Abstract
Context:
Brain metastasis is one of the most feared complications of cancer that poses significant mortality and morbidity in patients with advanced cancer. The incidence is rising because of greater use of magnetic resonance imaging and spectroscopy; and increased survival from recent advances in immunotherapy and modern radiotherapy techniques. Despite all, the prognosis remains poor.
Aims:
This study aimed to analyze prognostic factors and overall survival in patients with brain metastases.
Subjects and Methods:
A total of 145 patients were analyzed from July 2014 to June 2015 for various prognostic factors prospectively. Survival analysis was done using Kaplan–Meier curve.
Results:
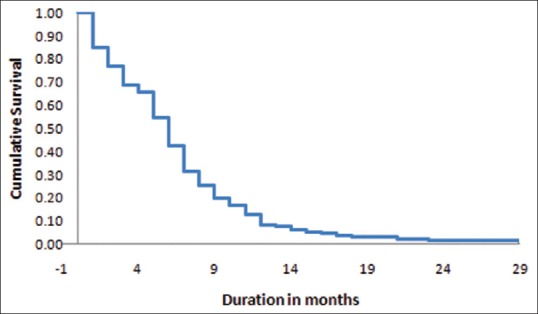
The median overall survival was 6 months, while 1- and 2-year survival rates were 8.3% and 1.4%, respectively. Median survival was highest with surgery followed by radiotherapy (11 months). Whole-brain radiotherapy (WBRT) significantly improved the survival (P = 0.006). The most common primary was lung cancer (58%) and the most common histology was adenocarcinoma (36%). Most patients (38%) were diagnosed upfront with brain metastases. Most of the lesions were multiple (80%) and located in cerebrum (58%). Survival was significantly improved with female gender (P = 0.003), Eastern Cooperative Oncology Group performance status (PS) 0–2 (P = 0.006), breast primary (P = 0.004), time lag of >6 months (P < 0.001), solitary lesion (P = 0.002), and controlled primary (P = 0.017).
Conclusions:
WBRT remains the cornerstone of the management of brain metastases. The present study concludes that the survival of patients with brain metastases is significantly improved with female gender, good PS, primary breast cancer, time lag of >6 months between diagnosis of the primary tumor and development of brain metastases, solitary lesion, and controlled primary tumor.
Keywords: Brain metastases, survival, whole-brain radiotherapy
INTRODUCTION
Brain metastasis is one of the most feared complications of cancer that poses significant mortality and morbidity in patients with advanced stage. The incidence of brain metastasis is rising not only because of greater use of magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS), but also due to increased survival from recent advances in systemic therapy and modern radiotherapy techniques. Metastatic brain tumors outnumber primary ones by a factor of 10:1. The most common primary tumor is the lung cancer followed by breast, accounting for around two-third of the cases; however for about 5%–10% of the cases, primary remains unknown. Only 15%–30% of cases are diagnosed clinically; 10%–30% are diagnosed with autopsy. The median age of presentation is around 60 years.[1,2,3,4]
Headache is the most common symptom and hemiparesis is the most common sign. A new-onset neurologic symptom in a known cancer patient should always be presumed to be from brain metastasis until proven otherwise. MRI is the imaging of choice for suspected brain metastases.[5,6,7] Multiple well-circumscribed lesions, gray-white junction location, and a small tumor nidus with a large amount of associated vasogenic edema favor metastases. More than 60% of patients with brain metastases will have primary tumor demonstrated on mammogram, contrast-enhanced computed tomography (CECT), or MRI. However, for those patients in whom primary lesion still remains undetectable, positron emission tomography should be considered.
For solitary lesion, surgical excision followed by whole-brain radiotherapy (WBRT) is the preferred modality of treatment.[8,9,10,11,12] For multiple lesions, WBRT is the only treatment option. Steroids are prescribed in almost all the patients.[13,14,15,16,17] The median survival is around 7 months in most of the studies for patients treated with WBRT, while <2 months for patients continued on steroids alone. Good prognostic features described in literature include age < 60 years, female sex, good performance status (PS), breast as primary, controlled primary, absence of extracranial disease, ≤3 lesions, supratentorial lesions, and absence of neurocognitive abnormalities, apart from WBRT.[18,19,20,21,22]
SUBJECTS AND METHODS
The present study was carried out at the department of Radiotherapy, SMS Medical College, Jaipur, from July 2014 to June 2015. A total of 152 patients presented with brain metastases, of which 7 patients having a history of previous WBRT were excluded. All patients were evaluated for histopathological confirmation of either the primary tumor or of the brain lesion itself (in case of the postoperated solitary brain lesion) and imaging of the brain, which included either CECT or MRI.
Various prognostic factors that were evaluated include baseline patient characteristics (age, gender, and Eastern Cooperative Oncology Group [ECOG] PS), tumor characteristics (site of primary tumor, histopathology, number and location of lesions), clinical characteristics (presenting sign or symptom, time lag between diagnosis of primary tumor and development of brain metastases, status of primary tumor [whether controlled or not], and presence or absence of extracranial metastasis), and treatment characteristics (treatment modality [steroids only, WBRT, or surgical excision followed by WBRT] and survival period). Out of 145 patients included in the study, 29 (20%) patients were continued on steroids only and 11 (7.6%) patients underwent surgical resection of the brain lesion followed by WBRT. WBRT was delivered over cobalt teletherapy machine (ATC-C9/Bhabhatron II) using conventional planning with dose of 30 Gy in 10 fractions with 3 Gy per fraction for 2 weeks. For survival analysis, the surviving time was the time between diagnosis of brain metastases and the death of the patient.
Statistical analysis
For statistical analysis, all data were recorded and analyzed on Microsoft Excel 2007 and XLSTAT software version 2017 for Windows (Addinsoft, NY, USA). Chi-square test was used for all categorical data. P value reports were two tailed and an alpha level of 0.05 was used to assess statistical significance. Survival analysis was done using Kaplan–Meier survival curve.
RESULTS
The association of brain metastases with various parameters is shown in Table 1. In the present study, the most common cohort was of young male patients with primary lung adenocarcinoma presenting with headache, diagnosed upfront with more than four metastases in both cerebral hemispheres with uncontrolled primary and presence of metastases in other sites. The Kaplan–Meier survival curve of the patients is shown in Figure 1. The median survival period in our study was 6 months (range, 2 weeks–29 months), whereas 1- and 2-year survival rates were 8.3% and 1.4%, respectively.
Table 1.
Univariate analysis of various baseline patient, tumor, clinical, and treatment characteristics of patients with brain metastases
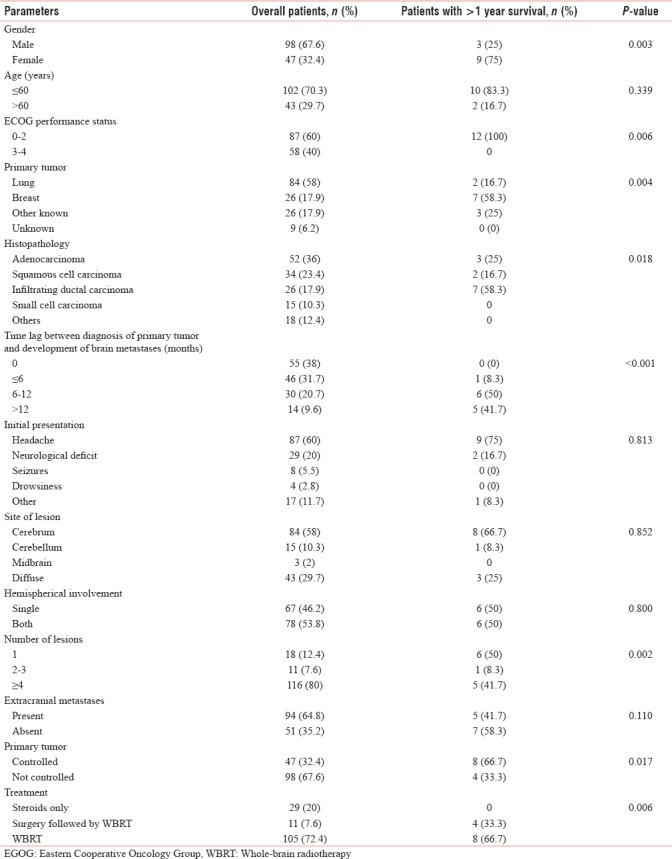
Figure 1.
Kaplan–Meier survival plot of the patients with brain metastases
Institution of WBRT remained the most important significant factor affecting survival of patients with brain metastases (P = 0.006). Patients with single lesion who underwent surgery followed by adjuvant WBRT had the longest survival. Other factors with which survival was found to be significantly associated in the present study were female gender (P = 0.003), ECOG PS 0–2 (P = 0.006), breast being the primary tumor (P = 0.004), infiltrating ductal carcinoma histopathology (P = 0.018), time lag of >6 months between diagnosis of the primary tumor and development of brain metastases (P < 0.001), solitary lesion (P = 0.002), and controlled primary tumor (P = 0.017).
DISCUSSION
The baseline patient and tumor characteristics of patients with brain metastases in the present study are matched with most of the previously reported studies. Ekici et al. evaluated the clinical status, prognostic factors, and treatment modalities affecting survival in 315 patients with brain metastasis treated with WBRT in Turkey between 2004 and 2014.[23] They found that the average patient age of onset was 58 years. The primary tumor site was lung (68%) followed by breast (12%), the primary remained unknown in 4.4% of cases. Hazuka et al. have reported lung as the most common (56%) primary site and adenocarcinoma as the most common (46%) tumor histology.[24] Egawa et al. also reported lung (57.5%) as the most common primary site followed by breast (11%).[25] Saha et al. retrospectively analyzed the demographic and clinical profile of 72 patients with brain metastases and found that brain metastases were more common in males and occurred mostly in the sixth decade of life.[26] Carcinoma lung was the most common primary while adenocarcinoma was the most common histology of the primary that gave rise to metastases. However, Akhavan et al. concluded that breast cancer was the most common primary followed by carcinoma lung as majority of the cases were females in their study.[27] However, adenocarcinoma remained the most common histology of the primary tumor giving rise to brain metastases.
The clinical presentation of the patients in the present study is also in agreement with most of the studies cited in the literature. Ekici et al. reported in their study that 26.6% of patients had single brain metastasis, 22.5% had two or three lesions and 50.4% patients had more than three lesions. About 15.8% of patients underwent surgical resection. Akhavan et al. in their study had shown that 20.4% patients had a single brain metastasis, 8.7% had two or three lesions, while 42.2% patients had more than three lesions. Saha et al. concluded that multiple metastases were more common than the single metastasis, supratentorial lesions were more common than infratentorial lesions, and parietal lobe was the most common site of involvement. However, Delattre et al. have reported single brain metastasis in 49%, two in 21%, three in 13%, four in 6%, and five or more in 11% of the patients.[28] Posner and Chernik,[29] and Victor have reported headache as the most common symptom in 49% and 42% of the patients, respectively. Victor had also shown that the primary disease was not controlled at the time of detection of metastases in two-third of the patients.
The treatment characteristics and survival outcome of the patients in the present study also replicate the results of previous studies. Ekici et al. showed that median overall survival of patients treated with a combination of surgery and WBRT was significantly better compared with those treated with WBRT alone (13.5 vs. 5.5 months, P < 0.001) and 1- and 2-year survival rates were 17 and 4.7%, respectively. Akhavan et al. reported an overall survival of 10.1 months and 1- and 2-year survival rates of 27% and 12%, respectively. Lagerwaard and Levendag reported the median survival of 1.6 months only in patients treated with steroids alone.[30] Lagerwaard et al. have reported an overall median survival of 3.4 months, with 6-month, 1-year, and 2-year survival rates of 36%, 12%, and 4%, respectively. Survival was statistically significantly different between treatment modalities, with median survival of 1.3 months in patients treated with steroids only, 3.6 months in patients treated with radiotherapy, and 8.9 months in patients treated with neurosurgery followed by radiotherapy (P < 0.001). Hazuka et al. have reported the median survival of 11 months and the 1- and 2-year survival rates of 40% and 12%, respectively. Egawa et al. have reported the 50% survival period of 4.1 months for radiotherapy only, 4.2 months for radiotherapy and surgery, and 6.9 months for combined radiotherapy and chemotherapy. Sen et al. have reported the median survival of 5 months.[31] Lentzsch et al. have reported the median survival of 82 weeks for 10/162 surgical patients, 26 weeks for 145/162 patients treated with radiotherapy, and 5 weeks for the 17/162 patients who received symptomatic (corticosteroid) therapy only.[32]
The factors associated significantly with survival in patients with brain metastases in the present study are more or less the same with what has been reported in the literature. Lagerwaard et al. concluded that PS, response to steroid treatment, systemic tumor activity, and serum lactate dehydrogenase were independent prognostic factors with the strongest impact on survival, second only to treatment modality. In patients with lung primaries, sex was found to have a significant impact on survival. In patients with breast primaries, interval between primary tumor and development of brain metastases appeared to be a statistically significant prognostic factor. Lagerwaard and Levendag concluded that PS, age, extracranial metastases, and primary tumor status were the major determinants of survival for patients with brain metastasis. Hazuka et al. concluded that neurologic impairment at the time of diagnosis and the presence of multiple brain metastases were associated with a significantly poorer survival; solitary metastasis, gross total resection, and tumor histopathology of adenocarcinoma significantly prolonged survival; whereas, primary tumor site, presence of active extracranial disease, and radiation dose had no significant effect on survival. Sen et al. concluded that survival was significantly decreased in the presence of symptoms related to the primary tumor (P = 0.001). Lentzsch et al. concluded Karnofsky Index, dose of radiation (P < 0.001), solitary metastases (P < 0.04), and primary tumor size (P < 0.04) as significant prognostic factors for survival. Saha et al. did not find any relationship of occupation or socioeconomic status with the incidence of brain metastases.
CONCLUSIONS
WBRT remains the cornerstone of the management of multiple brain metastases, whereas for solitary lesion, surgical excision followed by WBRT is the preferred treatment modality. Survival of patients with brain metastases is significantly improved with female gender, good PS, primary breast cancer, infiltrating ductal carcinoma histology, time lag of >6 months between diagnosis of the primary tumor and development of brain metastases, solitary lesion, and controlled primary tumor apart from institution of WBRT.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Victor TS. Brain Metastasis. Medscape Reference. [Last accessed on 2016 May 05]. Available from: http://www.emedicine.medscape.com/article .
- 2.Posner JB. Brain metastases: 1995. A brief review. J Neurooncol. 1996;27:287–93. doi: 10.1007/BF00165486. [DOI] [PubMed] [Google Scholar]
- 3.Lagerwaard FJ, Levendag PC, Nowak PJ, Eijkenboom WM, Hanssens PE, Schmitz PI, et al. Identification of prognostic factors in patients with brain metastases: A review of 1292 patients. Int J Radiat Oncol Biol Phys. 1999;43:795–803. doi: 10.1016/s0360-3016(98)00442-8. [DOI] [PubMed] [Google Scholar]
- 4.Wen PY, Loeffler JS. Management of brain metastases. Oncology (Williston Park) 1999;13:941–54. 957-61. [PubMed] [Google Scholar]
- 5.Davis PC, Hudgins PA, Peterman SB, Hoffman JC., Jr Diagnosis of cerebral metastases: Double-dose delayed CT vs. contrast-enhanced MR imaging. AJNR Am J Neuroradiol. 1991;12:293–300. [PMC free article] [PubMed] [Google Scholar]
- 6.Schellinger PD, Meinck HM, Thron A. Diagnostic accuracy of MRI compared to CCT in patients with brain metastases. J Neurooncol. 1999;44:275–81. doi: 10.1023/a:1006308808769. [DOI] [PubMed] [Google Scholar]
- 7.Sze G, Milano E, Johnson C, Heier L. Detection of brain metastases: Comparison of contrast-enhanced MR with unenhanced MR and enhanced CT. AJNR Am J Neuroradiol. 1990;11:785–91. [PMC free article] [PubMed] [Google Scholar]
- 8.Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoekstra FH, et al. Treatment of single brain metastasis: Radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993;33:583–90. doi: 10.1002/ana.410330605. [DOI] [PubMed] [Google Scholar]
- 9.Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 10.Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA. 1998;280:1485–9. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 11.Mintz AH, Kestle J, Rathbone MP, Gaspar L, Hugenholtz H, Fisher B, et al. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer. 1996;78:1470–6. doi: 10.1002/(sici)1097-0142(19961001)78:7<1470::aid-cncr14>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 12.DeAngelis LM, Mandell LR, Thaler HT, Kimmel DW, Galicich JH, Fuks Z, et al. The role of postoperative radiotherapy after resection of single brain metastases. Neurosurgery. 1989;24:798–805. doi: 10.1227/00006123-198906000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Ruderman NB, Hall TC. Use of glucocorticoids in the palliative treatment of metastatic brain tumors. Cancer. 1965;18:298–306. doi: 10.1002/1097-0142(196503)18:3<298::aid-cncr2820180306>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 14.Mahmood U, Kwok Y, Regine WF, Patchell RA. Whole-brain irradiation for patients with brain metastases: Still the standard of care. Lancet Oncol. 2010;11:221–2. doi: 10.1016/S1470-2045(09)70389-4. [DOI] [PubMed] [Google Scholar]
- 15.Pease NJ, Edwards A, Moss LJ. Effectiveness of whole brain radiotherapy in the treatment of brain metastases: A systematic review. Palliat Med. 2005;19:288–99. doi: 10.1191/0269216305pm1017oa. [DOI] [PubMed] [Google Scholar]
- 16.Yuile PG, Tran MH. Survival with brain metastases following radiation therapy. Australas Radiol. 2002;46:390–5. doi: 10.1046/j.1440-1673.2002.01092.x. [DOI] [PubMed] [Google Scholar]
- 17.Borgelt B, Gelber R, Kramer S, Brady LW, Chang CH, Davis LW, et al. The palliation of brain metastases: Final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1980;6:1–9. doi: 10.1016/0360-3016(80)90195-9. [DOI] [PubMed] [Google Scholar]
- 18.Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–51. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 19.Lutterbach J, Bartelt S, Ostertag C. Long-term survival in patients with brain metastases. J Cancer Res Clin Oncol. 2002;128:417–25. doi: 10.1007/s00432-002-0354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soffietti R, Rudā R, Mutani R. Management of brain metastases. J Neurol. 2002;249:1357–69. doi: 10.1007/s00415-002-0870-6. [DOI] [PubMed] [Google Scholar]
- 21.Hoskin PJ, Brada M. Second Workshop on Palliative Radiotherapy and Symptom Control. Radiotherapy for brain metastases. Clin Oncol (R Coll Radiol) 2001;13:91–4. doi: 10.1053/clon.2001.9226. [DOI] [PubMed] [Google Scholar]
- 22.Vecht CJ. Clinical management of brain metastasis. J Neurol. 1998;245:127–31. doi: 10.1007/s004150050191. [DOI] [PubMed] [Google Scholar]
- 23.Ekici K, Temelli O, Dikilitas M, Halil Dursun I, Bozdag Kaplan N, Kekilli E, et al. Survival and prognostic factors in patients with brain metastasis: Single center experience. J BUON. 2016;21:958–63. [PubMed] [Google Scholar]
- 24.Hazuka MB, Burleson WD, Stroud DN, Leonard CE, Lillehei KO, Kinzie JJ, et al. Multiple brain metastases are associated with poor survival in patients treated with surgery and radiotherapy. J Clin Oncol. 1993;11:369–73. doi: 10.1200/JCO.1993.11.2.369. [DOI] [PubMed] [Google Scholar]
- 25.Egawa S, Tukiyama I, Akine Y, Kajiura Y, Yanagawa S, Watai K, et al. Radiotherapy of brain metastases. Int J Radiat Oncol Biol Phys. 1986;12:1621–5. doi: 10.1016/0360-3016(86)90288-9. [DOI] [PubMed] [Google Scholar]
- 26.Saha A, Ghosh SK, Roy C, Choudhury KB, Chakrabarty B, Sarkar R, et al. Demographic and clinical profile of patients with brain metastases: A retrospective study. Asian J Neurosurg. 2013;8:157–61. doi: 10.4103/1793-5482.121688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akhavan A, Binesh F, Heidari S. Survival of brain metastatic patients in Yazd, Iran. Asian Pac J Cancer Prev. 2014;15:3571–4. doi: 10.7314/apjcp.2014.15.8.3571. [DOI] [PubMed] [Google Scholar]
- 28.Delattre JY, Krol G, Thaler HT, Posner JB. Distribution of brain metastases. Arch Neurol. 1988;45:741–4. doi: 10.1001/archneur.1988.00520310047016. [DOI] [PubMed] [Google Scholar]
- 29.Posner JB, Chernik NL. Intracranial metastases from systemic cancer. Adv Neurol. 1978;19:579–92. [PubMed] [Google Scholar]
- 30.Lagerwaard FJ, Levendag PC. Prognostic factors in patients with brain metastases. Forum (Genova) 2001;11:27–46. [PubMed] [Google Scholar]
- 31.Sen M, Demiral AS, Cetingöz R, Alanyali H, Akman F, Sentürk D, et al. Prognostic factors in lung cancer with brain metastasis. Radiother Oncol. 1998;46:33–8. doi: 10.1016/s0167-8140(97)00124-2. [DOI] [PubMed] [Google Scholar]
- 32.Lentzsch S, Reichardt P, Weber F, Budach V, Dörken B. Brain metastases in breast cancer: Prognostic factors and management. Eur J Cancer. 1999;35:580–5. doi: 10.1016/s0959-8049(98)00421-3. [DOI] [PubMed] [Google Scholar]


