Highlights
-
•
This is the first meta-analysis of individual data in chronic Trypanosoma cruzi infection after treatment.
-
•
The probability of seroreversion is variable along the course of follow-up.
-
•
An interaction was found between age at treatment and country setting.
-
•
The course of parasitological/molecular tests after treatment needs to be assessed.
Keywords: Trypanosoma cruzi, Chronic disease, Follow-up studies, Individual participant data, Meta-, analysis, Serologic tests
Abstract
Objective
To determine the course of serological tests in subjects with chronic Trypanosoma cruzi infection treated with anti-trypanosomal drugs.
Methods
A systematic review and meta-analysis was conducted using individual participant data. Survival analysis and the Cox proportional hazards regression model with random effects to adjust for covariates were applied. The protocol was registered in the PROSPERO database (http://www.crd.york.ac.uk/PROSPERO; CRD42012002162).
Results
A total of 27 studies (1296 subjects) conducted in eight countries were included. The risk of bias was low for all domains in 17 studies (63.0%). Nine hundred and thirteen subjects were assessed (149 seroreversion events, 83.7% censored data) for enzyme-linked immunosorbent assay (ELISA), 670 subjects (134 events, 80.0% censored) for indirect immunofluorescence assay (IIF), and 548 subjects (99 events, 82.0% censored) for indirect hemagglutination assay (IHA). A higher probability of seroreversion was observed within a shorter time span in subjects aged 1–19 years compared to adults. The chance of seroreversion also varied according to the country where the infection might have been acquired. For instance, the pooled adjusted hazard ratio between children/adolescents and adults for the IIF test was 1.54 (95% confidence interval 0.64–3.71) for certain countries of South America (Argentina, Bolivia, Chile, and Paraguay) and 9.37 (95% confidence interval 3.44–25.50) for Brazil.
Conclusions
The disappearance of anti-T. cruzi antibodies was demonstrated along the course of follow-up. An interaction between age at treatment and country setting was found.
Introduction
Chagas disease is a potentially life-threatening endemic illness in the Latin America region (Bulla et al., 2014, Pérez-Molina and Molina, 2018). It is caused by a protozoan parasite called Trypanosoma cruzi. This parasite has been classified into six genetic variants with approximate geographical distribution in domestic and wild transmission cycles (Zingales et al., 2012). It is mainly transmitted through vectors (namely triatomine bugs) in impoverished rural areas (Houweling et al., 2016, Pérez-Molina and Molina, 2018). Blood transfusion and congenital transmission are other mechanisms for acquiring the disease. Alternative mechanisms are accidental, oral, and via organ transplantation (Dias and Amato Neto, 2011, Ministerio de Salud de la Nación, 2012).
The duration and clinical presentation of the initial acute phase of the infection may be variable, depending on the patient’s age, immunological status, presence of comorbidities, and the transmission pathway. It usually lasts a few months and may be symptomatic (prolonged febrile syndrome, asthenia, hepatosplenomegaly, and other characteristic but less frequent signs, such as Romaña sign and ‘chagoma’ of inoculation) or asymptomatic (Ministerio de Salud de la Nación, 2012).
Most subjects who do not receive specific treatment during the acute phase go on to develop a chronic infection. If untreated, the chronic phase usually continues for the subject’s lifetime, and 30% to 40% of patients will progress to the chronic phase with a cardiac, digestive, neurological, or mixed form at 15 to 30 years after the initial infection. Progressive heart failure and sudden death are the main causes of death in patients with chronic Chagas heart disease (Ministerio de Salud de la Nación, 2012, Pérez-Molina and Molina, 2018).
Several systematic reviews on the effectiveness of treatment in chronically infected subjects have been published (Villar et al., 2014, Fuentes et al., 2012, Pérez-Molina et al., 2009, Vallejo and Reyes, 2005). The current recommendation of the World Health Organization (WHO) is to offer anti-trypanosomal drugs (benznidazole or nifurtimox) to subjects with chronic Trypanosoma cruzi infection, particularly those who are asymptomatic (Bulla et al., 2014). Based on current techniques and their attributes, the general consensus is that treatment success is confirmed by conversion from a positive to a negative serological state (seroreversion), while treatment failure is demonstrated through a positive molecular or parasitological test. The assessment of the response to treatment is uncertain in a large number of subjects because of the long span needed to demonstrate the disappearance of anti-T. cruzi antibodies (de Lana and Martins-Filho, 2009, Viotti et al., 2014).
In this context, it was sought to answer the following question: When and to what extent does the administration of anti-trypanosomal drugs result in the negativization of serological tests through the course of follow-up? The present research group previously published a prognostic systematic review of follow-up studies including chronically infected and treated subjects as a first attempt to address the research question. The results of that review showed a dynamic pattern of serological response; however, pre-planned secondary analyses were incomplete due to the limited utility of aggregate data (Sguassero et al., 2015). As a consequence, a meta-analysis of individual participant data (IPD) was conducted considering all laboratory tests performed after treatment (Riley et al., 2010, Stewart et al., 2015). The purpose of this article is to present the course of serological tests and describe the effect of explanatory factors on the time to seroreversion in treated subjects with chronic Trypanosoma cruzi infection. The hypothesis was based on previous research suggesting an earlier reversion of conventional serology to negative results in treated subjects with an ‘early chronic infection’ (children and adolescents) compared to those with a ‘late chronic infection’ (adults).
Methods
Study design
This meta-analysis was performed according to the protocol registered in the international database of prospectively registered systematic reviews, PROSPERO (http://www.crd.york.ac.uk/PROSPERO, registration number CRD42012002162) in March 2012 (Sguassero et al., 2012). All studies shared unidentified data; hence individual consent for the reuse of participant data was not sought.
The inclusion and exclusion criteria have been detailed elsewhere (Sguassero et al., 2015). Cohort studies and randomized controlled trials (RCTs) with follow-up data on serological, molecular, and parasitological outcomes measured in subjects with a definite diagnosis of chronic Trypanosoma cruzi infection, who received benznidazole and/or nifurtimox, were included. Subjects in the acute phase, children aged 12 months or younger born to infected mothers, immunocompromised participants, and pregnant women were excluded. Two authors, one with training in systematic reviews (YS) and one expert in the field (SSE), identified potentially eligible studies. YS also screened the references of relevant studies. Disagreements were resolved by discussion. The electronic search strategies used have been described previously; there was no restriction on language or year of publication (Sguassero et al., 2015).
The electronic searches in the Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE), MEDLINE (Supplementary Material, Annex A, Table S1), Embase, and LILACS were updated on July 1, 2015. Three new eligible studies were identified (Bianchi et al., 2015, Andrade et al., 2013, de Oliveira, 2013). In addition, the protocol was presented to experts in the field and three further studies were identified by contacting the principal investigators (Morillo et al., 2015, Lacunza et al., 2015, Guhl et al., 2004).
Two review authors (YS and AC) independently assessed the risk of bias of included studies using the tools recommended by the Cochrane Collaboration (Higgins and Green, 2011, Sterne et al., 2016). A data extraction template with the following principal domains of bias was used: selection of participants into the study, measurement of the intervention, measurement of outcomes, and missing data. The judgment regarding the overall risk of bias for each serological outcome was rated as low, moderate, or high. Studies with a low risk of bias for all key domains or studies where it seemed unlikely that bias would have altered the results were considered to have a low risk of bias. Studies with a risk of bias in at least one domain were considered to have an overall risk matching the higher level of bias identified that decreases the grade of certainty. Discrepancies were resolved through discussion with a third author (SSE).
Outcomes
Primary outcomes were the results of conventional serological tests, such as the enzyme-linked immunosorbent assay (ELISA), indirect immunofluorescence (IIF) assay, and indirect hemagglutination assay (IHA). Secondary outcomes were the results of non-conventional ‘in-house’ serological tests based on recombinant/synthetic or biochemically purified antigens specifically used to monitor changes after anti-trypanosomal treatment. The results of serological tests were dichotomized as reactive or non-reactive (negative result) independently of each other. It was decided to analyze the techniques separately given the variability of their performance in demonstrating serological changes over time. The dependent variable was defined as the time elapsed from the end of treatment to the first negative result of each serological test as measured by the authors. No composite outcomes were defined at the protocol stage.
Data collection
A systematic approach to request, collect, and manage IPD was undertaken (Stewart et al., 2015). All corresponding or primary authors were invited to participate in this research by e-mail. In the case of a non-response, the contact details were checked and the website of universities or other research organizations explored to find a new e-mail address. At least three rounds of e-mails per investigator were launched by the same person (YS). In some cases, a member of the review team (SSE) sent a letter of invitation or made a phone call. The strategy developed had three basic steps. The first e-mail intended to present the research and provided a link to the systematic review protocol. The importance of the meta-analytical approach was explained in brief and the investigators were invited to participate. A second e-mail was sent to those collaborators expressing their willingness to contribute; this e-mail included a link to a short online survey aimed at gathering baseline information (availability of primary study protocol, the format of the study dataset, the time needed to send the original data, etc.), and a section to update their contact details. Following this, a third e-mail was sent that included a detailed description of the piece of information needed for each outcome of interest, an agreement on data security and co-authorship to be signed, and recommendations for encrypted data sharing.
Concerning the variables of interest, collaborators provided individual-level data at baseline (sex, age at treatment, age at study entry, country of origin, the name of the anti-trypanosomal drug, the dose and length of treatment) and during the follow-up (dates of measurements and results of all available laboratory tests). The internal consistency of IPD for each study dataset was checked independently by two authors (YS and KR), taking into account the information published in the original reports. Minor queries were resolved by reaching a consensus; however, any specific IPD inconsistency was resolved by seeking clarification from the collaborators (YS). Any inaccuracies or errors were properly discussed and all amendments were registered before merging a study dataset into the master file (YS and KR). Missing data were also requested, whenever appropriate (YS). All collaborators answered an e-mail about the risk of reinfection in their study population.
Statistical analysis
The one-stage IPD meta-analysis was followed as stated at the protocol stage. Two functions that are dependent on time for describing the distribution of event times (the survival and hazard functions) were estimated (Pocock et al., 2002, Collett, 2003). The Kaplan–Meier method was used to obtain univariate descriptive survival data on two or more groups of subjects (Collett, 2003). The Cox proportional hazards regression model with random effects was applied to adjust for pre-specified covariates of clinical relevance. In this model, the time was assembled in years as provided in most datasets. The excess risk or frailty for distinct studies was described using the variance component of random effects and the corresponding Kendall’s Tau coefficient () (Therneau and Grambsch, 2000). Hazard ratios (HR) and 95% confidence intervals (CI) were calculated.
Subgroup analyses were performed based on potential predictors of treatment response in the chronic phase of Trypanosoma cruzi infection, such as the age of the subject at treatment (children/adolescents vs. adults) and the country where the subject was born, as a surrogate for the lineage of the parasite (Argentina, Bolivia, Chile, and Paraguay vs. Brazil) (Zingales et al., 2012).
Taking advantage of available IPD on polymerase chain reaction (PCR) or xenodiagnosis after treatment, the study participants were assigned to the following three post-hoc categories: ‘potential responders’, i.e. subjects with three or more negative PCR or xenodiagnosis results; ‘uncertain responders’, i.e. subjects with at least two negative PCR or xenodiagnosis results; and ‘non-responders/potential non-responders’, i.e. subjects with just one PCR or xenodiagnosis (positive or negative).
The effect of the risk of bias was explored through a sensitivity analysis. The survival analyses for this study were done using SAS version 9.4. A p-value of less than 0.05 was considered statistically significant. It was not possible to use aggregate data from studies for which IPD were not obtained because time-to-event analysis requires individual-level data.
Results
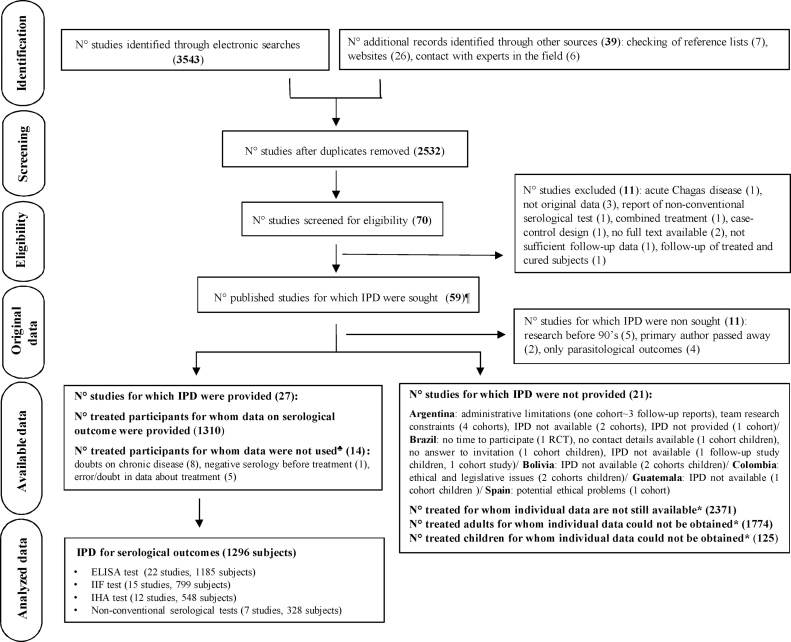
A total of 24 cohort studies and three RCTs (1296 subjects) reporting on serological outcomes were included (Figure 1; Table 1) (Lacunza et al., 2015, Molina et al., 2014, Fabbro et al., 2014, Jackson et al., 2013, Machado-de-Assis et al., 2013, Muñoz et al., 2013, Rumi et al., 2013, Aguiar et al., 2012, Machado-de-Assis et al., 2012, Hasslocher-Moreno, 2010, Murcia et al., 2010, de Lana et al., 2009, Escribà et al., 2009, Fernandes et al., 2009, Sosa-Estani et al., 2009, Sánchez Negrette et al., 2008, de Castro et al., 2006, Vera de Bilbao et al., 2006, Meira et al., 2004, Streiger et al., 2004, Vera de Bilbao et al., 2004, Solari et al., 2001, Silveira et al., 2000, de Andrade et al., 1996, Maldonado et al., 1995). For studies conducted before the 1990s or for which the primary author had died, no attempt to obtain original raw data was made. It was not possible to obtain IPD from 21 cohort studies (Figure 1); some of them are known to be lost (Supplementary Material, Annex B). Three eligible studies involving children could be reachable in the future: two studies were from Colombia (n = 79) and reported up to 42.0% of seroreversion after 30 months of treatment, and one was from Brazil (n = 46), which reported almost 20.0% of seroreversion after 24 months of follow-up. Regarding the studies conducted in adults that may still be accessible, the published rate of seroreversion ranged from 5% after 5–10 years of treatment to 45.0% in just one cohort study reporting on 55 treated subjects living in a non-endemic area after more than 20 years of follow-up (Supplementary Material, Annex A, Table S2).
Figure 1.
PRISMA IPD flow diagram.
¶One multi-country study including four cohorts of children. *Number obtained from published reports. ♣A subject could have more than one reason for exclusion. IPD, individual participant data; RCT, randomized controlled trial.
Table 1.
Studies for which individual participant data on serological outcomes was provided, categorized by country, duration of follow-up, area of endemicity, treatment, and type of test (27 studies, 1296 treated subjects with chronic Trypanosoma cruzi infection).
| Source | Study IDa | Maximum duration follow-up (months) | Area of endemicity | Risk of reinfection | Age at treatment (years) | Anti-trypanosomal drug | Number of treated subjects with IPD for time-to-event analysis |
Total number of subjects with serologyb | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parasitological tests |
Conventional serological test |
Non-conventional serological test | |||||||||||
| XD | PCR | ELISA | IIF | IHA | |||||||||
| Argentina | Lacunza et al. (2015) | 69 | Yes | No | 16–34 | Benznidazole | – | 52 | 38 | – | 39 | – | 39 |
| Fabbro et al. (2014) | 401 | No | No | 6–45 | Benznidazole and Nifurtimox | – | – | 47 | 52 | 52 | – | 52 | |
| Rumi et al. (2013) | 60 | Yes | No | 3–16 | Benznidazole | – | 45 | 45 | – | 45 | – | 45 | |
| Streiger et al. (2004) | 288 | No | No | 1–14 | Benznidazole and Nifurtimox | 21 | – | 26 | 48 | 48 | – | 48 | |
| Sosa-Estani et al. (2009) | 144 | Yes | No | 6–14 | Benznidazole | – | – | 16 | 16 | 16 | 16 | – | |
| Sosa Estani et al. (1998) | 48 | 49 | 46 | 53 | 53 | 52 | 53 | 53 | |||||
| Sánchez Negrette et al. (2008) | 66 | Yes | No | 19–41 | Benznidazole | – | – | 18 | – | 18 | 18 | 18 | |
| Total number of subjects – Argentina | 255 | ||||||||||||
| Brazil | Machado-de-Assis et al. (2013) | 156 | Yes | No | 6–37 | Benznidazole | – | 27 | 26 | – | – | 22 | 26 |
| de Lana et al. (2009) | |||||||||||||
| Aguiar et al. (2012) | 348 | Yes | No | 8–56 | Benznidazole | – | 29 | 29 | 29 | – | – | 29 | |
| Machado-de-Assis et al. (2012) | 432 | Yes | No | 2–60 | Benznidazole | – | 94 | 94 | 94 | 94 | 94 | 94 | |
| Hassslocher-Moreno (2010) | 204 | No | No | 16–56 | Benznidazole | 62 | – | – | 62 | – | – | 62 | |
| Fernandes et al. (2009) | 36 | Yes | No | 17–48 | Benznidazole | – | 80 | 80 | 80 | – | – | 80 | |
| de Castro et al. (2006) | 44 | Yes | NK | 23–76 | Benznidazole | – | 37 | 37 | 37 | 37 | – | 37 | |
| Meira et al. (2004) | 50 | Yes | No | 10–61 | Benznidazole | – | 31 | 31 | – | 31 | – | 31 | |
| Silveira et al. (2000) | 204 | Yes | NK | 7–12 | Benznidazole and Nifurtimox | 38 | 28 | 37 | 37 | 37 | – | 37 | |
| de Andrade et al. (1996) | 36 | Yes | No | 8–11 | Benznidazole | – | – | 58 | 58 | 58 | 58 | 58 | |
| Total number of subjects – Brazil | 454 | ||||||||||||
| Bolivia | Flores-Chavez et al. (2006) | 12 | Yes | No | 5–10 | Benznidazole | 22 | 33 | 33 | 22 | – | 33 | 33 |
| Total number of subjects – Bolivia | 33 | ||||||||||||
| Chile | Muñoz et al. (2013) | 57 | Yes | No | 22–48 | Nifurtimox | 21 | 21 | – | 21 | – | – | 21 |
| Solari et al. (2001) | 36 | Yes | No | 1–10 | Nifurtimox | 37 | 37 | 37 | – | 37 | – | 37 | |
| Total number of subjects – Chile | 58 | ||||||||||||
| Honduras | Escribà et al. (2009) | 51 | Yes | No | 1–18 | Benznidazole | – | – | 227 | – | – | – | 227 |
| Total number of subjects – Honduras | 227 | ||||||||||||
| Paraguay | Vera de Bilbao et al. (2006) | 120 | Yes | No | 9–11 | Benznidazole | – | – | 5 | 5 | – | – | 5 |
| Vera de Bilbao et al. (2004) | 24 | Yes | No | 7–14 | Benznidazole | 20 | 20 | 20 | 20 | – | – | 20 | |
| Maldonado et al. (1995) | |||||||||||||
| Total number of subjects – Paraguay | 25 | ||||||||||||
| Spain | Molina et al. (2014) | 12 | No | No | 27–60 | Benznidazole | – | 25 | 26 | – | – | – | 26 |
| Murcia et al. (2010) | 30 | No | No | 2–74 | Benznidazole | – | 138 | 181 | 181 | – | – | 181 | |
| Total number of subjects – Spain | 207 | ||||||||||||
| Switzerland | Jackson et al. (2013) | 36 | No | No | 25–59 | Nifurtimox | – | 37 | 37 | – | – | – | 37 |
| Total number of subjects – Switzerland | 37 | ||||||||||||
| Grand total | 1296 | ||||||||||||
ELISA, enzyme-linked immunosorbent assay; IIF, indirect immunofluorescence assay; IHA, indirect hemagglutination assay; IPD, individual participant data; NK, not known; PCR, polymerase chain reaction; XD, xenodiagnosis.
The study identification includes surname of primary author and year of publication.
The estimate of the total number of subjects was based on the test with the best number of individual participant data.
The total available follow-up for serological outcomes was 6847 person-years (median 3.0 years per person, interquartile range 1.5–7.0 years). Benznidazole was used as the only treatment of choice in 24 out of 27 included studies. The most common duration of treatment was 31 to 60 days (959 subjects) (Supplementary Material, Annex A, Table S3). Most of the included studies reported at least two serological tests. Non-conventional serology results were provided in seven studies and molecular or parasitological test results were provided in 20 studies (Table 1). The risk of bias was low for all domains in 17 studies (63.0%), moderate in five (18.5%), and high in another five (Supplementary Material, Annex A, Figure S1).
No issues were encountered when checking IPD integrity. The highest rate of missing IPD was around 15.0% for ELISA. Sample sizes beyond 10 years of follow-up were small. The approach used for measuring the variation among studies revealed that was 0.63 for ELISA, 0.58 for IIF, and 0.47 for IHA.
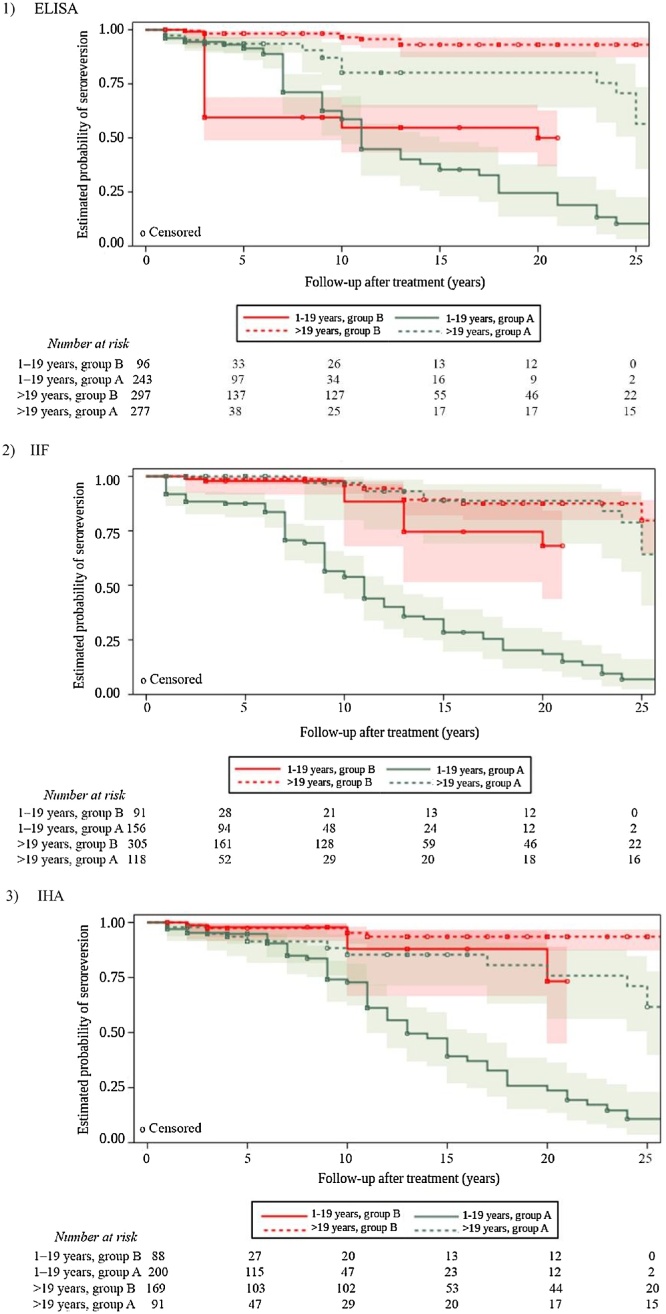
A total 913 subjects (149 seroreversion events, 83.7% censored data) were assessed for ELISA, 670 subjects (134 events, 80.0% censored data) for IIF, and 548 subjects (99 events, 82.0% censored data) for IHA. A higher probability of seroreversion was observed within a shorter time span in treated subjects aged 1–19 years compared to adults. In this respect, after 11–13 years of follow-up, there was a 0.50 probability of seroreversion in the subgroup of treated children and adolescents living in Argentina, Bolivia, Chile, and Paraguay. When considering the same time after treatment for the subgroup of Brazilian children and adolescents, a similar probability of 0.55 was observed for the ELISA test. In subjects treated as adults, the probability of seroreversion after 11 years of treatment was around 0.90 (Figure 2). There were no statistically significant differences in the risk of seroreversion between female and male subjects for any of the serological tests (data not shown).
Figure 2.
Kaplan–Meier plots of the progression of conventional serology in treated subjects with chronic Trypanosoma cruzi infection stratified by age at treatment and country setting, with 95% confidence intervals. Plots show the proportion of treated subjects progressing towards seroreversion according to (1) ELISA, (2) IIF, and (3) IHA tests during the follow-up, stratified by age at treatment (1–19 years vs. >19 years) and country setting (group A: Argentina, Bolivia, Chile and Paraguay vs. group B: Brazil).
ELISA, enzyme-linked immunosorbent assay; IIF, indirect immunofluorescence assay; IHA, indirect hemagglutination assay.
By using non-conventional serology test results, an earlier probability of seroreversion could also be inferred compared to conventional serology in children and adolescents (Supplementary Material, Annex A, Table S4 and Figure S2). No statistically significant difference among survival curves stratified by category using available PCR or xenodiagnosis IPD was found (Supplementary Material, Annex A, Table S5 and Figure S3).
The adjusted Cox model demonstrated the interaction between age at treatment and country setting. The pooled adjusted HR between subjects aged 1–19 years at treatment in comparison to adults varied according to the country where the infection might have been acquired. In the region of Brazil, a higher chance of seroreversion was found in children and adolescents compared to adults: HR 6.60 (95% CI 2.03–21.42) for ELISA, HR 9.37 (95% CI 3.44–25.50) for IIF, and HR 5.55 (95% CI 1.46–21.11) for IHA. No statistically significant differences were found for this comparison in Argentina, Bolivia, Chile, and Paraguay (Table 2). The sensitivity analysis was not informative (Supplementary Material, Annex A, Table S6).
Table 2.
Hazard ratios (95% confidence intervals) corresponding to the adjusted Cox interaction model for conventional serology in treated children or adolescents (1–19 years) versus adults (>19 years) with chronic Trypanosoma cruzi infection.
| Serological test | HR (95% CI) |
p-valuea | |
|---|---|---|---|
| Brazil | Argentina, Bolivia, Chile, and Paraguay | ||
| ELISA | 6.60 (2.03–2.42) | 1.71 (0.77–3.81) | 0.062 |
| IIF | 9.37 (3.44–25.50) | 1.54 (0.64–3.71) | 0.007 |
| IHA | 5.55 (1.46–21.11) | 1.09 (0.44–2.70) | 0.047 |
CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; IIF, indirect immunofluorescence assay; IHA, indirect hemagglutination assay; HR, hazard ratio.
The p-value corresponds to the effect of the interaction obtained from the adjusted Cox proportional hazards model.
Discussion
This appears to be the first meta-analysis using IPD in subjects with chronic Trypanosoma cruzi infection who had received anti-trypanosomal drugs. The results provide an improved picture of the kinetics of anti-T. cruzi antibodies in different country settings and show that the serological course after treatment is variable along the duration of follow-up, as reported previously (de Lana and Martins-Filho, 2009, Viotti et al., 2014). Nevertheless, the single-arm study design poses limitations with regard to the inferences that can be drawn from this meta-analysis. This approach relates directly to the aim of the research and the preliminary published results showing scatter plots of negative serological tests in non-treated subjects with chronic Trypanosoma cruzi infection based on aggregate data (Sguassero et al., 2015). The techniques used to measure the serological course presented differences that could be explained by the affinity during the formation of the antigen–antibody complex, with the ELISA test performing better for the detection of seroreversion. This phenomenon has been described before in two RCTs in the medium-term (de Andrade et al., 1996; Sosa-Estani et al., 1998). Non-conventional serological tests seem to detect early the disappearance of antibodies after treatment in children and adolescents compared to ELISA, IIF and IHA tests.
Possible patterns of interaction with the probability of occurrence of seroreversion were explored. The age at treatment and the country setting are two variables that merit further attention when assessing treatment success during the chronic phase. Whereas a positive molecular or parasitological test confirms the failure of treatment, a negative result cannot rule out the absence of infection because of the lack of sensitivity in the chronic phase of Chagas disease. The pattern of serology shown according to the number and results of available PCR or xenodiagnosis tests merits a more comprehensive assessment. The preliminary observations suggested a better course in treated subjects with persistent parasite clearance measured by three or more negative PCR or xenodiagnosis tests after treatment. Among other plausible explanations, seroreversion in subjects classified as ‘non-responders/potential non-responders’ might be explained by the transient nature of the PCR results and by the conservative approach adopted to define the categories on a post-hoc basis.
This study has the following strengths. A pragmatic approach was taken in adopting the current recommendations for IPD meta-analysis (Supplementary Material, Annex C). The rate of response to the invitation to participate was high and most of the studies for which IPD were not provided included adults. The risk of bias assessment was restricted to those domains that were relevant to the meta-analysis. Included studies benefited from a well-defined intervention status and objective outcome measures, hence the measurement of interventions and the measurement of outcomes were judged as having a low risk of bias in all studies. A pilot test was conducted for the selection of the statistical methodology to ensure a rigorous IPD analysis.
This study has some limitations. To be relevant, a meta-analysis should include all of the available studies. The inherent conflicts in the provision of IPD were in line with the barriers to data sharing described in the literature, including concerns about protecting subject confidentiality and anonymity, lack of time, and unavailable or unusable datasets (Mello et al., 2013). It was not possible to explore potential sources of heterogeneity as planned given the lack of comparison groups to estimate the hazard ratios for each study. Some datasets included children while others included only adults, and all studies were conducted in a single country. The meta-analysis also did not include sufficient IPD from countries where the TcI Trypanosoma cruzi lineage may be prevalent (Zingales et al., 2012).
The electronic searches were updated on August 28, 2017 to find new studies published since the last search. At that time, three potentially eligible studies reporting serological outcomes (two studies including adults living in Argentina and Spain, and one study including children living in Guatemala) were identified; these will be considered in future updates.
The survival analyses may be useful in regard to current recommendations for the clinical management of chronic Trypanosoma cruzi infection. Factors such as the age of the patient attending the clinical appointment and the age at treatment (time elapsed between treatment and clinical assessment), the country of origin (or the country where the infection was acquired), and the complementary assessment of conventional serology and PCR results are critical to monitoring after treatment.
The use of participant-level data was intended to overcome the pragmatic constraints of a new follow-up study, such as the long time between exposure to anti-trypanosomal drugs and seroreversion, the need for appropriate sample sizes for children and adults, and adequate rates of follow-up. It is hoped that this study provides an encouraging example of the importance of anonymizing and sharing IPD for secondary analysis. This strategy may decrease the burden on research resources through the meaningful reuse of existing data.
The next steps will be to determine the course of molecular and parasitological tests after treatment during chronic Trypanosoma cruzi infection and to conduct a new analysis using available IPD according to the standard definition of cure (seroreversion defined as having negative results for at least two different serology techniques) as the primary result. The time period covered will likely be that during which healthcare providers expect to make decisions based on official guidelines, e.g., up to 3 years. The ultimate goal is to provide a monitoring tool to help in the assessment of treatment in chronically infected patients.
Acknowledgements
The authors thank Professor Khalid Khan from the Women’s Health Research Unit, Multi-disciplinary Evidence Synthesis Hub, Barts and the London School of Medicine & Dentistry, Queen Mary University of London, and Fundación Mundo Sano for supporting the TDR grant application. The authors would like to thank all researchers working in this field who sent a response or feedback to our invitation to participate in this project.
Acknowledgments
Funding
YS was granted a UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme for Research and Training in Tropical Diseases (TDR), World Health Organization, Geneva, Switzerland (grant number B20393). This article was supported by the European project BERENICE, a collaborative project which is funded under the European Community’s 7th Framework Programme (grant agreement number: HEALTH-30593). Each of the authors was supported by their individual institutions. The views expressed are those of the authors and are not necessarily those of the WHO or any of the institutions with which the authors are affiliated.
Ethical approval
The protocol was approved by the Bioethics Committee of the Faculty of Medical Sciences of the National University of Rosario, Argentina (record number 42705/0345). All studies shared unidentified data; hence individual consent for the reuse of participant data was not sought.
Conflict of interest
All of the authors declare no competing interests.
Corresponding Editor: Eskild Petersen, Aarhus, Denmark
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ijid.2018.05.019.
Contributor Information
Yanina Sguassero, Email: ysguassero@crep.org.ar.
Sergio Sosa-Estani, Email: ssosaestani@gmail.com.
Annexes A–C. Supplementary data
The following are Supplementary data to this article:
References
- Aguiar C., Batista A.M., Pavan T.B., Almeida E.A., Guariento M.E., Wanderley J.S. Serological profiles and evaluation of parasitaemia by PCR and blood culture in individuals chronically infected by Trypanosoma cruzi treated with benznidazole. Trop Med Int Health. 2012;17:368–373. doi: 10.1111/j.1365-3156.2011.02936.x. [DOI] [PubMed] [Google Scholar]
- Andrade M.C., de Oliveira M.F., Nagao Dias A.T., Coelho I.C.B., Candido D., Freitas E.C. Clinical and serological evolution in chronic Chagas disease patients in a 4-year pharmacotherapy follow-up: a preliminary study. Rev Soc Bras Med Trop. 2013;46:776–778. doi: 10.1590/0037-8682-1646-2013. [DOI] [PubMed] [Google Scholar]
- Bianchi F., Cucunubá Z., Guhl F., González N.L., Freilij H., Nicholls R.S. Follow-up of an asymptomatic chagas disease population of children after treatment with nifurtimox (Lampit) in a sylvatic endemic transmission area of Colombia. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0003465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulla D., Luquetti A., Sánchez M., Sosa Estani S., Salvatella R. Decálogo básico de la atención de la enfermedad de Chagas a nivel primario. Rev Chilena infectol. 2014;31(5):588–589. doi: 10.4067/S0716-10182014000500011. [DOI] [PubMed] [Google Scholar]
- Collett D. 2nd ed. Chapman & Hall/CRC; Philadelphia, PA: 2003. Modelling survival data in medical research. (Texts In Statistical Science) [Google Scholar]
- de Andrade A.L., Zicker F., de Oliveira R.M., Almeida Silva S., Luquetti A., Travassos L.R. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet. 1996;348:1407–1413. doi: 10.1016/s0140-6736(96)04128-1. [DOI] [PubMed] [Google Scholar]
- de Castro A.M., Luquetti A.O., Rassi A., Chiari E., Galvão L.M. Detection of parasitemia profiles by blood culture after treatment of human chronic Trypanosoma cruzi infection. Parasitol Res. 2006;99:379–383. doi: 10.1007/s00436-006-0172-5. [DOI] [PubMed] [Google Scholar]
- de Lana M., Martins-Filho O.A. Serological methods as a tool to monitor post-therapeutic cure in Chagas disease. Rev Soc Bras Med Trop. 2009;2:54–56. [Google Scholar]
- de Lana M., Lopes L.A., Martins H.R., Bahia M.T., Machado-de-Assis G.F., Wendling A.P. Clinical and laboratory status of patients with chronic Chagas disease living in a vector-controlled area in Minas Gerais, Brazil, before and nine years after aetiological treatment. Mem Inst Oswaldo Cruz. 2009;104:1139–1147. doi: 10.1590/s0074-02762009000800011. [DOI] [PubMed] [Google Scholar]
- de Oliveira C.C.S. Instituto Oswaldo Cruz; Rio de Janeiro: 2013. Acompanhamento da parasitemia, dos níveis sorológicos e da evolução clínica de portadores da Doença de Chagas crónica residentes no Mato Grosso do Sul, onze anos após tratamento com benznidazol. [Google Scholar]
- Dias J.C.P., Amato Neto V. Prevention concerning the different alternative routes for transmission of Trypanosoma cruzi in Brazil. Rev Soc Bras Med Trop. 2011;44(2):68–72. doi: 10.1590/s0037-86822011000800011. [DOI] [PubMed] [Google Scholar]
- Escribà J.M., Ponce E., de Dios Romero A., Viñas P.A., Marchiol A., Bassets G. Treatment and seroconversion in a cohort of children suffering from recent chronic Chagas infection in Yoro, Honduras. Mem Inst Oswaldo Cruz. 2009;104:986–991. doi: 10.1590/s0074-02762009000700008. [DOI] [PubMed] [Google Scholar]
- Fabbro D.L., Danesi E., Olivera V., Codebó M.O., Denner S., Heredia C. Trypanocide treatment of women infected with Trypanosoma cruzi and its effect on preventing congenital Chagas. PLoS Negl Trop Dis. 2014;8 doi: 10.1371/journal.pntd.0003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes C.D., Tiecher F.M., Balbinot M.M., Liarte D.B., Scholl D., Steindel M. Efficacy of benznidazol treatment for asymptomatic chagasic patients from state of Rio Grande do Sul evaluated during a three years follow-up. Mem Inst Oswaldo Cruz. 2009;104:27–32. doi: 10.1590/s0074-02762009000100004. [DOI] [PubMed] [Google Scholar]
- Flores-Chavez M., Bosseno M.F., Bastrenta B., Alcazar Dalenz J.L., Hontebeyrie M., Revollo S. Polymerase chain reaction detection and serologic follow-up after treatment with benznidazole in Bolivian children infected with a natural mixture of Trypanosoma cruzi I and II. Am J Trop Med Hyg. 2006;75:497–501. [PubMed] [Google Scholar]
- Fuentes B.R., Maturana A.M., de la Cruz M.R. Eficacia de nifurtimox para el tratamiento de pacientes con enfermedad de Chagas crónica [Efficacy of nifurtimox for the treatment of chronic Chagas disease] Rev Chilena Infectol. 2012;29:82–86. doi: 10.4067/S0716-10182012000100013. [DOI] [PubMed] [Google Scholar]
- Guhl F., Nicholls R., Montoya F., Rosas F., Velasco E.M., Herrera C. IX European Multicolloquium of Parasitology; Spain: 2004. Rapid negativization of serology after treatment with benznidazole for Chagas disease in a group of Colombian school children. [Google Scholar]
- Hasslocher-Moreno A.M. Instituto Oswaldo Cruz; Rio de Janeiro: 2010. Eletrocardiográfica, Parasitológica e Sorológica de Pacientes com Doença de Chagas na Forma Indeterminada tratados com Benzonidazol comparados com Grupo Controle. http://arca.icict.fiocruz.br/handle/icict/3796. [Accessed 13 January 2015] [Google Scholar]
- Higgins J.P.T., Green S., editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. The Cochrane Collaboration; 2011. [Google Scholar]
- Houweling T.A.J., Karim-Kos H.E., Kulik M.C., Stolk W.A., Haagsma J.A., Lenk E.J. Socioeconomic inequalities in neglected tropical diseases: a systematic review. PLoS Negl Trop Dis. 2016;10(5) doi: 10.1371/journal.pntd.0004546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson Y., Chatelain E., Mauris A., Holst M., Miao Q., Chappuis F. Serological and parasitological response in chronic Chagas patients 3 years after nifurtimox treatment. BMC Infect Dis. 2013;13:85. doi: 10.1186/1471-2334-13-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacunza C.D., Sánchez Negrette O., Mora M.C., García Bustos M.F., Basombrío M.A. Uso de la reacción en cadena de la polimerasa para el control terapéutico de la infección crónica por Tripanosoma cruzi. Rev Patol Trop. 2015;44:21–32. [Google Scholar]
- Machado-de-Assis G.F., Silva A.R., DoBem V.A.L., Bahia M.T., Martins-Filho O.A., Dias J.C. Posttherapeutic cure criteria in Chagas’ disease: conventional serology followed by supplementary serological, parasitological, and molecular tests. Clin Vaccine Immunol. 2012;19:1283–1291. doi: 10.1128/CVI.00274-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-de-Assis G.F., Diniz G.A., Montoya R.A., Dias J.C., Coura J.R., Machado-Coelho G.L. A serological, parasitological and clinical evaluation of untreated Chagas disease patients and those treated with benznidazole before and thirteen years after intervention. Mem Inst Oswaldo Cruz. 2013;108:873–880. doi: 10.1590/0074-0276130122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado M., Vera de Bilbao N., Samudio M., Schinini A., Acosta N., López E. Tratamiento con benznidazol en niños de seis a doce años infectados con T. cruzi. Annual Reports II CS. 1995:154–163. [Google Scholar]
- Meira W.S.F., Galvão L.M.C., Gontijo E.D., Machado Coelho G.L.L., Norris K.A., Chiari E. Use of the Trypanosoma cruzi recombinant complement regulatory protein to evaluate therapeutic efficacy following treatment of chronic chagasic patients. J Clin Microbiol. 2004;42:707–712. doi: 10.1128/JCM.42.2.707-712.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello M.M., Francer J.K., Wilenzick M., Teden P., Bierer B.E., Barnes M. Preparing for responsible sharing of clinical trial data. N Engl J Med. 2013;369:1651–1658. doi: 10.1056/NEJMhle1309073. [DOI] [PubMed] [Google Scholar]
- Ministerio de Salud de la Nación . Ministerio de Salud de la Nación; Buenos Aires: 2012. Guías para la atención al paciente infectado con Trypanosoma cruzi (Enfermedad de Chagas) https://www.google.com.ar/url?sa=t&rct=j&q=&esrc=s&source=web&cd=3&cad=rja&uact=8&ved=0ahUKEwjim6CyrPjXAhVIQpAKHTe5AbAQFggvMAI&url=http%3A%2F%2Fwww.msal.gob.ar%2Fchagas%2Fimages%2Fstories%2FEquipos%2FGuia_Nacional_Chagas_version_27092012.pdf&usg=AOvVaw1FwWcgGCQqZvhwcb2CeFHa. [Accessed 6 November 2017] [Google Scholar]
- Molina I., Gómez I Prat J., Salvador F., Treviño B., Sulleiro E., Serre N. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N Engl J Med. 2014;370:1899–1908. doi: 10.1056/NEJMoa1313122. [DOI] [PubMed] [Google Scholar]
- Morillo C.A., Marin-Neto J.A., Avezum A., Sosa-Estani S., Rassi A., Jr., Rosas F. Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy. N Engl J Med. 2015;373:1295–1306. doi: 10.1056/NEJMoa1507574. [DOI] [PubMed] [Google Scholar]
- Muñoz C., Zulantay I., Apt W., Ortiz S., Schijman A.G., Bisio M. Evaluation of nifurtimox treatment of chronic chagas disease by means of several parasitological methods. Antimicrob Agents Chemother. 2013;57:4518–4522. doi: 10.1128/AAC.00227-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murcia L., Carrilero B.M., Muñoz J.M., Iborra A., Segovia M. Usefulness of PCR for monitoring benznidazole response in patients with chronic Chagas’ disease: a prospective study in a non-disease endemic country. J Antimicrob Chemother. 2010;65:1759–1764. doi: 10.1093/jac/dkq201. [DOI] [PubMed] [Google Scholar]
- Pérez-Molina J.A., Molina I. Chagas disease. Lancet. 2018;10115(39):82–94. doi: 10.1016/S0140-6736(17)31612-4. [DOI] [PubMed] [Google Scholar]
- Pérez-Molina J.A., Pérez-Ayala A., Moreno S., Fernández-González M.C., Zamora J., López Vélez R. Use of benznidazole to treat chronic Chagas’ disease: a systematic review with a meta-analysis. J Antimicrob Chemother. 2009;64:1139–1147. doi: 10.1093/jac/dkp357. [DOI] [PubMed] [Google Scholar]
- Pocock S.J., Clayton T.C., Altman D.G. Survival plots of time-to-event outcomes in clinical trials: good practice and pitfalls. Lancet. 2002;359:1686–1689. doi: 10.1016/S0140-6736(02)08594-X. [DOI] [PubMed] [Google Scholar]
- Riley R.D., Lambert P.C., Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340:c221. doi: 10.1136/bmj.c221. [DOI] [PubMed] [Google Scholar]
- Rumi M.M., Pérez Brandán C., Gil J.F., D’Amato A.M., Ragone P.G., Lauthier J.J. Benznidazole treatment in chronic children infected with Trypanosoma cruzi: serological and molecular follow-up of patients and identification of discrete typing units. Acta Trop. 2013;128:130–136. doi: 10.1016/j.actatropica.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Sánchez Negrette O., Sánchez Valdéz F.J., Lacunza C.D., García Bustos M.F., Mora M.C., Uncos A.D. Serological evaluation of specific-antibody levels in patients treated for chronic Chagas’ disease. Clin Vaccine Immunol. 2008;15:297–302. doi: 10.1128/CVI.00106-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sguassero Y., Christensen C., Cuesta C., Roberts K., Comandé D., Ciapponi A. Course of chronic Trypanosoma cruzi infection after treatment based on parasitological and serological tests: a systematic review of follow-up studies. PROSPERO. 2012 doi: 10.1371/journal.pone.0139363. CRD42012002162. http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42012002162. [Accessed 6 November 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sguassero Y., Cuesta C.B., Roberts K.N., Hicks E., Comandé D., Ciapponi A. Course of chronic Trypanosoma cruzi infection after treatment based on parasitological and serological tests: a systematic review of follow-up studies. PLoS One. 2015;10 doi: 10.1371/journal.pone.0139363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira C.A.N., Edwin Castillo E., Castro C. Specific treatment evaluation for Trypanosoma cruzi in children in the evolution of the indeterminate phase. Rev Soc Bras Med Trop. 2000;33:191–196. doi: 10.1590/s0037-86822000000200006. [DOI] [PubMed] [Google Scholar]
- Solari A., Ortíz S., Soto A., Arancibia C., Campillay R., Contreras M. Treatment of Trypanosoma cruzi-infected children with nifurtimox: a 3 year follow-up by PCR. J Antimicrob Chemother. 2001;48:515–519. doi: 10.1093/jac/48.4.515. [DOI] [PubMed] [Google Scholar]
- Sosa Estani S., Segura E.L., Ruiz A.M., Velazquez E., Porcel B.M., Yampotis C. Efficacy of chemotherapy with benznidazole in children in the indeterminate phase of Chagas’ disease. Am J Trop Med Hyg. 1998;59:526–529. doi: 10.4269/ajtmh.1998.59.526. [DOI] [PubMed] [Google Scholar]
- Sosa-Estani S., Cura E., Velazquez E., Yampotis C., Segura E.L. Etiological treatment of young women infected with Trypanosoma cruzi, and prevention of congenital transmission. Rev Soc Bras Med Trop. 2009;42:484–487. doi: 10.1590/s0037-86822009000500002. [DOI] [PubMed] [Google Scholar]
- Adapted from Sterne JAC, Higgins JPT, Reeves BC on behalf of the development group for ROBINS-I. ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. Version 5 July 2016. http://www.riskofbias.info. [Accessed 9 June 2016].
- Stewart L.A., Clarke M., Rovers M., Riley R.D., Simmonds M., Stewart G. Preferred reporting items for systematic reviews and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA. 2015;313:1657–1665. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- Streiger M.L., del Barco M.L., Fabbro D.L., Arias E.D., Amicone N.A. Longitudinal study and specific chemotherapy in children with chronic Chagas’ disease, residing in a low endemicity area of Argentina. Rev Soc Bras Med Trop. 2004;37:365–375. doi: 10.1590/s0037-86822004000500001. [DOI] [PubMed] [Google Scholar]
- Therneau T.M., Grambsch P.M. Springer-Verlag Publishers; New York, NY: 2000. Modeling survival data: extending the cox model (Statistics for Biology and Health) [Google Scholar]
- Vallejo M., Reyes P.P.A. Trypanocidal drugs for late stage, symptomatic Chagas disease (Trypanosoma cruzi infection) Cochrane Database Syst Rev. 2005;(4) doi: 10.1002/14651858.CD004102.pub2. Art. No.: CD004102. [DOI] [PubMed] [Google Scholar]
- Vera de Bilbao N., Samudio M., Schinini A., Acosta N., López E., González N. Evaluación a 24 meses post-tratamiento con benznidazol en niños de 6 a 12 años infectados por Tripanosoma Cruzi. Rev Patol Trop. 2004;33:301–312. [Google Scholar]
- Vera de Bilbao N., Elías E., Martínez J., Carpinelli de Tomassone M., Torres S., Sosa L. Evolución serológica y parasitológica post-tratamiento de pacientes con enfermedad de Chagas crónica reciente. Mem Inst Investig Cienc Salud. 2006;4:5–10. [Google Scholar]
- Villar J.C., Perez J.G., Cortes O.L., Riarte A., Pepper M., Marin-Neto J.A. Trypanocidal drugs for chronic asymptomatic Trypanosoma cruzi infection. Cochrane Database Syst Rev. 2014;(5) doi: 10.1002/14651858.CD003463.pub2. CD003463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viotti R., Alarcón de Noya B., Araujo-Jorge T., Grijalva M.J., Guhl F., López M.C. Towards a paradigm shift in the treatment of chronic Chagas disease. J Antimicrob Chemother. 2014;58:635–639. doi: 10.1128/AAC.01662-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingales B., Miles M.A., Campbell D.A., Tibayrenc M., Macedo A.M., Teixeira M.M. The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect Genet Evol. 2012;12:240–253. doi: 10.1016/j.meegid.2011.12.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.