Abstract
We have identified a hydrated bicarbonate formed by chemisorption of 13CO2 on both dimethylaminopropylsilane (DMAPS) and aminopropylsilane (APS) pendant molecules grafted on SBA-15 mesoporous silica. The most commonly-used sequence in solid-state NMR, 13C CPMAS, failed to detect bicarbonate in these solid amine sorbent samples; here, we have employed a Bloch decay (“pulse-acquire”) sequence (with 1H decoupling) to detect such species. The water that is present contributes to dynamic motion of the bicarbonate product, thwarting CPMAS but enabling direct 13C detection by shortening the spin-lattice relaxation time. Since solid-state NMR plays a major role in characterizing chemisorption reactions, these new in-sights that allow for the routine detection of previously elusive bicarbonate species (which are also challenging to observe in IR spectroscopy) represents an important advance. We note that employing this straightforward NMR technique can reveal the presence of bicarbonate that has often otherwise been overlooked, as demonstrated in APS, that has been thought to only contain adsorbed CO2 as carbamate and carbamic acid species. As in other systems (e.g. proteins), dynamic species that sample multiple environments tend to broaden as their motion is frozen out. Here, we show two distinct bicarbonate species upon freezing, and coupling to different protons is shown through preliminary 13C-1H HETCOR measurements. This work demonstrates that bicarbonates have likely been formed in the presence of water but have gone unobserved by NMR due to the nature of the experiments most routinely employed, perspective that will transform the way the sorption community will view CO2 capture by amines.
Graphical Abstract
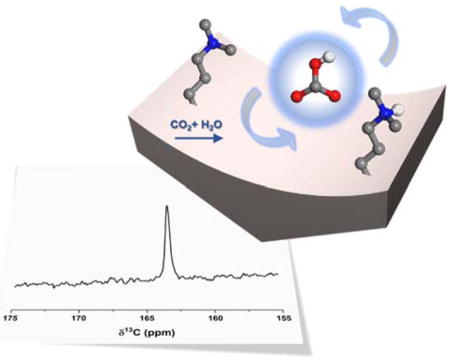
Solid amine sorbents capture CO2 with relatively lower regeneration energy and are less corrosive to equipment compared to existing aqueous amine solution.1 However, the mechanism(s) of solid amines reacting with CO2 are still not fully understood. Three chemisorbed species are proposed based on well-studied reactions with amines: carbamic acid, carbamate and bicarbonate.2,3 Carbamic acid and carbamate formed on amines grafted on mesoporous silica SBA-15 have been observed by IR and solid-state NMR (SSNMR) numerous times.4–11
The formation of bicarbonate in CO2 chemisorption reactions on solid amines has been debated in the past.7,12–15 Solid-state NMR is used extensively to characterize chemisorption products because it can determine structures of these non-crystalline systems and can be employed in a manner that leads to quantification of products, as well as the consumption of reactants, and can detect side products that are formed as well.16–19 In NMR in particular, the bicarbonate 13C chemical shift appears at a range of values, depending on the environment that contributes to the shielding experienced by the 13C nucleus, and hence is sensitive to surface pH.20 As a result, bicarbonate moieties may appear at different frequencies in similar samples, and the NMR resonance may even be masked by that of more prominent carbamate or carbamic acid resonances.21 Carbonate (CO32−) is not observed here, because conditions that favor its formation (pH > 11) are not present.
In this work, dimethylaminopropylsilane species (DMAPS) grafted onto SBA-15 was selected as an ideal “model” system for characterization by solid-state NMR. (Scheme 1.) While tertiary amines such as DMAPS are not of practical importance in carbon capture applications due to their low CO2 uptake, they have been chosen for study here because they can only adsorb CO2 by a bicarbonate route in the presence of water. The highly-utilized (and studied) primary and secondary amines (such as APS) can adsorb CO2, forming both carbamate and bicarbonate species. For DMAPS there is no obfuscation of the bicarbonate signal with that of carbamate or carbamic acid, which is critical since all three moieties appear in a similar chemical shift region and are therefore very difficult to discriminate from one another.22,23
Scheme 1.
Proposed reaction of CO2- with DMAPS.
Figure 1 shows 13C{1H} NMR spectra for 13CO2-reacted DMAPS that was dampened substantially with water prior to gas exposure (a detailed description of the sample preparation is given in the Supporting Information.) Figure 1a is the result of a room temperature Bloch decay magic-angle spinning (MAS) NMR experiment and shows a very narrow (for solid-state NMR) 270 Hz 13C resonance centered at 163 ppm (experiments with and without 1H decoupling were identical; here 1H de-coupling is included for all experiments for consistency). Figure 1b is a 13C{1H} CPMAS spectrum and shows that no signal is observed. (Note: a very small signal can be observed on occasion, with no discernable correlation to experimental conditions). These affects are similar to studies of carbonate in biomineral interfaces.24,25 Below, are two low-temperature 100 K NMR experiments: 1c) is 13C{1H} Bloch decay MAS NMR, and 1d) is a 13C{1H} CPMAS experiment. In both, the single room-temperature resonance that was narrowed by dynamic motion now is separated into two species, which are broadened considerably and shifted from that of the hydrated-bicarbonate. The appearance between the two spectra shows that both are in hydrogen-rich sites.
Figure 1.
Bicarbonate 13C NMR from CO2-reacted DMAPS: a) and b) at room temperature (RT) via 13C{1H} MAS Bloch decay NMR (“pulse-acquire”), and 13C{1H} CPMAS, respectively; c) and d) at 100 K by the same sequences, as indicated on the figure. Rotational frequency for MAS, νr, was 10 kHz.
What is significant is that the low temperature experiment shows that 13C{1H} CPMAS is now feasible under low temperature conditions where dynamic motion can be arrested. Upon freezing, there is an induced speciation into two magnetically-inequivalent sites. We find that the Figure 1 observations can be “cycled” between low and high temperatures, and the speciation is reversible. That is, when the sample is warmed, a single resonance is observed again at room temperature. We surmise that one of the two signals is bicarbonate still surrounded by water, but now “motionally-arrested”, thereby permitting efficient 1H-13C dipole-dipole coupling. The other resonance is slightly more challenging to assign. The two species are shifted in frequency from one another, indicating these are different chemical environments; however, both species are assigned as bicarbonate, as other forms of chemisorbed CO2 on tertiary amines are not suggested. Carbonate (CO32−) is a possibility in highly basic environments; however, it has not been found in these solid amine systems and cannot be conclusively ruled out. In order to determine the chemical nature of the second species, we look to the example of a related system: 15N{1H} CPMAS of pyridine in SBA-15 at 120 K to 130 K.26,27 In this work, two pyridine signals were observed by 15N NMR, assigned to a surface-bound species, and a “free” pyridine. At room temperature, one pyridine resonance was observed. Two different characteristic adsorbate sites were identified—a so-called “frozen” conformation arising from interactions with the surface, and one where the adsorbate adopts an “isotropic-like motional state.”28,29
We have examined a series of spectra over temperatures ranging from 100K to room temperature. What is evident (selected data shown in the Supporting Information, Figure S2) is the appearance of two-site exchange between these resonances, where the NMR signals arise as a weighted average between two sites, with a “coalescence region” in between. Such exchange phenomena have also been observed in a dipeptide of glycine-(2,2)-d2-alanine adsorbed on SBA-15 in the presence of minimal amounts of water,28 at 4 to 7 H2O molecules/nm2.
Figure 2 shows 2-dimensional 13C-1H HETCOR NMR dataset that indicates two bicarbonate signals are present at low temperatures, but are coupled to different protons, leading to their chemical- and magnetic-inequivalence. Further experiments are underway to fully characterize these species, but the protons in this region are consistent with H2O in different environments.30
Figure 2.
13C-1H HETCOR of bicarbonate from 13CO2 – reacted DMAPS at 100 K.
Finally, to demonstrate the generality of the presence of bicarbonate in other pendant amine systems, in Figure 3 we show a pair of NMR spectra for a related 13CO2-reacted primary amine pendant molecule, aminopropylsilane (APS), in SBA-15. Adsorbents containing these functional groups have been widely studied by 13C NMR after exposure to CO2.5,23 Again, in the presence of water, using 13C{1H} MAS Bloch decay, we observe both a bicarbonate signal (at 161 ppm) and a second signal that we have assigned previously23 at 164 ppm to carbamate (Figure 3a). Also, notable in the 13C{1H} CPMAS spectrum shown in Figure 3b is a tiny signal attributable to bicarbonate. Substantial motional averaging of bicarbonate decreases the intensity of this signal when relying on dipole-dipole coupling between 1H and 13C spins in CPMAS. Using conventional data collection procedures at room temperature, the bicarbonate signal is significantly attenuated—therefore the chemisorption products may potentially be missed or “mis-assigned” inadvertently by using this standard technique. Because Bloch decay is so infrequently successful owing to the long 13C T1 relaxation times that are typically found in solids, it is a sequence that is not often employed. Thus, these data show how researchers in the community might have overlooked important chemisorbed products, and therefore it is imperative that both CPMAS and MAS Bloch decay sequences be applied to study these carbon dioxide capture reactions in solid amine sorbents in the future.
Figure 3.

13CO2- reacted “wet” aminopropylsilane (APS) solid-amine sorbent forms both carbamate and bicarbonate, evidenced by 13C NMR at room temperature: a) via 13C{1H} MAS Bloch decay, and b) via 13C{1H} CPMAS.
In summary, a single narrow resonance at 163 ppm observed in 13C{1H} Bloch decay NMR of both DMAPS and APS grafted onto mesoporous silica, assigned to bicarbonate. We show in this work, to our surprise, that bicarbonates have likely been formed all along in the presence of water, but they have been missed by solid-state NMR studies due to the nature of the experiments that are routinely employed (namely, 13C CPMAS). We selected DMAPS, a tertiary amine adsorbent, to identify the adsorbed product—bicarbonate--but then extend the methodology to show that the same species also form on a primary amine-containing sample. CPMAS of hydrated-bicarbonate formed from DMAPS is only possible at very low temperatures, because it shows dynamic motion caused by surrounding water.4,22 The motion is arrested at 100 K, where bicarbonate then appears as two chemically-distinct species: one that retains a hydrated “shell” and one that we postulate is chemically- and magnetically distinct, potentially associated with the surface of the solid-amine sorbent. The two-site exchange model between bicarbonate surrounded by water and surface-bound bicarbonate is consistent with other literature precedent and our data. Dynamic motion of bicarbonate averages out the 13C-1H dipolar interaction, thereby leading to poor signal in 13C{1H} CPMAS. Therefore, researchers exploring CO2 adsorption in materials are cautioned to perform both Bloch decay and 13C{1H} CPMAS in order to examine the possible existence of bicarbonate.
Supplementary Material
Acknowledgments
This work is supported by the Center for Understanding and Control of Acid Gas-Induced Evolution of Materials for Energy (UNCAGE-ME), an Energy Frontier Research Center funded by U.S. Department of Energy (US DOE), Office of Science, Basic Energy Sciences (BES) under Award no. DE-SC0012577. The National High Magnetic Field Laboratory is supported by the NSF (DMR-1644779) and by the State of Florida. The 14.1 T DNP system at NHMFL is funded in part by NIH S10 OD018519 (magnet and console), and NSF CHE-1229170 (probe). We thank Dr. Johannes E. Leisen at the Georgia Institute of Technology NMR Center, and Dr. Tanya L. Whitmer in NMR Laboratory in the Department of Chemistry, The Ohio State University facility for acquisition of some of the data shown. C.-H. Chen acknowledges fellowship support from the Taiwan Ministry of Education.
Footnotes
Experimental procedures, 13C NMR background from empty coil, and 13C{1H} CPMAS of 13CO2 loaded DMAPS at 250 K and 223 K.
References
- 1.Didas SA, Choi S, Chaikittisilp W, Jones CW. Acc Chem Res. 2015;48:2680–2687. doi: 10.1021/acs.accounts.5b00284. [DOI] [PubMed] [Google Scholar]
- 2.Kortunov PV, Siskin M, Baugh LS, Calabro DC. Energy & Fuels. 2015;29:5919–5939. [Google Scholar]
- 3.Kortunov PV, Siskin M, Paccagnini M, Thomann H. Energy and Fuels. 2016;30:1223–1236. [Google Scholar]
- 4.Foo GS, Lee JJ, Chen CH, Hayes SE, Sievers C, Jones CW. Chem Sus Chem. 2017;10:266–276. doi: 10.1002/cssc.201600809. [DOI] [PubMed] [Google Scholar]
- 5.Pinto ML, Mafra L, Guil JM, Pires J, Rocha J. Chem Mater. 2011;23:1387–1395. [Google Scholar]
- 6.Shimon D, Chen CH, Lee JJ, Didas SA, Sievers C, Jones CW, Hayes SE. Environ Sci Technol. 2018;52:1488–1495. doi: 10.1021/acs.est.7b04555. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Schwartz V, Clark JC, Ma X, Overbury SH, Xu X, Song C. J Phys Chem C. 2009;113:7260–7268. [Google Scholar]
- 8.Bacsik Z, Ahlsten N, Ziadi A, Zhao G, Garcia-Bennett AE, Martinn-Matute B, Hedin N. Langmuir. 2011;27:11118–11128. doi: 10.1021/la202033p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knöfel C, Martin C, Hornebecq V, Llewellyn PL. J Phys Chem C. 2009;113:21726–21734. [Google Scholar]
- 10.Danon A, Stair PC, Weitz E. J Phys Chem C. 2011;115(23):11540–11549. [Google Scholar]
- 11.Wilfong WC, Srikanth CS, Chuang SSC. ACS Appl Mater Interfaces. 2014;6:13617–13626. doi: 10.1021/am5031006. [DOI] [PubMed] [Google Scholar]
- 12.Hahn MW, Steib M, Jentys A, Lercher JA. J Phys Chem C. 2015;119:4126–4135. [Google Scholar]
- 13.Moore JK, Sakwa-Novak MA, Chaikittisilp W, Mehta AK, Conradi MS, Jones CW, Hayes SE. Environ Sci Technol. 2015;49:13684–13691. doi: 10.1021/acs.est.5b02930. [DOI] [PubMed] [Google Scholar]
- 14.Didas SA, Sakwa-novak MA, Foo GS, Sievers C, Jones CW. J Phys Chem Lett. 2014;5:4194–4200. doi: 10.1021/jz502032c. [DOI] [PubMed] [Google Scholar]
- 15.Bacsik Z, Ahlsten N, Ziadi A, Zhao GY, Garcia-Bennett AE, Martín-Matute B, Hedin N. Langmuir. 2011;27:11118–11128. doi: 10.1021/la202033p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernin D, Hedin N. Curr Opin Colloid Interface Sci. 2018;33:53–62. [Google Scholar]
- 17.Flaig RW, Osborn Popp TM, Fracaroli AM, Kapustin EA, Kalmutzki MJ, Altamimi RM, Fathieh F, Reimer JA, Yaghi OM. J Am Chem Soc. 2017 doi: 10.1021/jacs.7b06382. jacs.7b06382. [DOI] [PubMed] [Google Scholar]
- 18.Hung C-T, Yang C-F, Lin J-S, Huang S-J, Chang Y-C, Liu S-B. Micropouous mesoporous Mater. 2017;238:2–13. [Google Scholar]
- 19.Andreoli E, Dillon EP, Cullum L, Alemany LB, Barron AR. Sci Rep. 2014;4:7304. doi: 10.1038/srep07304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mani F, Peruzzini M, Stoppioni P. Green Chmiestry. 2006;8:995–1000. [Google Scholar]
- 21.Li X, Hagaman E, Tsouris C, Lee JW. Energy Fuels. 2003;17:69–74. [Google Scholar]
- 22.Lee JJ, Chen C-H, Shimon D, Hayes SE, Sievers C, Jones CW. J Phys Chem C. 2017:23480–23487. [Google Scholar]
- 23.Chen CH, Shimon D, Lee JJ, Didas SA, Mehta AK, Sievers C, Jones CW, Hayes SE. Environ Sci Technol. 2017;51:6553–6559. doi: 10.1021/acs.est.6b06605. [DOI] [PubMed] [Google Scholar]
- 24.Akiva-Tal A, Kababya S, Balazs YS, Glazer L, Berman A, Sagi A, Schmidt A. Proc Natl Acad Sci. 2011;108:14763–14768. doi: 10.1073/pnas.1102608108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ben Shir I, Kababya S, Katz I, Pokroy B, Schmidt A. Chem Mater. 2013;25:4595–4602. [Google Scholar]
- 26.Gurinov AA, Mauder D, Akcakayiran D, Findenegg GH, Shenderovich IG. Chem Phys Chem. 2012;13:2282–2285. doi: 10.1002/cphc.201200204. [DOI] [PubMed] [Google Scholar]
- 27.Shenderovich IG, Buntkowsky G, Schreiber A, Gedat E, Sharif S, Albrecht J, Golubev NS, Findenegg GH, Limbach HH. J Phys Chem B. 2003;107:11924–11939. [Google Scholar]
- 28.Jayanthi S, Kababya S, Schmidt A, Vega S. J Phys Chem C. 2016;120:2797–2806. [Google Scholar]
- 29.Ben Shir I, Kababya S, Schmidt A. J Phys Chem C. 2012;116:9691–9702. [Google Scholar]
- 30.Grünberg B, Emmler T, Gedat E, Shenderovich I, Findenegg GH, Limbach HH, Buntkowsky G. Chem - A Eur J. 2004;10:5689–5696. doi: 10.1002/chem.200400351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





