Abstract
Background:
Polycystic ovarian syndrome (PCOS) is a condition characterized by insulin resistance (IR) and hormonal dysfunction. We conducted a randomized, controlled trial comparing the effects of metformin, oral contraceptive pills (OCP) and their combination in PCOS.
Materials and Methods:
We randomized 90 newly diagnosed PCOS (age 18–40 year, symptom duration >6 months) patients into three groups (Group 1–Metformin, Group 2–OCP, and Group 3– Metformin + OCP) in this prospective study. We excluded patients with past use of insulin sensitizers and hormone therapy. We evaluated for the hyperandrogenism (acne, acanthosis, hirsutism, and hormone panel), IR by homeostasis model assessment (HOMA-IR), inflammation (high-sensitivity C-reactive protein, fibrinogen, and ferritin), and body composition (% fat, android/gynoid ratio) markers at baseline and 6 months after therapy. The data were analyzed using appropriate statistical methods and P < 0.05 was considered statistically significant.
Results:
The study population had a mean age 23.2 ± 4.4 years and body mass index of 28.4 ± 6.1 kg/m2. The improvement in the clinical parameters was similar in all the groups. The combination therapy showed a better response in reducing inflammatory markers, IR, and body composition than either of the groups using a single drug. Metformin alone has resulted in a minor reduction of the androgens. None of the patients developed significant adverse effect to the given therapy.
Conclusion:
PCOS is managed with either metformin or OCP in many patients. The combination improves the hyperandrogenism, body composition, and reduces the inflammatory markers.
Keywords: India, insulin resistance, metformin, oral contraceptive pill, polycystic ovarian syndrome
Introduction
Polycystic ovarian syndrome (PCOS) is the most common endocrine disorder in women of reproductive age group. PCOS is characterized by hyperandrogenic chronic anovulation, dysfunctional uterine bleeding, and altered ovarian morphology.[1] PCOS is a chronic disorder with long-term reproductive and metabolic consequences. Insulin resistance (IR) is an invariable component of the PCOS, and few researchers have reported the metabolic differences between the obese and lean PCOS patients.[2] The pathophysiology of PCOS is complex precluding clear-cut guidelines on the management. The management approach varies as per the age and the presenting complaint of the patients.[3] Moreover, there is a wide variation in the approach toward PCOS between the Endocrinologists, Gynecologists, and Dermatologists. The commonly used drugs in the PCOS include insulin sensitizers, oral contraceptive pills (OCP), and anti-androgens.[4]
The need for prolonged drug therapy poses an enormous psychological burden on the young females and their parents. A holistic view of therapy is required and involvement of the patient in selecting the therapy helps in improving the compliance. The previous reports have given conflicting results about the clinical, hormonal, and reproductive outcomes.[5,6,7] The observed variation in the results is a source of confusion that prompted the researchers to look at alternate therapeutic options. They include the use of Vitamin D, Acarbose, Berberine, pioglitazone and other preparations which are under various phases of evaluation.[8,9,10] A limited number of studies exist from our country regarding the effects of the commonly used drugs in the management of PCOS.[11,12] Hence, we conducted this study to determine the effect of metformin and OCP either alone or in combination in women with PCOS.
Materials and Methods
Study population
We conducted this randomized, controlled, prospective interventional study in a tertiary care, teaching hospital of the armed forces. The participants were recruited from the endocrinology/medicine/gynecology clinic of our hospital. We included 90 newly diagnosed patients with PCOS (aged between 18 and 40 years, symptom duration >6 months, premenopausal, and normal thyroid function) in this study. We excluded patients with a history of using any drug therapy (insulin sensitizers, hormone therapy, calcium, and Vitamin D), use of other drugs that affect the body composition and androgen levels (insulin, glucocorticoids), and pregnancy or lactation. The patients were divided into three groups using a computer-generated random sequence numbering based on the intervention received: Group 1 (OCP), Group 2 (Metformin), and Group 3 (Metformin + OCP). All the patients were explained about healthy lifestyle measures and dietary advice to reduce weight. All patients provided informed consent after receiving detailed counseling about the nature and conduct of the study procedures. The study was approved by the Institutional Ethics Committee as part of the postgraduate thesis conducted in our department.
Study measures
Clinical data were collected from the participants including demographic details such as age, presenting complaints (hirsutism, menstrual history, and symptoms of androgen excess), and dietary history (special reference to the daily calorie intake) and drug history (nutritional and vitamin supplements). Weight was recorded on a digital weighing scale using OMRON HN 286 (Omron Corporation, Kyoto, Japan with a sensitivity of 100 g), height by using a SWWS05 stadiometer (Multicare Company, Delhi, India with a sensitivity of 0.1 cm), and body mass index (BMI) was calculated as weight in kilograms divided height in meters squared. A detailed general examination was conducted for the identification of temporal balding, acne, hirsutism (scored by the Ferriman and Gallwey system), lipodystrophy, acanthosis nigricans, and skin tags. The details of the study procedure and their interventions are given in Figure 1. The starting dose of the metformin was 500 mg/day, which was increased up to 2000 mg/day gradually over 1 month time. The OCP used was a standard combination pill comprising of the ethinyl estradiol 35 mcg and cyproterone acetate 2 mg.
Figure 1.
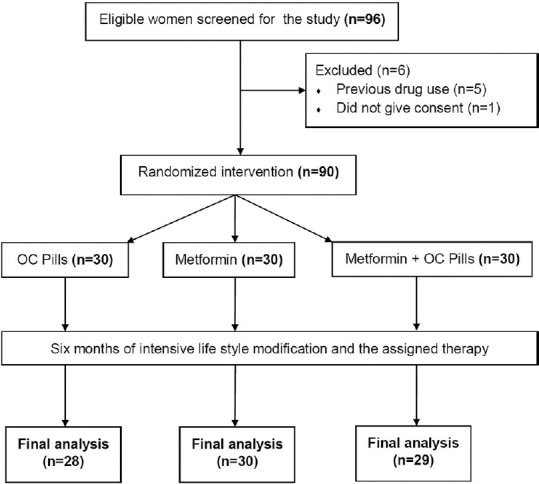
Flow diagram of the study
Study interventions
Fasting venous blood samples were collected after an overnight fast for >12 h and analyzed for hematological and biochemical parameters. They include glucose, insulin, lipid profile (total cholesterol, triglycerides [TG], high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol), hormonal profile (luteinizing hormone, follicle-stimulating hormone, dehydroepiandrosterone sulfate, and testosterone) and inflammatory markers (high sensitivity C-reactive protein, fibrinogen, and ferritin). Plasma glucose was estimated by the glucose oxidase method and the coefficients of variation for all biochemical tests were <10% in our laboratory. Body fat percentage and android/gynoid ratio were determined in the fasting state at the same time of the day using dual-energy X-ray absorptiometry (DEXA) (Hologic QDR 2000, Hologic®, Bedford, MA 01730, USA) technique. The local ethics committee approved the study protocol, and all patients provided written informed consent. The patients were followed up every month in the OPD for a total period of 6 months. All the biochemical parameters and the DEXA scan have been repeated at the end of 6 months of therapy.
Study definitions
We used the Rotterdam criteria for the diagnosis of PCOS.[13] Acanthosis nigricans was graded as per the classification given by Burke et al.[14] The severity of acne was graded as per the Global Acne Grading System.[15] In this study, we considered the HOMA-IR >3 as suggestive of IR.[16]
Statistical analysis
Data are presented as mean, standard deviation, and descriptive statistics were used for the data analysis. ANOVA test and Chi-square test were used to compare the data between the groups. A two-tailed P < 0.05 was considered statistically significant for all the tests, and the statistical analysis was done using the GraphPad Prism Software, Version 6 (Graph Pad Software, San Deigo, CA, USA).
Results
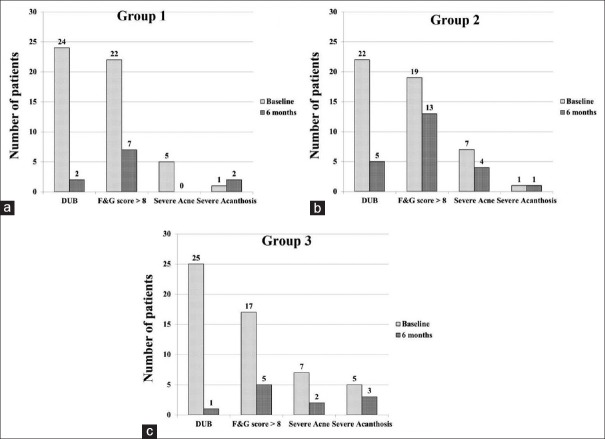
The study population had a mean age 23.2 ± 4.4 year and BMI of 28.4 ± 6.1 kg/m2. The baseline demographic and clinical parameters of the three groups were given in Table 1. Menstrual irregularities, hirsutism, and weight gain were the most common presenting symptoms in all the three groups. Briefly, all the three groups were comparable in the clinical presentation and the symptomatology. However, patients in Group 3 had higher BMI and IR in comparison to other groups. The entire study population falls into the obese category and had significant hirsutism. Other hormonal, inflammatory and body composition parameters were not significantly different between the three groups. The observed changes in all the clinical parameters are shown in Figure 2. Menstrual irregularity and hirsutism improved significantly in all the groups. Group 2 (Metformin alone) showed an insignificant improvement in reducing the number of patients with severe hirsutism. The grade of acanthosis was not changed significantly in any of the groups.
Table 1.
Comparison between three groups regarding the baseline demographic and biochemical parameters
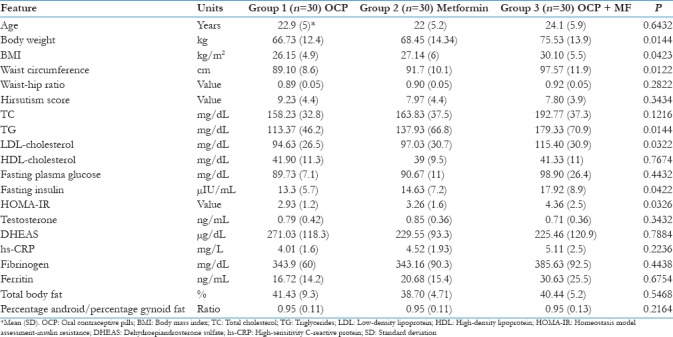
Figure 2.
Changes in the clinical parameters in Groups 1 (a) 2 (b) and 3 (c) before and after therapy
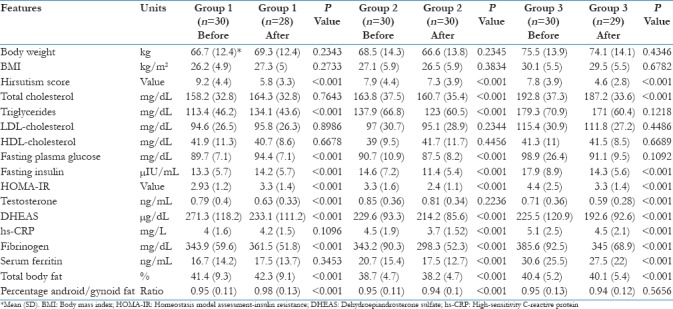
The observed changes in clinical, biochemical, hormonal, inflammatory, and body composition markers are given in Table 2. Briefly, the data suggests that the use of OCP resulted in reduction of hirsutism, IR, and increases the body fat percentage. The use of metformin alone has resulted in the reduction of all parameters including hyperandrogenism, dyslipidemia, inflammation, and body fat. The combination group also has shown similar results akin to that of the Group 2. The body weight was not significantly different in all the three groups after 6 months of intervention and lifestyle advice. The use of metformin has resulted in a definite trend toward changing the metabolic milieu of the patient. None of the patients developed significant adverse effect to the given therapy. Two patients developed transient gastrointestinal intolerance with metformin and one patient developed a self-limiting rash after OCP.
Table 2.
Comparison of all three groups at baseline and after 6 months of therapy
Discussion
Our study showed that treatment with OCP has resulted in significant reduction in hyperandrogenism with worsening of lipid profile, IR, and body composition. Metformin has shown improvement in lipid profile, IR, inflammatory markers, and a favorable change in the body composition. The combination of metformin and OCP also showed similar beneficial results. Our data have shown that metformin is comparable to OCP in restoring the menstrual pattern, unlike previous studies.[17,18] This discrepancy could be due to the high prevalence of the IR in our patients, which is ameliorated using metformin. The use of OCP has shown beneficial effects on the hirsutism and acne when compared to metformin as reported earlier.[19] This is because the OCP reduces the free androgen levels by multiple mechanisms.[20] The use of metformin alone has not been shown to significantly reduce the hirsutism akin to the previous studies on the subject.[6,17] The effect of acne has also been noted maximum with the use of the combination therapy. There was no significant change observed in the severity of the acanthosis in our data. This could be due to the small sample size and also the limited follow-up duration of 6 months only.
Previous studies have shown variable effects on the body weight and BMI with any of the interventions in PCOS.[21] Our data also did not show any significant changes in these parameters. Lifestyle modification plays a major role in losing weight and is difficult to follow over a prolonged period. Few studies have shown that patients using metformin have lost weight whereas those using OCP have gained weight.[22] However, our data did not show any such discrepant observation. In our study, all the patients lost weight at the end of 6 months, except those in the Group 1, albeit not significantly. This could be explained by the continuous emphasis of lifestyle measures and also being armed forces service hospital which emphasizes good lifestyle measures routinely.
Our data on the lipids have shown that the use of OCP resulted in slight worsening of the lipid profile which is offset by the combination with Metformin. The use of metformin has improved the lipids by improving the IR. TG showed maximum reduction in Group 3 due to a high-baseline value. Our study showed that the testosterone changes were comparable between the OCP and metformin groups. Metformin improves the IR, alters the sex hormone binding globulin and reduces the androgen level.[23] In accordance with previous studies, our data suggested that the metformin resulted in reduction of the insulin levels and HOMA-IR.[24] This could also be contributed by the improvement in the lifestyle and regular exercise. The use of metformin has blunted the observed rise in the IR with the use of OCP in our subjects. The reduction in inflammatory markers is observed maximum in the groups that used Metformin, which has a potent anti-inflammatory action also.[25] Our study has also demonstrated that the use of metformin has improved the body composition and reduced the body fat percentage in comparison to OCP.[26] The strengths of our study include randomized design with a robust follow-up and comprehensive evaluation of all the parameters. However, the limitations include small sample size, limited period of observation, and lack of a control arm.
Conclusion
Our study suggests that the addition of metformin has resulted in significant beneficial changes over the use of OCP alone in PCOS. Further studies with large sample size and long-term observation are required to support our findings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Trikudanathan S. Polycystic ovarian syndrome. Med Clin North Am. 2015;99:221–35. doi: 10.1016/j.mcna.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Kulshreshtha B, Ganie MA, Praveen EP, Gupta N, Lal Khurana M, Seith A, et al. Insulin response to oral glucose in healthy, lean young women and patients with polycystic ovary syndrome. Gynecol Endocrinol. 2008;24:637–43. doi: 10.1080/09513590802342858. [DOI] [PubMed] [Google Scholar]
- 3.Balen A. The pathophysiology of polycystic ovary syndrome: Trying to understand PCOS and its endocrinology. Best Pract Res Clin Obstet Gynaecol. 2004;18:685–706. doi: 10.1016/j.bpobgyn.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Setji TL, Brown AJ. Polycystic ovary syndrome: Update on diagnosis and treatment. Am J Med. 2014;127:912–9. doi: 10.1016/j.amjmed.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Consensus on women's health aspects of polycystic ovary syndrome (PCOS) Hum Reprod. 2012;27:14–24. [Google Scholar]
- 6.Elter K, Imir G, Durmusoglu F. Clinical, endocrine and metabolic effects of metformin added to ethinyl estradiol-cyproterone acetate in non-obese women with polycystic ovarian syndrome: A randomized controlled study. Hum Reprod. 2002;17:1729–37. doi: 10.1093/humrep/17.7.1729. [DOI] [PubMed] [Google Scholar]
- 7.Hu L, Shen H, Wu QF, Tian L, Hu MH. Treatment of polycystic ovarian syndrome with insulin resistance by insulin-sensitizer. Clin Exp Obstet Gynecol. 2014;41:288–92. [PubMed] [Google Scholar]
- 8.Geller DH, Pacaud D, Gordon CM, Misra M of the Drug and Therapeutics Committee of the Pediatric Endocrine Society. State of the art review: Emerging therapies: The use of insulin sensitizers in the treatment of adolescents with polycystic ovary syndrome (PCOS) Int J Pediatr Endocrinol. 2011;2011:9. doi: 10.1186/1687-9856-2011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho LW, Kilpatrick ES, Keevil BG, Coady AM, Atkin SL. Effect of metformin, orlistat and pioglitazone treatment on mean insulin resistance and its biological variability in polycystic ovary syndrome. Clin Endocrinol (Oxf) 2009;70:233–7. doi: 10.1111/j.1365-2265.2008.03309.x. [DOI] [PubMed] [Google Scholar]
- 10.Artini PG, Di Berardino OM, Simi G, Papini F, Ruggiero M, Monteleone P, et al. Best methods for identification and treatment of PCOS. Minerva Ginecol. 2010;62:33–48. [PubMed] [Google Scholar]
- 11.Dasari P, Pranahita G. The efficacy of metformin and clomiphene citrate combination compared with clomiphene citrate alone for ovulation induction in infertile patients with PCOS. J Hum Reprod Sci. 2009;2:18–22. doi: 10.4103/0974-1208.51337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joshi B, Mukherjee S, Patil A, Purandare A, Chauhan S, Vaidya R, et al. A cross-sectional study of polycystic ovarian syndrome among adolescent and young girls in Mumbai, India. Indian J Endocrinol Metab. 2014;18:317–24. doi: 10.4103/2230-8210.131162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fauser B, Tarlatzis B, Chang J Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 14.Burke JP, Hale DE, Hazuda HP, Stern MP. A quantitative scale of acanthosis nigricans. Diabetes Care. 1999;22:1655–9. doi: 10.2337/diacare.22.10.1655. [DOI] [PubMed] [Google Scholar]
- 15.Hacivelioglu S, Gungor AN, Gencer M, Uysal A, Hizli D, Koc E, et al. Acne severity and the global acne grading system in polycystic ovary syndrome. Int J Gynaecol Obstet. 2013;123:33–6. doi: 10.1016/j.ijgo.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Majid H, Masood Q, Khan AH. Homeostatic model assessment for insulin resistance (HOMA-IR): A Better marker for evaluating insulin resistance than fasting insulin in women with polycystic ovarian syndrome. J Coll Physicians Surg Pak. 2017;27:123–6. [PubMed] [Google Scholar]
- 17.Morin-Papunen L, Vauhkonen I, Koivunen R, Ruokonen A, Martikainen H, Tapanainen JS, et al. Metformin versus ethinyl estradiol-cyproterone acetate in the treatment of nonobese women with polycystic ovary syndrome: A randomized study. J Clin Endocrinol Metab. 2003;88:148–56. doi: 10.1210/jc.2002-020997. [DOI] [PubMed] [Google Scholar]
- 18.Rautio K, Tapanainen JS, Ruokonen A, Morin-Papunen LC. Effects of metformin and ethinyl estradiol-cyproterone acetate on lipid levels in obese and non-obese women with polycystic ovary syndrome. Eur J Endocrinol. 2005;152:269–75. doi: 10.1530/eje.1.01840. [DOI] [PubMed] [Google Scholar]
- 19.Ibáñez L, de Zegher F. Ethinylestradiol-drospirenone, flutamide-metformin, or both for adolescents and women with hyperinsulinemic hyperandrogenism: Opposite effects on adipocytokines and body adiposity. J Clin Endocrinol Metab. 2004;89:1592–7. doi: 10.1210/jc.2003-031281. [DOI] [PubMed] [Google Scholar]
- 20.Cibula D, Fanta M, Vrbikova J, Stanicka S, Dvorakova K, Hill M, et al. The effect of combination therapy with metformin and combined oral contraceptives (COC) versus COC alone on insulin sensitivity, hyperandrogenaemia, SHBG and lipids in PCOS patients. Hum Reprod. 2005;20:180–4. doi: 10.1093/humrep/deh588. [DOI] [PubMed] [Google Scholar]
- 21.Nestler JE. Metformin for the treatment of the polycystic ovary syndrome. N Engl J Med. 2008;358:47–54. doi: 10.1056/NEJMct0707092. [DOI] [PubMed] [Google Scholar]
- 22.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Diagnosis and treatment of polycystic ovary syndrome: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565–92. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lord JM, Flight IH, Norman RJ. Metformin in polycystic ovary syndrome: Systematic review and meta-analysis. BMJ. 2003;327:951–3. doi: 10.1136/bmj.327.7421.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al Khalifah RA, Florez ID, Dennis B, Thabane L, Bassilious E. Metformin or oral contraceptives for adolescents with polycystic ovarian syndrome: A Meta-analysis. Pediatrics. 2016:137. doi: 10.1542/peds.2015-4089. pii: e20154089. [DOI] [PubMed] [Google Scholar]
- 25.Tan BK, Adya R, Shan X, Aghilla M, Lehnert H, Keay SD, et al. The anti-atherogenic aspect of metformin treatment in insulin resistant women with the polycystic ovary syndrome: Role of the newly established pro-inflammatory adipokine acute-phase serum amyloid A; evidence of an adipose tissue-monocyte axis. Atherosclerosis. 2011;216:402–8. doi: 10.1016/j.atherosclerosis.2010.08.069. [DOI] [PubMed] [Google Scholar]
- 26.Arslanian SA, Lewy V, Danadian K, Saad R. Metformin therapy in obese adolescents with polycystic ovary syndrome and impaired glucose tolerance: Amelioration of exaggerated adrenal response to adrenocorticotropin with reduction of insulinemia/insulin resistance. J Clin Endocrinol Metab. 2002;87:1555–9. doi: 10.1210/jcem.87.4.8398. [DOI] [PubMed] [Google Scholar]