-of-Function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371:32–41. doi: 10.1056/NEJMoa1308027. [DOI] [PubMed] [Google Scholar]
- 15.Wang F, Kohan AB, Dong HH, Yang Q, Xu M, Huesman S, Lou D, Hui DY, Tso P. Overexpression of apolipoprotein C-III decreases secretion of dietary triglyceride into lymph. Physiol Rep. 2014;2:e00247. doi: 10.1002/phy2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jattan JJ, Rodia CN, Li D, Diakhate AC, Dong H, Bataille AM, Shroyer NF, Kohan AB. Using murine-derived primary intestinal enteroids for studies of dietary triglyceride absorption and lipoprotein synthesis, and to determine the role of intestine-specific apoC-III. J Lipid Res. 2017 doi: 10.1194/jlr.M071340. jlr.M071340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong S, Khalil MB, Links PH, Zhao Y, Iqbal J, Hussain M, Parks RJ, Wang Y, Yao Z. Expression of apolipoprotein C-III in McA-RH7777 cells enhances VLDL assembly and. J Lipid Res. 2010;51 doi: 10.1194/jlr.M900346-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin W, Sundaram M, Wang Y, Zhou H, Zhong S, Chang CC, Manhas S, Yao EF, Parks RJ, McFie PJ, et al. Missense mutation in APOC3 within the C-terminal lipid binding domain of human ApoC-III results in impaired assembly and secretion of triacylglycerol-rich very low density lipoproteins: evidence that ApoC-III plays a major role in the formation of lipid p. J Biol Chem. 2011;286:27769–27780. doi: 10.1074/jbc.M110.203679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caron S, Verrijken A, Mertens I, Samanez CH, Mautino G, Haas JT, Duran-Sandoval D, Prawitt J, Francque S, Vallez E, et al. Transcriptional activation of apolipoprotein CIII expression by glucose may contribute to diabetic dyslipidemia. Arterioscler Thromb Vasc Biol. 2011;31:513–519. doi: 10.1161/ATVBAHA.110.220723. [DOI] [PubMed] [Google Scholar]
- 20.Altomonte J, Cong L, Harbaran S, Richter A, Xu J, Meseck M, Dong HH, Nauli AM, Sun Y, Whittimore JD, et al. Foxo1 mediates insulin action on apoC-III and triglyceride metabolism. J Clin … Elsevier Inc. 2004;114:1493–1503. doi: 10.1172/JCI19992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen M, Breslow J, Li W, Leff T. Transcriptional regulation of the apoC-III gene by insulin in diabetic mice: correlation with changes in plasma triglyceride levels. J Lipid Res. 1994;35:1918–1924. [PubMed] [Google Scholar]
- 22.Chen Y-J, Chen C-C, Li T-K, Wang P-H, Liu L-R, Chang F-Y, Wang Y-C, Yu Y-H, Lin S-P, Mersmann HJ, et al. Docosahexaenoic acid suppresses the expression of FoxO and its target genes. J Nutr Biochem. 2012;23:1609–1616. doi: 10.1016/j.jnutbio.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Nauli AM, Sun Y, Whittimore JD, Atyia S, Krishnaswamy G, Nauli SM, Albers JJ, Kennedy H, Marcovina SM, Chantret I, et al. Chylomicrons produced by Caco-2 cells contained ApoB-48 with diameter of 80-200 nm. Physiol Rep. 2014;2:192–196. doi: 10.14814/phy2.12018. Physiological Reports. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. Nature. Vol. 459. Nature Publishing Group; 2009. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche; pp. 262–265. [DOI] [PubMed] [Google Scholar]
- 25.Mendivil CO, Rimm EB, Furtado J, Chiuve SE, Sacks FM. Low-density lipoproteins containing apolipoprotein C-III and the risk of coronary heart disease. Circulation. 2011;124:2065–2072. doi: 10.1161/CIRCULATIONAHA.111.056986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altomonte J, Cong L, Harbaran S, Richter A, Xu J, Meseck M, Dong HH. Foxo1 mediates insulin action on apoC-III and triglyceride metabolism. 2004;114:1493–1503. doi: 10.1172/JCI19992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao Z, Wang Y. Apolipoprotein C-III and hepatic triglyceride-rich lipoprotein production. Curr Opin Lipidol. 2012;23:206–212. doi: 10.1097/MOL.0b013e328352dc70. [DOI] [PubMed] [Google Scholar]
- 28.Caron S, Staels B. Apolipoprotein CIII: a link between hypertriglyceridemia and vascular dysfunction? Circ Res. 2008;103:1348–1350. doi: 10.1161/CIRCRESAHA.108.189860. [DOI] [PubMed] [Google Scholar]
- 29.Bouchi R, Foo KS, Hua H, Tsuchiya K, Ohmura Y, Sandoval PR, Ratner LE, Egli D, Leibel RL, Accili D. FOXO1 inhibition yields functional insulin-producing cells in human gut organoid cultures. Nat Commun. 2014;5:4242. doi: 10.1038/ncomms5242. [DOI] [PMC free article] [PubMed] [Google Scholar]