Abstract
Prolactin is a 23 kDa single chain protein of 199 amino acids synthesised and released principally by lactotrophs in the anterior pituitary gland. The secretion is mainly under inhibitory control by hypothalamic dopamine and regulated in a negative feedback manner, with prolactin itself providing the afferent signal: short-loop feedback. The main function of prolactin is during pregnancy and lactation in the development of mammary glands, milk synthesis and maintenance of milk secretion. Serum prolactin levels rise rapidly during pregnancy with increase in the size and number of lactotrophs. During lactation suckling induces rapid secretion of prolactin via a neuroendocrine reflex pathway. In the absence of pregnancy, hyperprolactinaemia may present with symptoms of hypogonadotropic hypogonadism including menstrual disturbance and infertility or visual symptoms from a pituitary mass effect by a prolactinoma, the most common pituitary tumour. Hyperprolactinaemia is diagnosed by laboratory measurement of serum prolactin. There is considerable variability in routinely available prolactin immunoassays as a result of differing reactivity towards monomeric prolactin and macroprolactin and lack of commutability of the WHO 3rd International Standard between routine methods. Macroprolactinaemia is a relatively common cause of interference in the prolactin assay that may lead to incorrect diagnosis and unnecessary investigations. Measurement of prolactin post polyethylene glycol precipitation (PEG) when prolactin levels are above the reference interval is routinely used to identify macroprolactin, however harmonisation of PEG precipitation process and reporting may improve clinical care.
Introduction
A lactogenic effect from injecting anterior pituitary extracts into castrated virgin rabbits was first demonstrated in 1928 by Stricher and Greuter.1 Then in 1933 Riddle and colleagues purified the responsible hormone and named it prolactin in recognition of its major role in lactation.2 Since that time prolactin has been shown to have more than 300 actions across reproduction, metabolic, fluid and immune regulation systems and mediated via endocrine, autocrine and paracrine activities.3 In this review we aim to update the reader on molecular, biological and analytical aspects of this fascinating hormone.
Prolactin Chemistry and Biology
Molecular Forms
In humans, prolactin is encoded by a single gene on chromosome 6 which consists of six exons and four introns.4 Following cleavage of the 28 amino acid signal peptide, the mature 23 kDa protein consists of 199 amino acids.5 It belongs to the cytokine family of proteins, characterised by a 3D structure comprising four antiparallel α helices, and has strong structural homology with growth hormone and placental lactogen.6
Numerous variants of the prolactin protein have been identified, many of which result from post-translational modifications of the mature protein including phosphorylation, glycosylation, sulfation and deamidation.6,7 In addition to monomeric 23 kDa prolactin, two other major forms are present in the circulation: ‘big prolactin’ and ‘big-big prolactin’ (macroprolactin). Big prolactin is the dimer of monomeric form and big-big prolactin comprises high molecular mass (>150 kDa) complexes of 23 kDa prolactin and IgG autoantibodies. Both these forms have minimal biological activity.8 Figure 1 depicts the structure of monomeric prolactin, big prolactin and big-big prolactin.9
Figure 1.
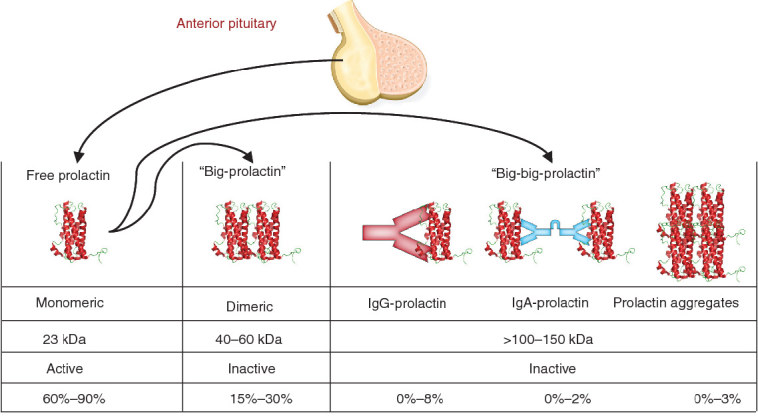
Structure of monomeric prolactin, “big-prolactin” and “big-big prolactin”. Figure 1 adapted from reference 9 with permission.
Proteolytic cleavage of the 23 kDa protein also generates smaller prolactin variants of 14 kDa, 16 kDa and 22 kDa prolactin.6 The 16 kDa variant is a product of cleavage of prolactin at the long loop that connects the third and fourth α helices. This cleavage can occur outside the cells in the interstitial medium and, therefore, in the vicinity of blood capillaries. The 16 kDa prolactin is also known as vasoinhibin due to its inherent antiangiogenic properties by binding to endothelial cells.10
Prolactin Receptor
The prolactin receptor (PRLR) is a member of the haematopoietic cytokine receptor superfamily.3 It comprises of an extracellular domain, a single transmembrane domain and an intracellular signal-transducing domain. The extracellular domain contains two disulfide bridges that are essential for ligand binding. The cytoplasmic domain contains two regions (Box 1 and Box 2) that are highly conserved among cytokine receptors. Box 1 is a membrane-proximal region composed of eight amino acids, is very rich in proline and hydrophobic residues and adopts a consensus folding conformation that is specifically recognised by transducing tyrosine kinases.
The human PRLR gene, located on chromosome 5, contains at least 10 exons, but alternative splicing results in several different isoforms.3 These isoforms have an identical extracellular domain, but differ in the size and sequence of the intracellular portion which can be short, intermediate or long. A soluble PRLR (PRL-binding protein), which contains only the extracellular domain of the membrane receptor has also been identified.11 The main isoform of PRLR found in humans is the long form.11 In addition to prolactin, the human PRLR can also bind placental lactogen and growth hormone.12
Molecular Signalling and Effect
Human PRLR dimers associated via the transmembrane domain are constitutively expressed on cell surfaces.13,14 PRLR heterodimers of long and short isoforms are functionally inactive and homodimers require binding by a ligand for signal transmission because PRLR does not have intrinsic tyrosine kinase activity. Binding of ligand (prolactin) to two extracellular interaction sites on pre-dimerised PRLRs triggers a change in the conformation of the receptor dimer leading to the initiation of intracellular signal transduction.15
Prolactin signalling through the long isoform activates many kinases including receptor associated JAK-2, Src family of tyrosine kinases (Src) and phosphatidylinositol 3-kinase (PI3K)/AKT, mitogen-activated protein kinase (MAPK)3 and serine/threonine kinase Nek3–Vav2–Rac1 pathways.16–19 JAK2 phosphorylates tyrosine residues on the intracellular part of the receptor and autophosphorylates residues within itself. JAK2 also phosphorylates cytoplasmic members of the signal transducer and activator of transcription (Stat) family. Activation of Src is required for cell proliferation (including Src kinase) which stimulates expression of the long PRLR isoform as well as downstream signalling pathways in the mammary gland. An emergent member of the prolactin signalling cascade, Arf-GAP with GTPase, ANK-repeat and PH-domain-containing protein 2 (commonly known as PI3-kinase enhancerA; PIKEA), associates directly with both STAT5 and PRLR, which is an essential event for prolactin-induced activation of STAT5 and subsequent gene transcription.20 These signalling events induce several prolactin-responsive genes, such as those encoding proteins involved in cell proliferation and cell differentiation.
Regulation of Prolactin Secretion
Although inhibitory regulation of prolactin secretion was clearly demonstrated in the 1950s, it was not until much later that dopamine was established as the principal factor responsible for the inhibition of prolactin synthesis and secretion.21 The critical role of dopamine in suppressing endogenous prolactin secretion was clearly demonstrated by the identification of dopamine receptors in anterior pituitary lactotrophs and the observation that mice lacking the dopamine D2 receptor were hyperprolactinaemic.22,23
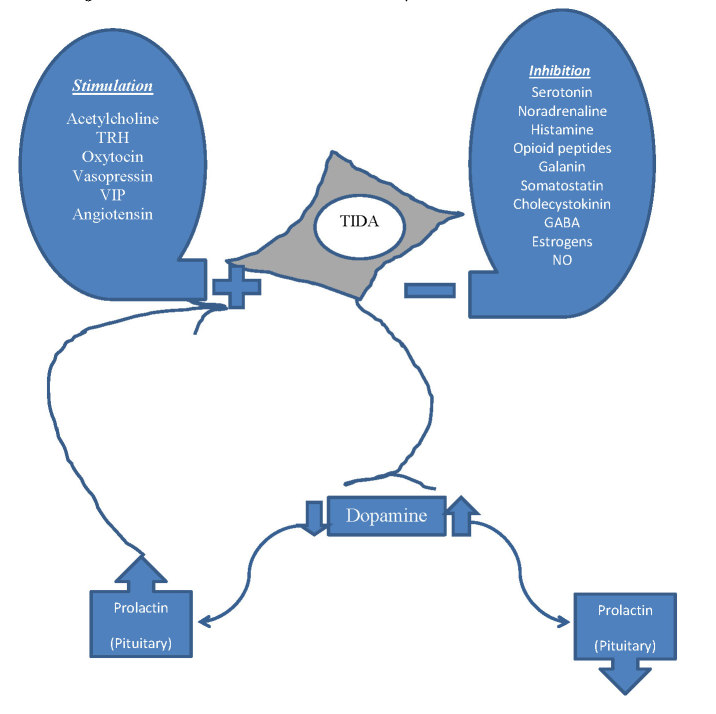
The dopamine neurons that control prolactin secretion are located within the arcuate nucleus of the hypothalamus. These neurons have been subdivided into three sub-populations based on the anatomy of their projections: the tuberoinfundibular (TIDA), tuberohypophyseal (THDA), and periventricular hypophyseal (PHDA) dopaminergic neurons.5 TIDA neurons arise from the dorsomedial arcuate nucleus and project to the external zone of the median eminence secreting dopamine into the pituitary portal blood vessels. THDA and PHDA neurons have their cell bodies located slightly more rostrally, but their projections pass through the median eminence to the hypophysis and dopamine secretion into the short portal vessels. While anatomically distinct, all three populations appear to be regulated similarly.24 TIDA neurons exhibit a robust oscillation between hyperpolarised and depolarised states, and a spontaneous firing during the depolarised ‘up-state’. Patterns of firing of an individual TIDA neuron were reflected in the pattern of dopamine release from the population, suggesting that the neurons act as a synchronous network to release dopamine in a pulsatile or phasic fashion.25
Of the five dopamine receptors, the two D2-like receptors, D2 and D4 are found in the pituitary gland. Lactotrophs are the only anterior pituitary cells that display spontaneous electrical activity in the absence of hypothalamic stimulation. Ca2+ influx through voltage-gated Ca2+ channels (VGCC) stimulates prolactin secretion which accounts for the basal prolactin secretion, and is consistent with a regulatory mechanism primarily based on inhibition. Dopamine inhibits calcium influx resulting in membrane hyperpolarisation which inhibits prolactin secretion. Furthermore it suppresses adenylate cyclase thereby inhibiting prolactin gene expression.26 Dopamine also plays a significant role in the regulation of lactotroph proliferation as well as in suppression of oestradiol-induced proliferation.
Numerous factors have been identified to act on dopamine neurones, thereby indirectly regulating prolactin secretion (Figure 2). Factors that inhibit actions of the dopamine neurons thereby stimulating prolactin release include serotonin, noradrenaline, histamine, opioids, galanin, somatostatin, cholecystokinin, ɣ amino butyric acid (GABA), nitric oxide and oestrogen.5 Oestradiol also regulates prolactin gene expression to promote prolactin secretion.27 Factors that stimulate dopamine neurons and thereby inhibit prolactin release include acetylcholine, thyrotropin releasing hormone, oxytocin, vasopressin, vasoactive intestinal peptide, pituitary adenylate cyclase-activating peptide, angiotensin II, neurotensin, neuropeptide Y, calcitonin, bombesin like peptides, atrial natriuretic peptide and prolactin itself.28
Figure 2.
Factors affecting dopamine neurons and control of prolactin secretion and short loop feedback. TIDA = Tuberoinfundibular dopaminergic neurons, TRH = Thyrotropin releasing hormone, VIP = Vasoactive intestinal polypeptide, GABA = ɣ amino butyric acid, NO = nitric oxide
Short-loop Feedback
Although hypothalamo-prolactin lacks the classical anterior pituitary hormone-mediated negative feedback pathway, it is still regulated in a negative feedback manner, with prolactin itself providing the afferent signal. Prolactin regulation of dopamine neurons is mediated by multiple mechanisms over different time courses. The time course of prolactin action has a ‘rapid’ component of increased activity and a delayed component.
Electrophysiological data have shown that prolactin induces a fourfold increase in firing rate within seconds to minutes, acutely changing the firing pattern from a basal phasic pattern to a tonically active pattern.25,29,30 The very rapid action involves two components: a low voltage component from transient receptor potential (TRP)-like current and high voltage component from inhibition of a Ca2+-dependent current. The slower component, with a time course of minutes to hours, likely involves prolactin induced serine phosphorylation of tyrosine hydroxylase resulting in increased enzyme activity. The delayed component of prolactin action on dopamine neurons is likely through reduced expression tyrosine hydroxylase.31
Together, these three layers of regulation provide tight homeostatic control, with prolactin rapidly increasing the firing rate of these neurons to induce increased dopamine secretion and rapid suppression of further prolactin secretion from the lactotroph. At the same time, slower but more prolonged changes in tyrosine hydroxylase phosphorylation and transcriptional events regulate dopamine production and secretion over a much longer time-course.31
Biological Functions/Effects of Prolactin
Lactotrophic Functions
The main role of prolactin is promoting milk synthesis and maintaining lactation postpartum. In pregnant women, increasing oestrogen secretion stimulates proliferation of the lactotrophs, resulting in increased prolactin secretion. Prolactin stimulates mammary gland growth and, together with oestradiol, progesterone, placental lactogen, insulin and cortisol prepares the breast for postpartum lactation. At the same time, high oestrogen concentrations inhibit the lactotropic effect of prolactin in the mammary gland. The fall in oestrogen levels to non-pregnant levels after delivery results in the initiation of lactation.
Adaptations to Short-loop Feedback during Lactation
Normal negative feedback regulation of prolactin secretion dominates until late pregnancy. Placental lactogens produced during pregnancy bind to and activate PRLR stimulating prolactin responsive functions and bypasses the feedback inhibition although prolactin remains dominant over placental lactogens in human pregnancy. The continued increase in prolactin during human pregnancy is thought to be due to stimulatory effect of oestrogen on TIDA neurons thereby inhibiting dopamine release as well as its stimulatory effect on lactotrophs resulting in hyperplasia.32
In late pregnancy there is a decrease in activity of the hypothalamic dopamine neurons associated with a nocturnal surge in pituitary prolactin secretion immediately before parturition. Hypothalamic dopamine neurons no longer release dopamine in response to prolactin or placental lactogen, rendering the short-loop negative feedback system functionally inactive and this adaptation persists during lactation.33 Although PRLR expression in the dopamine neurons is maintained and acute electrophysiological responses to prolactin persist, there is a change in the cellular response downstream of the PRLR. Phosphorylation of tyrosine hydroxylase is decreased and activation of STAT5b in dopamine neurons is also reduced during lactation.34,35 At the same time, there is an increase in met-enkephalin expression in the dopamine neurons and it seems possible that elevated prolactin may drive this met-enkephalin expression.36,37 Hence, the neurons essentially change their phenotype, changing from being dopaminergic to enkephalinergic possibly mediating a completely different function of prolactin in the brain during lactation.
There is a highly coordinated release of prolactin during lactation, caused by the suckling stimulus. It has been postulated that a suckling induced ‘prolactin-releasing factor’ may be involved in stimulating prolactin secretion at this time.38 It is well established that enkephalin can promote prolactin secretion.39 While most evidence suggests that this effect is mediated centrally through regulation of TIDA neurons, it can also act in the pituitary gland to antagonise dopaminergic inhibition of lactotrophs.40
Recent advances in understanding neuronal pathways suggest a direct neuronal pathway conveying the suckling stimulus by transmitting the somatosensory afferent information from the nipple via the spinal cord to the hypothalamus. Neurons in this pathway express the peptide tuberoinfundibular peptide of 39 residues (TIP39).41,42 This peptide, acting through the parathyroid hormone 2 receptor to suppress activity of TIDA neurons, may be a critical regulator of prolactin secretion in response to suckling.43
Reproductive Effects
The main mechanism by which prolactin influences the gonads is by inhibition of gonadotropin-releasing hormone (GnRH) secretion leading to hypogonadotropic hypogonadism. Although GnRH neurons were thought to be directly regulated by prolactin, it has been demonstrated that only a very small percentage of GnRH neurons express the PRLRs and membrane excitability of GnRH neurons is not acutely modulated by prolactin.44,45 Recent studies have shown that prolactin may modulate the reproductive axis by acting on a specific population of hypothalamic neurons that express the Kiss1 gene.46,47 The Kiss1 gene encodes neuropeptides, known as kisspeptins, that are critically involved in reproduction. Most of these neurons co-express the PRLRs and an acute prolactin stimulus can induce pSTAT5 in Kiss1-expressing neurons. Kisspeptin1 reduces secretion of GnRHI from hypothalamic neurons resulting in reduced LH and FSH secretion and loss of ovarian stimulation, which can result in infertility.48 Prolactin also decreases sensitivity of the luteinizing hormone (LH) and of the follicle-stimulating hormone (FSH) receptors in the gonads.
Effects on Other Hormones
Prolactin improves glucose homeostasis by increasing β-cell mass under certain conditions such as pregnancy, whereas hyperprolactinaemia due to a pituitary gland adenoma exacerbates insulin resistance.49,50 In diabetic rats, it was observed that high levels of prolactin exacerbate insulin resistance and impair the insulin-secretory capacity, in contrast to the normal adaptive increases in glucose-stimulated insulin secretion and insulin sensitivity realised with moderately increased prolactin levels.50 Prolactin also enhances dihydroepiandrosterone (DHEA), cortisol and aldosterone secretion by the adrenal cortex cells.51
Effects on the Immunological System
Prolactin is also produced by lymphocytes and other immune cells. It acts as a cytokine and plays an important role in human immune responses.52 Prolactin effects on immunological systems may depend on concentration, resulting in immunostimulation at modest levels and inhibition at high levels.53,54 For example, in many cases of autoimmune diseases, the severity of the disease is lower or even remitted during pregnancy when serum prolactin level is elevated.53 On the other hand, there is an association between autoimmune diseases and moderate hyperprolactinaemia suggesting that prolactin is implicated in the initiation of the autoimmune reactions.54
Other Functions
Prolactin is involved in osmoregulation, acting to increase water and salt absorption in all segments of the bowel and reduce renal Na+ and K+ excretion.55 It can also stimulate proliferation, differentiation and migration of neuronal stem cells.56 It has been observed that prolactin has a proliferative effect on glial progenitors and oligodendrocyte precursor cells, leading to myelination of central nervous system (CNS).55
Hyperprolactinaemia
‘Hyperprolactinaemia’ refers to an increase in circulating prolactin levels, usually producing reproductive problems in both sexes, particularly anovulatory infertility in women. The prevalence of hyperprolactinaemia is difficult to establish due to the non-specific nature of the symptoms and because not all patients are symptomatic or undergo prolactin measurements. Estimates of prevalence have been reported as approximately 0.4% in unselected normal population, 5% in a family planning clinic population and 17% in women with reproductive disorders.57
Table 1 shows some clinical settings where prolactin measurements are commonly requested.
Table 1.
Clinical settings where prolactin is commonly requested.
| Investigation of: |
|---|
| Menstrual irregularities including amenorrhea and oligomenorrhea |
| Infertility |
| Galactorrhea |
| Sexual dysfunction |
| Gynaecomastia in men |
| Suspected pituitary dysfunction |
| Suspected pituitary mass lesion |
| Prior to initiation of some antipsychotic medications (baseline) |
| Monitoring of prolactin levels in patients on antipsychotic medications |
Causes of Hyperprolactinaemia
Hyperprolactinaemia may be divided into three types: functional/physiological, factitious/analytical, and pathological hyperprolactinaemia.
An example of physiological hyperprolactinaemia is the marked prolactin increase seen in pregnancy and during lactation. Increased concentrations may also occur with high-protein diets, stress (including venepuncture), physical exertion, hypoglycaemia, or sexual intercourse,58 prolactin levels in these situations are typically less than 1000 mIU/L. Functional hyperprolactinaemia secondary to the use of therapeutic medications that inhibit secretion of dopamine or its action in the pituitary is common;59,60 increases up to ten-fold over baseline may be seen. Some medications known to cause hyperprolactinaemia are shown in Table 2.
Table 2.
Medications that cause hyperprolactinaemia.
| Drug Class | Examples |
|---|---|
| Dopamine receptor 2 antagonists | |
| First generation antipsychotics | Chlorpromazine Fluphenazine Haloperidol |
| Second generation antipsychotics | Paliperidone Risperidone Quetiapine Amisulpride |
| Other D2 receptor antagonists | Amoxapine Metoclopramide Domperidone |
| Antidepressants | Amitriptyline Desipramine Clomipramine Amoxapine |
| Mono amine oxidase inhibitors | Pargyline Clorgyline |
| Antihypertensives | Methyldopa Verapamil |
| Opioid analgesics | Morphine Methadone |
Analytical causes of falsely elevated prolactin levels are also not uncommon. These include macroprolactinaemia and analytical interference by human anti-mouse monoclonal antibody (HAMA) or heterophile antibodies. Macroprolactinaemia is present in 3.7% of the general population61 and in 10–25% in patients with raised prolactin results.62–66
Pathologic hyperprolactinaemia is mainly due to sellar and parasellar lesions, including pituitary adenomas (prolactinoma, growth hormone/prolactin-secreting and adrenocorticotropic hormone/prolactin-secreting adenomas), non-pituitary tumours and infiltrative conditions such as sarcoidosis, craniopharyngioma, empty sella syndrome, vascular malformations, pituitary metastases or non-functioning adenomas compressing the pituitary stalk). Prolactin levels >2000 mIU/L are rare in hyperprolactinaemia secondary to a non-functioning pituitary adenoma.67
Prolactinomas are the most frequent organic cause of prolactin excess and the most common hormone-secreting pituitary tumours. They represent approximately 40% of all pituitary tumours with an estimated prevalence of 100 per 1 million populations.68,69 Prolactinomas are mainly diagnosed in women aged 20–40 years. After the fifth decade of life, frequency of prolactinomas becomes similar in both sexes. Prolactinomas may infrequently present as a component of multiple endocrine neoplasia type 1 (MEN1) when they seem to be more aggressive than sporadic prolactinomas.70 Women present more commonly with microadenomas (<1 cm in size) than macroadenomas (>1 cm in size), while men present more frequently with macroadenomas than microadenomas. 69
Prolactin values between the upper limits of normal and 2000 mIU/L may be due to psychoactive drugs, oestrogen, renal failure or functional (idiopathic) causes, although these levels can also be caused by microprolactinomas.71 In general, serum prolactin levels parallel tumour size. Most patients with prolactin levels over 3000 mIU/L (five times higher than the normal values) will have a prolactinoma. However it should be noted that some drugs such as metoclopramide, risperidone, or phenothiazines may induce prolactin levels higher than 3000 mIU/L. Macroadenomas are typically associated with levels of over 5000 mIU/L, and in some cases over 20000 mIU/L.72 These values are not absolute as prolactinomas can present with variable elevations in prolactin, because there may be dissociation between tumour mass and hormonal secretion. Furthermore, the cause of the hyperprolactinaemia may be due to compression of the pituitary stalk by a tumour other than a prolactinoma.
Hyperprolactinaemia often occurs in polycystic ovary syndrome (PCOS), chronic renal failure, hepatic cirrhosis, epilepsy, injuries of the chest, polycystic ovary syndrome, pseudopregnancy, primary hypothyroidism, Cushing’s disease, and Addison’s disease.73,74
Patients are considered to have idiopathic hyperprolactinaemia when secondary causes have been ruled out and no lesion is seen on pituitary magnetic resonance imaging (MRI). Idiopathic hyperprolactinaemia may have a microadenoma <2 mm which is too small for detection by MRI or may be familial. Familial idiopathic hyperprolactinaemia is caused by a germline heterozygous mutation in PRLR disrupting ligand binding and downstream signalling resulting in prolactin insensitivity.75
Clinical Presentation
Although hyperprolactinaemia is associated with hypogonadotrophic hypogonadism in both sexes, the clinical manifestations are gender-specific. Women generally present with oligomenorrhea or amenorrhea and anovulatory cycles (due to inhibition of LH and FSH pulsatile secretion), galactorrhea and/or infertility. They may be affected by premenstrual syndrome, dyspareunia, hirsutism and tendency to anxiety and depression. In men hyperprolactinaemia typically presents with symptoms such as impotence and loss of libido, gynecomastia/galactorrhea and infertility.76 However, in men, symptoms can be subtle and are often only recognised at an advanced stage.77
Bone loss and progressive atherosclerosis due to an indirect decrease in oestrogens secretion can also occur as a consequence of hypogonadism in both sexes. Furthermore, patients with hyperprolactinaemia have an altered body composition with increased fat mass and reduced lean mass.68
In pre-menopausal women, menstrual disturbance is a common consequence of drug-induced hyperprolactinaemia. Over 30% of women on antipsychotics with hyperprolactinaemia have serum oestradiol levels in the post-menopausal range, and there is an association between the prolactin elevation and the subsequent sex hormone deficiency.78 Symptomatic galactorrhoea may occur in up to 20% of patients.79 A higher rate of sexual dysfunction in patients treated with the prolactin-elevating drugs has also been reported.80 Antipsychotic-induced hyperprolactinaemia results in hypogonadism and significant bone loss may occur in both genders.
Hyperprolactinaemia caused by a pituitary macroadenoma may present with headaches induced by elevated intracranial pressure, visual field defects (initially affecting upper-outer quadrants which may progress to complete bi-temporal hemianopia) caused by optic chiasm compression and diplopia secondary to compression of intracavernous segments of the cranial nerves (III, IV, ophthalmic V1, maxillary V2, VI).76
In 219 hospitalised patients with prolactinoma at diagnosis, men generally were admitted due to symptoms of tumour compression and women for gonadal dysfunction and galactorrhea.68
Diagnosis
The first step in the diagnosis and evaluation of hyperprolactinaemia is a detailed history of the clinical symptoms as well as possible causes of hyperprolactinaemia including medications, comorbidities and lifestyle factors. The physical examination is directed towards evaluation of signs of hypothyroidism, hypogonadism, renal failure and visual field defects.
The main investigations in the diagnosis of a hyperprolactinaemia are hormonal and radiological. A single serum prolactin result obtained without excessive venepuncture stress above the upper limit of normal is sufficient for the diagnosis of hyperprolactinaemia. A fasting blood sample at least 2–3 h after waking up from sleep should be collected. Reference intervals for any given assay are higher in women than in men. Although some dynamic functional tests to differentiate idiopathic from prolactinomas have been proposed, none is recommended. Laboratories should exclude macroprolactinaemia in all samples above the gender-specific reference interval. When the results are doubtful (mild prolactin elevations e.g. due to stress) or inconsistent with the clinical picture, the measurement may be repeated on another day with 2–3 samples taken at 15–20 min intervals to minimise the effect of pulsatility.72
After hyperprolactinaemia is confirmed, imaging with MRI or computerised tomography (CT) is necessary to define the presence of a lesion compatible with a pituitary tumour.
Hypoprolactinaemia
Hypoprolactinaemia has recently been associated with conditions that may be clinically significant, especially in males.81 Low prolactin has been associated with reduced ejaculate and seminal vesicle volume in infertile patients. In addition, low prolactin levels have been associated with erectile dysfunction, premature ejaculation, and have been identified as an independent predictor of major adverse cardiovascular events. Furthermore it has also been associated with anxiety or depressive symptoms in men.
Prolactin Assays and Macroprolactinaemia
Prolactin Assays
Prolactin concentrations in blood are predominantly measured in clinical laboratories by automated immunoassay methodology. Current immunoassays typically employ a two-site immunometric or sandwich principle whereby prolactin is allowed to react with both a capture antibody, which is often immobilised on a solid phase, and a labelled antibody which is used for detection. Following capture of the analyte-antibody sandwich and removal of unreacted reagents by a wash step, the signal generated is directly related to the amount of prolactin present. Although such assays show good within-method agreement over a wide concentration range there are considerable between-method differences despite wide adoption of World Health Organization’s third international standard for prolactin, 84/500. This reference material is exclusively 23 kDa monomeric prolactin derived from human pituitaries but demonstrates a lack of commutability between methods.82 Concentration differences are exacerbated in external quality assurance programs. Furthermore there is variation in the reactivity of the assay antibodies towards the different isoforms of prolactin.83 Therefore the reference intervals are assay dependent and vary considerably between methods. The performance of four commonly utilised prolactin assays currently in use in Australia is shown in Figure 3. Some of this bias may be attributable to non-commutability of the material used in the RCPA Endocrine program. True bias should be assessed using patient samples. For example, Figure 4 shows assay bias between Siemens and Roche Prolactin assays performed at the authors’ institution.
Figure 3.
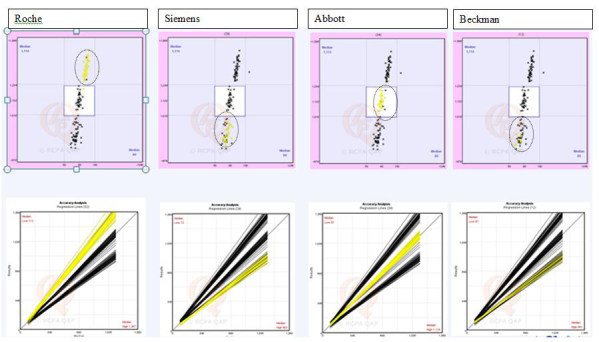
Performance of four commonly used prolactin assays in RCPA Endocrine Program Cycle 47. Top row-Youden plots; Bottom row - corresponding accuracy analysis for Youden plots. With permission from Royal College of Pathologists Australasia Quality Assurance Program (RCPAQAP).
Figure 4.
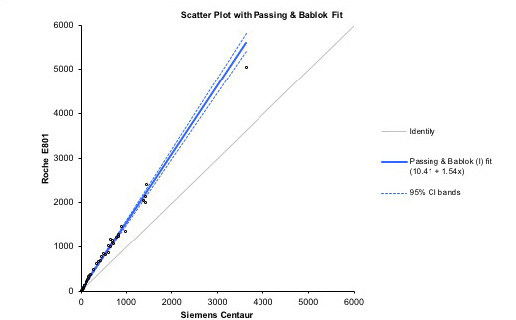
Comparative performance of prolactin between Roche Cobas vs Siemens Centaur on serum samples (n=40).
Cross reactivity in prolactin assays with structurally related molecules such as growth hormone and placental lactogen has been minimised by use of antibodies directed against two different epitopes on the prolactin molecule. However, all sandwich or immunometric assays are susceptible to interference from heterophilic antibodies which typically give falsely elevated prolactin level which may result in unnecessary investigation/interventions and patient anxiety. As manufacturers commonly add “blocking agents” to minimise such interference, heterophile antibody interference is uncommon and assay issues with macroprolactin are more prevalent. As with any immunometric assay, prolactin is also susceptible to high-dose hook effect which causes gross underestimation of prolactin and may consequently miss a prolactinoma.
Macroprolactin
As discussed above, macroprolactinaemia is characterised by the presence of a molecular mass of prolactin greater than 150 kDa as the predominant molecular form of circulating prolactin. Macroprolactin is mostly a complex of prolactin with IgG, especially anti-prolactin autoantibodies.84 Anti-prolactin autoantibodies bind to monomeric prolactin forming a large immune complex of prolactin with IgG. Delayed clearance of this complex increases measured serum prolactin concentrations. As previously mentioned, macroprolactinaemia may be present in 10–25% in patients with hyperprolactinaemia.62–66 Patients with macroprolactinaemia often lack typical clinical symptoms of hyperprolactinaemia as this form is biologically inactive.85 Macroprolactinaemia can lead to clinical dilemmas due to misinterpretations of biochemical testing leading to investigations and interventions which are not only unnecessary but costly and may cause harm. Current best practice recommends that sera with elevated prolactin are subfractionated using polyethylene glycol (PEG) precipitation to provide an indication for whether or not macroproalactin is present.86,87
Detection
Macroprolactin has been found to react in all immunoassays for prolactin that have been tested, albeit to variable extents.88,89 The variable reactivity in assays for prolactin probably reflects the autoantibody masking to different degrees the epitopes targeted by the immunoassay capture or detection antibodies. For example, in the case of the weakly reacting assays, the epitope to which the capture or detection antibody is directed may be sterically inaccessible due to binding of the endogenous anti-prolactin auto-antibody, thus the macroprolactin complex will not be detected.
The reference method for the determination of macroprolactin is size exclusion chromatography (SEC) which allows quantitation of all high molecular mass forms of prolactin in addition to providing an estimate of their molecular mass.8 This technique is time consuming and expensive and generally not routinely available in most clinical laboratories. Alternative techniques based on immunoassay of serum prolactin before and after removal of macroprolactin by ultrafiltration, immune-adsorption of IgG species with protein A, protein G or anti-human IgG and by precipitation with PEG have been described.90 Of these, PEG precipitation is the technique that has been most widely adopted. The technique involves mixing equal volumes of sera and 25% (w/v) PEG reagent. Following centrifugation, the residual prolactin in the supernatant is quantified. Proteins are separated according to their solubility; PEG acts as an inert, molecular “sponge” which absorbs water of hydration from proteins, reducing their solubility and leading to their precipitation. When applied to serum, PEG is relatively specific for precipitation of immunoglobulins and immunoglobulin complexes and so precipitates the most common form of macroprolactin, that containing IgG. PEG has the added advantage of precipitating big prolactin and macroprolactin, however a limitation of PEG precipitation is that approximately 20% of the monomeric prolactin in serum is co-precipitated with IgG. The presence of macroprolactin has also been falsely reported in rare cases where patient sera contained very high concentrations of gamma globulin.91 Furthermore IgA is only partially precipitated with PEG and rare cases of IgA-macroprolactin may not be recognised.92 A high pressure liquid chromatography method for separation of prolactin forms which is suitable for routine use has also been described.93
Reporting Macroprolactin
The results of PEG precipitation tests have traditionally been reported as percent total prolactin recovered after PEG treatment. Recovery post-PEG has been shown to correlate with the proportion of macroprolactin present following SEC and a cut-off of <40% is commonly used to indicate that macroprolactin is the predominant form of immunoreactive prolactin present.8,94 However it does not identify patients with macroprolactin as the predominant form present when the monomeric form is also elevated to a clinically significant level. Furthermore there is a recognition that macroprolactinaemia should be defined as hyperprolactinaemia due to the presence of macroprolactin with normal concentrations of monomeric prolactin. It may be argued that laboratory results should provide a measurement of the clinically relevant monomeric prolactin, which provides the most useful information for clinicians.95 However, because of the wide variation in prolactin assay reactivity and the characteristics of PEG precipitation, reference intervals for monomeric prolactin following PEG precipitation must be determined for each particular assay method.8 There is a wide variability in reporting of prolactin post PEG precipitation; some laboratories report only the pre–PEG prolactin concentration with a comment to clarify whether the predominant form is macroprolactin or otherwise while others report post PEG prolactin with assay specific post PEG reference intervals.94 Professional groups now recommend the latter approach to reporting.87,94
Conclusion
This review brings the reader up to date with developments in understanding the molecular signalling pathways that regulate prolactin synthesis and secretion. Monomeric prolactin is recognised as the biologically active form and accurate monomeric prolactin concentration measurement is essential for the diagnosis of hyperprolactinaemia. While biologically, the major role of prolactin is to promote and maintain lactation, over 300 actions have been described. Clinically prolactin measurement is of particular importance in the evaluation of menstrual irregularities and infertility in women and loss of libido and infertility in men as well as in the investigation of headache with visual field disturbance. Currently the accuracy of prolactin measurement is hampered by a lack of commutability of the widely used reference material, WHO 3rd International Standard, among routine assays as well as variable reactivity of assay antibodies with different prolactin isoforms. As well, all routine assays detect macroprolactin to some extent and laboratory practice in detection and reporting of macroprolactin has yet to be harmonised.
Acknowledgements
We would like to acknowledge the authors of reference 9 for giving permission to adapt Figure 1 from their publication and RCPAQAP for giving permission to use RCPA Endocrine Program cycle 47 data in Figure 3.
Footnotes
Competing interests: None declared.
References
- 1.Stricker P, Grueter F. Action du lobe anterieur de l’hypophyse sur la montee laiteuse. CR Soc Biol Paris. 1928;99:1978–80. [Google Scholar]
- 2.Riddle O, Bates RW, Dykshorn SW. The preparation, identification and assay of prolactin – a hormone of the anterior pituitary. Am J Physiol. 1933;105:191–216. [Google Scholar]
- 3.Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev. 1998;19:225–68. doi: 10.1210/edrv.19.3.0334. [DOI] [PubMed] [Google Scholar]
- 4.Truong AT, Duez C, Belayew A, Renard A, Pictet R, Bell GI, et al. Isolation and characterization of the human prolactin gene. EMBO J. 1984;3:429–37. doi: 10.1002/j.1460-2075.1984.tb01824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- 6.Horseman ND, Yu-Lee LY. Transcriptional regulation by the helix bundle peptide hormones: growth hormone, prolactin, and hematopoietic cytokines. Endocr Rev. 1994;15:627–49. doi: 10.1210/edrv-15-5-627. [DOI] [PubMed] [Google Scholar]
- 7.Walker AM. S179D prolactin: antagonistic agony! Mol Cell Endocrinol. 2007;276:1–9. doi: 10.1016/j.mce.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fahie-Wilson M, Smith TP. Determination of prolactin: the macroprolactin problem. Best Pract Res Clin Endocrinol Metab. 2013;27:725–42. doi: 10.1016/j.beem.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Lippi G, Plebani M. Macroprolactin: searching for a needle in a haystack? Clin Chem Lab Med. 2016;54:519–22. doi: 10.1515/cclm-2015-1283. [DOI] [PubMed] [Google Scholar]
- 10.Clapp C, Aranda J, González C, Jeziorski MC, Martínez de la Escalera G. Vasoinhibins: endogenous regulators of angiogenesis and vascular function. Trends Endocrinol Metab. 2006;17:301–7. doi: 10.1016/j.tem.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Trott JF, Hovey RC, Koduri S, Vonderhaar BK. Multiple new isoforms of the human prolactin receptor gene. Adv Exp Med Biol. 2004;554:495–9. doi: 10.1007/978-1-4757-4242-8_71. [DOI] [PubMed] [Google Scholar]
- 12.Goffin V, Shiverick KT, Kelly PA, Martial JA. Sequence-function relationships within the expanding family of prolactin, growth hormone, placental lactogen, and related proteins in mammals. Endocr Rev. 1996;17:385–410. doi: 10.1210/edrv-17-4-385. [DOI] [PubMed] [Google Scholar]
- 13.Qazi AM, Tsai-Morris CH, Dufau ML. Ligand-independent homo- and heterodimerization of human prolactin receptor variants: inhibitory action of the short forms by heterodimerization. Mol Endocrinol. 2006;20:1912–23. doi: 10.1210/me.2005-0291. [DOI] [PubMed] [Google Scholar]
- 14.Gadd SL, Clevenger CV. Ligand-independent dimerization of the human prolactin receptor isoforms: functional implications. Mol Endocrinol. 2006;20:2734–46. doi: 10.1210/me.2006-0114. [DOI] [PubMed] [Google Scholar]
- 15.Goffin V, Martial JA, Summers NL. Use of a model to understand prolactin and growth hormone specificities. Protein Eng. 1995;8:1215–31. doi: 10.1093/protein/8.12.1215. [DOI] [PubMed] [Google Scholar]
- 16.Fresno Vara JA, Cáceres MA, Silva A, Martín-Pérez J. Src family kinases are required for prolactin induction of cell proliferation. Mol Biol Cell. 2001;12:2171–83. doi: 10.1091/mbc.12.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García-Martínez JM, Calcabrini A, González L, Martín-Forero E, Agulló-Ortuño MT, Simon V, et al. A non-catalytic function of the Src family tyrosine kinases controls prolactin-induced Jak2 signaling. Cell Signal. 2010;22:415–26. doi: 10.1016/j.cellsig.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Berlanga JJ, Gualillo O, Buteau H, Applanat M, Kelly PA, Edery M. Prolactin activates tyrosyl phosphorylation of insulin receptor substrate 1 and phosphatidylinositol-3-OH kinase. J Biol Chem. 1997;272:2050–2. doi: 10.1074/jbc.272.4.2050. [DOI] [PubMed] [Google Scholar]
- 19.Miller SL, DeMaria JE, Freier DO, Riegel AM, Clevenger CV. Novel association of Vav2 and Nek3 modulates signaling through the human prolactin receptor. Mol Endocrinol. 2005;19:939–49. doi: 10.1210/me.2004-0443. [DOI] [PubMed] [Google Scholar]
- 20.Chan CB, Liu X, Ensslin MA, Dillehay DL, Ormandy CJ, Sohn P, et al. PIKE-A is required for prolactin-mediated STAT5a activation in mammary gland development. EMBO J. 2010;29:956–68. doi: 10.1038/emboj.2009.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacLeod RM, Fontham EH, Lehmeyer JE. Prolactin and growth hormone production as influenced by catecholamines and agents that affect brain catecholamines. Neuroendocrinology. 1970;6:283–94. doi: 10.1159/000121933. [DOI] [PubMed] [Google Scholar]
- 22.Kelly MA, Rubinstein M, Asa SL, Zhang G, Saez C, Bunzow JR, et al. Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron. 1997;19:103–13. doi: 10.1016/s0896-6273(00)80351-7. [DOI] [PubMed] [Google Scholar]
- 23.Saiardi A, Bozzi Y, Baik JH, Borrelli E. Antiproliferative role of dopamine: loss of D2 receptors causes hormonal dysfunction and pituitary hyperplasia. Neuron. 1997;19:115–26. doi: 10.1016/s0896-6273(00)80352-9. [DOI] [PubMed] [Google Scholar]
- 24.van den Pol AN, Herbst RS, Powell JF. Tyrosine hydroxylase-immunoreactive neurons of the hypothalamus: a light and electron microscopic study. Neuroscience. 1984;13:1117–56. doi: 10.1016/0306-4522(84)90292-6. [DOI] [PubMed] [Google Scholar]
- 25.Romanò N, Yip SH, Hodson DJ, Guillou A, Parnaudeau S, Kirk S, et al. Plasticity of hypothalamic dopamine neurons during lactation results in dissociation of electrical activity and release. J Neurosci. 2013;33:4424–33. doi: 10.1523/JNEUROSCI.4415-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishida M, Mitsui T, Yamakawa K, Sugiyama N, Takahashi W, Shimura H, et al. Involvement of cAMP response element-binding protein in the regulation of cell proliferation and the prolactin promoter of lactotrophs in primary culture. Am J Physiol Endocrinol Metab. 2007;293:E1529–37. doi: 10.1152/ajpendo.00028.2007. [DOI] [PubMed] [Google Scholar]
- 27.Lieberman ME, Maurer RA, Claude P, Wiklund J, Wertz N, Gorski J. Regulation of pituitary growth and prolactin gene expression by estrogen. Adv Exp Med Biol. 1981;138:151–63. doi: 10.1007/978-1-4615-7192-6_9. [DOI] [PubMed] [Google Scholar]
- 28.Ciechanowska M, Misztal T, Przekop P. Prolactin and the physiological regulation of its secretion. A review. J Anim Feed Sci. 2013;22:79–89. [Google Scholar]
- 29.Brown RS, Piet R, Herbison AE, Grattan DR. Differential actions of prolactin on electrical activity and intracellular signal transduction in hypothalamic neurons. Endocrinology. 2012;153:2375–84. doi: 10.1210/en.2011-2005. [DOI] [PubMed] [Google Scholar]
- 30.Lyons DJ, Horjales-Araujo E, Broberger C. Synchronized network oscillations in rat tuberoinfundibular dopamine neurons: switch to tonic discharge by thyrotropin-releasing hormone. Neuron. 2010;65:217–29. doi: 10.1016/j.neuron.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 31.Grattan DR, LeTissier P. Hypothalamic control of prolactin secretion, and the multiple reproductive functions of prolactin. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill’s Physiology of Reproduction. 4th ed. Amsterdam: Elsevier; 2015. pp. 469–526. [Google Scholar]
- 32.Kletzky OA, Marrs RP, Howard WF, McCormick W, Mishell DR., Jr Prolactin synthesis and release during pregnancy and puerperium. Am J Obstet Gynecol. 1980;136:545–50. doi: 10.1016/0002-9378(80)90686-9. [DOI] [PubMed] [Google Scholar]
- 33.Grattan DR, Steyn FJ, Kokay IC, Anderson GM, Bunn SJ. Pregnancy-induced adaptation in the neuroendocrine control of prolactin secretion. J Neuroendocrinol. 2008;20:497–507. doi: 10.1111/j.1365-2826.2008.01661.x. [DOI] [PubMed] [Google Scholar]
- 34.Anderson ST, Barclay JL, Fanning KJ, Kusters DH, Waters MJ, Curlewis JD. Mechanisms underlying the diminished sensitivity to prolactin negative feedback during lactation: reduced STAT5 signaling and up-regulation of cytokine-inducible SH2 domain-containing protein (CIS) expression in tuberoinfundibular dopaminergic neurons. Endocrinology. 2006;147:1195–202. doi: 10.1210/en.2005-0905. [DOI] [PubMed] [Google Scholar]
- 35.Steyn FJ, Anderson GM, Grattan DR. Hormonal regulation of suppressors of cytokine signaling (SOCS) messenger ribonucleic acid in the arcuate nucleus during late pregnancy. Endocrinology. 2008;149:3206–14. doi: 10.1210/en.2007-1623. [DOI] [PubMed] [Google Scholar]
- 36.Merchenthaler I. Induction of enkephalin in tuberoinfundibular dopaminergic neurons during lactation. Endocrinology. 1993;133:2645–51. doi: 10.1210/endo.133.6.7694844. [DOI] [PubMed] [Google Scholar]
- 37.Nahi F, Arbogast LA. Prolactin modulates hypothalamic preproenkephalin, but not proopiomelanocortin, gene expression during lactation. Endocrine. 2003;20:115–22. doi: 10.1385/ENDO:20:1-2:115. [DOI] [PubMed] [Google Scholar]
- 38.Crowley WR. Neuroendocrine regulation of lactation and milk production. Compr Physiol. 2015;5:255–91. doi: 10.1002/cphy.c140029. [DOI] [PubMed] [Google Scholar]
- 39.Cusan L, Dupont A, Kledzik GS, Labrie F, Coy DH, Schally AV. Potent prolactin and growth hormone releasing activity of more analogues of Met-enkephalin. Nature. 1977;268:544–7. doi: 10.1038/268544a0. [DOI] [PubMed] [Google Scholar]
- 40.Enjalbert A, Ruberg M, Arancibia S, Priam M, Kordon C. Endogenous opiates block dopamine inhibition of prolactin secretion in vitro. Nature. 1979;280:595–7. doi: 10.1038/280595a0. [DOI] [PubMed] [Google Scholar]
- 41.Cservenák M, Bodnár I, Usdin TB, Palkovits M, Nagy GM, Dobolyi A. Tuberoinfundibular peptide of 39 residues is activated during lactation and participates in the suckling-induced prolactin release in rat. Endocrinology. 2010;151:5830–40. doi: 10.1210/en.2010-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dobolyi A. Novel potential regulators of maternal adaptations during lactation: tuberoinfundibular peptide 39 and amylin. J Neuroendocrinol. 2011;23:1002–8. doi: 10.1111/j.1365-2826.2011.02127.x. [DOI] [PubMed] [Google Scholar]
- 43.Dobolyi A, Dimitrov E, Palkovits M, Usdin TB. The neuroendocrine functions of the parathyroid hormone 2 receptor. Front Endocrinol (Lausanne) 2012;3:121. doi: 10.3389/fendo.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grattan DR, Jasoni CL, Liu X, Anderson GM, Herbison AE. Prolactin regulation of gonadotropin-releasing hormone neurons to suppress luteinizing hormone secretion in mice. Endocrinology. 2007;148:4344–51. doi: 10.1210/en.2007-0403. [DOI] [PubMed] [Google Scholar]
- 45.Kokay IC, Petersen SL, Grattan DR. Identification of prolactin-sensitive GABA and kisspeptin neurons in regions of the rat hypothalamus involved in the control of fertility. Endocrinology. 2011;152:526–35. doi: 10.1210/en.2010-0668. [DOI] [PubMed] [Google Scholar]
- 46.Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev. 2012;92:1235–316. doi: 10.1152/physrev.00037.2010. [DOI] [PubMed] [Google Scholar]
- 47.Iwata K, Ikehara M, Kunimura Y, Ozawa H. Interactions between kisspeptin neurons and hypothalamic tuberoinfundibular dopaminergic neurons in aged female rats. Acta Histochem Cytochem. 2016;49:191–6. doi: 10.1267/ahc.16027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bernard V, Young J, Chanson P, Binart N. New insights in prolactin: pathological implications. Nat Rev Endocrinol. 2015;11:265–75. doi: 10.1038/nrendo.2015.36. [DOI] [PubMed] [Google Scholar]
- 49.Sorenson RL, Brelje TC. Adaptation of islets of Langerhans to pregnancy: beta-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm Metab Res. 1997;29:301–7. doi: 10.1055/s-2007-979040. [DOI] [PubMed] [Google Scholar]
- 50.Park S, Kim DS, Daily JW, Kim SH. Serum prolactin concentrations determine whether they improve or impair β-cell function and insulin sensitivity in diabetic rats. Diabetes Metab Res Rev. 2011;27:564–74. doi: 10.1002/dmrr.1215. [DOI] [PubMed] [Google Scholar]
- 51.Glasow A, Breidert M, Haidan A, Anderegg U, Kelly PA, Bornstein SR. Functional aspects of the effect of prolactin (PRL) on adrenal steroidogenesis and distribution of the PRL receptor in the human adrenal gland. J Clin Endocrinol Metab. 1996;81:3103–11. doi: 10.1210/jcem.81.8.8768882. [DOI] [PubMed] [Google Scholar]
- 52.Chavez-Rueda K, Hérnández J, Zenteno E, Leaños-Miranda A, Legorreta-Haquet MV, Blanco-Favela F. Identification of prolactin as a novel immunomodulator on the expression of co-stimulatory molecules and cytokine secretions on T and B human lymphocytes. Clin Immunol. 2005;116:182–91. doi: 10.1016/j.clim.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 53.Zoli A, Lizzio MM, Ferlisi EM, Massafra V, Mirone L, Barini A, et al. ACTH, cortisol and prolactin in active rheumatoid arthritis. Clin Rheumatol. 2002;21:289–93. doi: 10.1007/s100670200076. [DOI] [PubMed] [Google Scholar]
- 54.Imrich R. The role of neuroendocrine system in the pathogenesis of rheumatic diseases (minireview) Endocr Regul. 2002;36:95–106. [PubMed] [Google Scholar]
- 55.Breves JP, Serizier SB, Goffin V, McCormick SD, Karlstrom RO. Prolactin regulates transcription of the ion uptake Na+/Cl- cotransporter (ncc) gene in zebrafish gill. Mol Cell Endocrinol. 2013;369:98–106. doi: 10.1016/j.mce.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pathipati P, Gorba T, Scheepens A, Goffin V, Sun Y, Fraser M. Growth hormone and prolactin regulate human neural stem cell regenerative activity. Neuroscience. 2011;190:409–27. doi: 10.1016/j.neuroscience.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 57.Biller BM, Luciano A, Crosignani PG, Molitch M, Olive D, Rebar R, et al. Guidelines for the diagnosis and treatment of hyperprolactinemia. J Reprod Med. 1999;44(Suppl):1075–84. [PubMed] [Google Scholar]
- 58.Karasek M, Pawlikowski M. Hyperprolactinemia – the Essentials. European Endocrinology. 2006;1:53–7. [Google Scholar]
- 59.Torre DL, Falorni A. Pharmacological causes of hyperprolactinemia. Ther Clin Risk Manag. 2007;3:929–51. [PMC free article] [PubMed] [Google Scholar]
- 60.Madhusoodanan S, Parida S, Jimenez C. Hyperprolactinemia associated with psychotropics—a review. Hum Psychopharmacol. 2010;25:281–97. doi: 10.1002/hup.1116. [DOI] [PubMed] [Google Scholar]
- 61.Hattori N, Ishihara T, Saiki Y. Macroprolactinaemia: prevalence and aetiologies in a large group of hospital workers. Clin Endocrinol (Oxf) 2009;71:702–8. doi: 10.1111/j.1365-2265.2009.03570.x. [DOI] [PubMed] [Google Scholar]
- 62.Bjøro T, Mørkrid L, Wergeland R, Turter A, Kvistborg A, Sand T, et al. Frequency of hyperprolactinaemia due to large molecular weight prolactin (150–170 kD PRL) Scand J Clin Lab Invest. 1995;55:139–47. doi: 10.3109/00365519509089606. [DOI] [PubMed] [Google Scholar]
- 63.Fahie-Wilson MN, Soule SG. Macroprolactinaemia: contribution to hyperprolactinaemia in a district general hospital and evaluation of a screening test based on precipitation with polyethylene glycol. Ann Clin Biochem. 1997;34:252–8. doi: 10.1177/000456329703400305. [DOI] [PubMed] [Google Scholar]
- 64.Olukoga AO, Kane JW. Macroprolactinaemia: validation and application of the polyethylene glycol precipitation test and clinical characterization of the condition. Clin Endocrinol (Oxf) 1999;51:119–26. doi: 10.1046/j.1365-2265.1999.00757.x. [DOI] [PubMed] [Google Scholar]
- 65.Vallette-Kasic S, Morange-Ramos I, Selim A, Gunz G, Morange S, Enjalbert A, et al. Macroprolactinemia revisited: a study on 106 patients. J Clin Endocrinol Metab. 2002;87:581–8. doi: 10.1210/jcem.87.2.8272. [DOI] [PubMed] [Google Scholar]
- 66.Gibney J, Smith TP, McKenna TJ. Clinical relevance of macroprolactin. Clin Endocrinol (Oxf) 2005;62:633–43. doi: 10.1111/j.1365-2265.2005.02243.x. [DOI] [PubMed] [Google Scholar]
- 67.Karavitaki N, Thanabalasingham G, Shore HC, Trifanescu R, Ansorge O, Meston N, et al. Do the limits of serum prolactin in disconnection hyperprolactinaemia need re-definition? A study of 226 patients with histologically verified non-functioning pituitary macroadenoma. Clin Endocrinol (Oxf) 2006;65:524–9. doi: 10.1111/j.1365-2265.2006.02627.x. [DOI] [PubMed] [Google Scholar]
- 68.Colao A. Pituitary tumours: the prolactinoma. Best Pract Res Clin Endocrinol Metab. 2009;23:575–96. doi: 10.1016/j.beem.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 69.Prabhakar VK, Davis JR. Hyperprolactinaemia. Best Pract Res Clin Obstet Gynaecol. 2008;22:341–53. doi: 10.1016/j.bpobgyn.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 70.Vergès B, Boureille F, Goudet P, Murat A, Beckers A, Sassolas G, et al. Pituitary disease in MEN type 1 (MEN1): data from the France-Belgium MEN1 multicenter study. J Clin Endocrinol Metab. 2002;87:457–65. doi: 10.1210/jcem.87.2.8145. [DOI] [PubMed] [Google Scholar]
- 71.Molitch ME. Prolactinoma. In: Melmed S, editor. Pituitary. Blackwell Science; Cambridge: 1995. pp. 433–477. [Google Scholar]
- 72.Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, et al. Endocrine Society. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:273–88. doi: 10.1210/jc.2010-1692. [DOI] [PubMed] [Google Scholar]
- 73.Cortet-Rudelli C, Sapin R, Bonneville JF, Brue T. Etiological diagnosis of hyperprolactinemia. Ann Endocrinol (Paris) 2007;68:98–105. doi: 10.1016/j.ando.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 74.Robin G, Catteau-Jonard S, Young J, Dewailly D. Physiopathological link between polycystic ovary syndrome and hyperprolactinemia: myth or reality? Gynecol Obstet Fertil. 2011;39:141–5. doi: 10.1016/j.gyobfe.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 75.Newey PJ, Gorvin CM, Cleland SJ, Willberg CB, Bridge M, Azharuddin M, et al. Mutant prolactin receptor and familial hyperprolactinemia. N Engl J Med. 2013;369:2012–20. doi: 10.1056/NEJMoa1307557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ignacak A, Kasztelnik M, Sliwa T, Korbut RA, Rajda K, Guzik TJ. Prolactin—not only lactotrophin. A “new” view of the “old” hormone. J Physiol Pharmacol. 2012;63:435–43. [PubMed] [Google Scholar]
- 77.Galdiero M, Pivonello R, Grasso LF. Growth hormone, prolactin, and sexuality. J Endocrinol Invest. 2012;35:782–94. doi: 10.1007/BF03345805. [DOI] [PubMed] [Google Scholar]
- 78.Kinon BJ, Gilmore JA, Liu H, Halbreich UM. Prevalence of hyperprolactinemia in schizophrenic patients treated with conventional antipsychotic medications or risperidone. Psychoneuroendocrinology. 2003;28S2:55–68. doi: 10.1016/s0306-4530(02)00127-0. [DOI] [PubMed] [Google Scholar]
- 79.Haddad PM, Wieck A. Antipsychotic-induced hyperprolactinaemia: mechanisms, clinical features and management. Drugs. 2004;64:2291–314. doi: 10.2165/00003495-200464200-00003. [DOI] [PubMed] [Google Scholar]
- 80.Inder WJ, Castle D. Antipsychotic-induced hyperprolactinaemia. Aust N Z J Psychiatry. 2011;45:830–7. doi: 10.3109/00048674.2011.589044. [DOI] [PubMed] [Google Scholar]
- 81.Rastrelli G, Corona G, Maggi M. The role of prolactin in andrology: what is new? Rev Endocr Metab Disord. 2015;16:233–48. doi: 10.1007/s11154-015-9322-3. [DOI] [PubMed] [Google Scholar]
- 82.WHO International Collaborative Study of the Proposed 4th International Standard for Prolactin, Human. [Accessed 20 Feb 2018]. http://apps.who.int/iris/bitstream/10665/253053/1/WHO-BS-2016.2292-eng.pdf.
- 83.Beltran L, Fahie-Wilson MN, McKenna TJ, Kavanagh L, Smith TP. Serum total prolactin and monomeric prolactin reference intervals determined by precipitation with polyethylene glycol: evaluation and validation on common immunoassay platforms. Clin Chem. 2008;54:1673–81. doi: 10.1373/clinchem.2008.105312. [DOI] [PubMed] [Google Scholar]
- 84.Hattori N, Ikekubo K, Ishihara T, Moridera K, Hino M, Kurahachi H. A normal ovulatory woman with hyperprolactinemia: presence of anti-prolactin autoantibody and the regulation of prolactin secretion. Acta Endocrinol (Copenhagen) 1992;126:497–500. doi: 10.1530/acta.0.1260497. [DOI] [PubMed] [Google Scholar]
- 85.Wallace IR, Satti N, Courtney CH, Leslie H, Bell PM, Hunter SJ, et al. Ten-year clinical follow-up of a cohort of 51 patients with macroprolactinemia establishes it as a benign variant. J Clinical Endocrinol Metab. 2010;95:3268–71. doi: 10.1210/jc.2010-0114. [DOI] [PubMed] [Google Scholar]
- 86.Zaninotto M, Mion MM, Altinier S, Varagnolo M, Venturini R, Plebani M. Performance characteristics of laboratory testing and clinical outcomes. Clin Chim Acta. 2009;404:41–5. doi: 10.1016/j.cca.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 87.Kavanagh L, McKenna TJ, Fahie-Wilson MN, Gibney J, Smith TP. Specificity and clinical utility of methods for the detection of macroprolactin. Clin Chem. 2006;52:1366–72. doi: 10.1373/clinchem.2005.065854. [DOI] [PubMed] [Google Scholar]
- 88.Fahie-Wilson MN, John R, Ellis AR. Macroprolactin; high molecular mass forms of circulating prolactin. Ann Clin Biochem. 2005;42:175–92. doi: 10.1258/0004563053857969. [DOI] [PubMed] [Google Scholar]
- 89.Smith TP, Suliman AM, Fahie-Wilson MN, McKenna TJ. Gross variability in the detection of prolactin in sera containing big big prolactin (macroprolactin) by commercial immunoassays. J Clin Endocrinol Metab. 2002;87:5410–5. doi: 10.1210/jc.2001-011943. [DOI] [PubMed] [Google Scholar]
- 90.Kasum M, Oreskovic S, Zec I, Jezek D, Tomic V, Gall V, et al. Macroprolactinemia: new insights in hyperprolactinemia. Biochem Med (Zagreb) 2012;22:171–9. doi: 10.11613/bm.2012.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ram S, Harris B, Fernando JJ, Gama R, Fahie-Wilson M. False-positive polyethylene glycol precipitation tests for macroprolactin due to increased serum globulins. Ann Clin Biochem. 2008;45:256–9. doi: 10.1258/acb.2008.007233. [DOI] [PubMed] [Google Scholar]
- 92.Fahie-Wilson M, Halsall D. Polyethylene glycol precipitation: proceed with care. Ann Clin Biochem. 2008;45:233–5. doi: 10.1258/acb.2008.007262. [DOI] [PubMed] [Google Scholar]
- 93.Bell DA, Hoad K, Leong L, Bakar JA, Sheehan P, Vasikaran SD. A high pressure liquid chromatography method for separation of prolactin forms. Ann Clin Biochem. 2012;49:285–8. doi: 10.1258/acb.2011.011209. [DOI] [PubMed] [Google Scholar]
- 94.Boscato L, Scott S. Endocrine Working Party – Macroprolactin: What do you do? (abstract) Clin Biochem Rev. 2012;33:S28. [Google Scholar]
- 95.Smith TP, Fahie-Wilson MN. Reporting of post-PEG prolactin concentrations: time to change. Clin Chem. 2010;56:484–5. doi: 10.1373/clinchem.2009.135210. [DOI] [PubMed] [Google Scholar]