Abstract
Background
Retinopathy of prematurity (ROP) remains a major cause of childhood blindness and current laser photocoagulation and anti-VEGF antibody treatments are associated with reduced peripheral vision and possible delayed development of retinal vasculatures and neurons. In this study, we advanced the translational potential of adenosine A2A receptor (A2AR) antagonists as a novel therapeutic strategy for selectively controlling pathological retinal neovascularization in oxygen-induced retinopathy (OIR) model of ROP.
Methods
Developing C57BL/6 mice were exposed to 75% oxygen from postnatal (P) day 7 to P12 and to room air from P12 to P17 and treated with KW6002 or vehicle at different postnatal developmental stages. Retinal vascularization was examined by whole-mount fluorescence and cross-sectional hematoxylin-eosin staining. Cellular proliferation, astrocyte and microglial activation, and tip cell function were investigated by isolectin staining and immunohistochemistry. Apoptosis was analyzed by TUNEL assay. The effects of oxygen exposure and KW6002 treatment were analyzed by two-way ANOVA or Kruskal-Wallis test or independent Student’s t-test or Mann-Whitney U test.
Results
The A2AR antagonist KW6002 (P7-P17) did not affect normal postnatal development of retinal vasculature, but selectively reduced avascular areas and neovascularization, with the reduced cellular apoptosis and proliferation, and enhanced astrocyte and tip cell functions in OIR. Importantly, contrary to our prediction that A2AR antagonists were most effective at the hypoxic phase with aberrantly increased adenosine-A2AR signaling, we discovered that the A2AR antagonist KW6002 mainly acted at the hyperoxic phase to confer protection against OIR as KW6002 treatment at P7-P12 (but not P12-P17) conferred protection against OIR; this protection was observed as early as P9 with reduced avascular areas and reduced cellular apoptosis and reversal of eNOS mRNA down-regulation in retina of OIR.
Conclusions
As ROP being a biphasic disease, our identification of the hyperoxic phase as the effective window, together with selective and robust protection against pathological (but not physiological) angiogenesis, elevates A2AR antagonists as a novel therapeutic strategy for ROP treatment.
Keywords: Retinopathy of prematurity, Oxygen-induced retinopathy, Adenosine A2A receptor, KW6002
Background
Retinopathy of prematurity (ROP) remains a major cause of childhood blindness in many parts of the world (Chen et al. 2008; Gilbert 2008; Dhaliwal et al. 2009; Husain et al. 2013; Smith et al. 2013; Hellstrom et al. 2013; Hartnett 2015). As this disease of premature infants is believed to be largely driven by hypoxia-induced factor-1α (HIF-1α) signaling pathway and vascular endothelial growth factor (VEGF) levels in retina (Penn et al. 2008; Cavallaro et al. 2014; Campochiaro 2015), with characteristic hypoxia-induced pathological angiogenesis (Cavallaro et al. 2014; Fleck and McIntosh 2008; Hartnett and Penn 2012), current study of therapeutic development for ROP has largely focused on anti-VEGF therapy. Anti-VEGF antibody has been shown successful in a clinical trial to reduce the recurrence rate in stage III of ROP infants (Mintz-Hittner et al. 2011). However, intraocular injections of anti-VEGF antibody are invasive and repeated injection is associated with the risk of endophthalmitis. Importantly, as VEGF being an important cellular survival factor, the anti-VEGF treatment has been associated with serious concerns about the unintended effects of anti-VEGF agents on delayed development of retinal vasculatures and neurons, and on brain development of preterm infants (Nishijima et al. 2007; Saint-Geniez et al. 2008). Thus, alternative and less-invasive ROP therapeutic strategies with targets other than VEGF are critically needed to improve the ROP management and the quality of life for a growing number of premature infants.
We propose that drugs targeting adenosine (particularly A2A) receptor signaling offer therapeutic advantage by selectively controlling pathological angiogenesis with minimal effect on normal retinal and brain development (for a review see Chen et al. 2017). The validity of A2AR signaling as a promising and novel therapeutic target is supported by rapid (minutes) local increase of adenosine level associated with upregulation of enzymes responsible for generating and maintaining adenosine concentration and delayed (~ 24 h) upregulation of A2AR in oxygen-induced retinopathy (OIR) model of ROP (Elsherbiny et al. 2013; Lutty and McLeod 2003; Liu et al. 2017), diabetic retina of rat with proliferative retinopathy (Vindeirinho et al. 2013) and other pathological conditions (Chen et al. 2013; Frick et al. 2009; Schingnitz et al. 2010; Linden 2011). Aberrantly increased adenosine-A2AR signaling thus represents a local “find-me” signal and renders a unique “purinergic chemotaxis” for a local resolution to pathological conditions (Chen et al. 2013). Moreover, the variants of human A2AR gene are associated with reduced risk of developing diabetic retinopathy in a prospective study (Charles et al. 2011). In support for this proposal, our recent studies with genetic knockout of A2ARs or A1Rs suggested that in OIR, the most frequently used model for ocular pathological angiogenesis, genetic inactivation of A2AR or A1R did not influence normal postnatal development of retinal vasculature, but selectively attenuated pathological angiogenesis (Liu et al. 2010; Zhang et al. 2015), whereas A1R activity differentially modulated hyperoxia-induced vaso-obliteration and hypoxia-induced revascularization (Zhang et al. 2015). This proposal is further substantiated by a recent large prospective phase III clinical study “Caffeine Therapy for Apnea of Prematurity” showing severe ROP (as a secondary outcome in a two-year follow-up observation) was less common in infants assigned to chronic caffeine (a non-selective adenosine receptor antagonist) treatment compared with the control (Schmidt et al. 2007), and by pharmacological studies revealing that chronic caffeine treatment selectively attenuates retinal vasculopathy in OIR model (Zhang et al. 2017; Aranda et al. 2016). Thus, A2AR antagonism may represent novel and promising pharmacological strategy to control retinal pathological angiogenesis in ROP with distinct advantage over other anti-VEGF antibody strategies, which may be necessary not only for pathological angiogenesis, but also for normal retinal vascularization and brain development (LeBlanc et al. 2013).
However, the effectiveness and selectivity of A2AR antagonist-mediated protection against pathological angiogenesis (without affecting normal retinal vascularization) in ROP models have not been tested directly. In this study, the A2AR antagonist KW6002, a drug already in clinical phase III trials for Parkinson’s disease treatment with noted safety profile for aging population (Chen et al. 2013), was used to demonstrate the efficacy and selectivity of A2AR antagonist control of ROP without affecting normal retinal vascular development. Importantly, contrary to our prediction that A2AR antagonists are most effective at the hypoxic phase with aberrantly increased adenosine-A2AR signaling, we discovered that the A2AR antagonist KW6002 mainly acted at the hyperoxic phase to confer protection against OIR. As ROP being a biphasic disease, targeting the initial vaso-obliteration stage offers therapeutic advantage to preserve developing retinal vascular function and prevent progression to the proliferative phase of ROP. Thus, our identification of the hyperoxic phase as the effective therapeutic window, together with selective and robust protection against pathological (but not physiological) angiogenesis and possible cellular mechanisms (i.e. neuronal cell death and endothelial nitric oxide synthase (eNOS) activity), elevates A2AR antagonists as a novel therapeutic strategy for treatment of ROP.
Methods
Mouse model of oxygen-induced retinopathy (OIR)
C57BL/6 J mice from the Animal Laboratory of Wenzhou Medical University (Wenzhou, China) were used in this study. The OIR model described previously by Smith and colleagues was adopted (Liu et al. 2010; Smith et al. 1994). Briefly, seven-days old C57BL/6 J mice kept with the foster/nursing mothers were exposed to 75% oxygen for 5 days [from postnatal day 7(P7) to P12 (with P0 being the day for pup delivery)] to induce vaso-obliteration. At P12, the mice were returned to room air (21% oxygen) to induce retinal neovascularizaion, which was maximal at P17. Age-matched mice kept in room air throughout postnatal development (P0-P17) served as “Room-air Controls”. The foster mothers were rotated between the mice exposed to hyperoxia and the mice kept in room air every 8 h.
All procedures with animals in this study were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee of Wenzhou Medical University.
KW6002 treatment
KW6002 was prepared freshly in 15% DMSO, 15% castrol oil and 70% phosphate buffer saline (PBS), as we described previously (Chen et al. 2001). For each drug treatment, littermates from the same breeding were randomly divided into the drug treatment and control groups. For each treatment condition, at least 2–3 litters were used for the experiment. KW6002 was administered by intraperitoneal injection (i.p.) at dose of 10 mg/kg at different postnatal developmental stages (P7 or P12) and for different period (P7-P17, P7-P12,P12-P17 or P7-P9) every other day or every day. The mice received vehicle in the same volume and with the same intervals served as the control group.
Fluorescence immunostaining of whole-mount retinas
Fluorescence staining of whole-mounted retinas was performed as previously described (Connor et al. 2009). Mice were euthanized at P12 and P17 and eyes were enucleated and fixed with 4% paraformaldehyde for 1 h. The corneas were removed with scissors along the limbus, and then intact retinas were dissected. Retinas were blocked and permeabilized in PBS containing 0.5% Triton-X-100 overnight at 4 °C. Then retinas were incubated with 10 μg/ml isolectin B4 (Molecular Probes, Life Technologies, Carlsbad, CA, USA)overnight at 4 °C. Retinas were then incubated with anti-glial fibrillary acidic protein (GFAP) mouse monoclonal antibody (1:500, Sigma-Aldrich, St. Louis, MO, USA) for 12 h at 4 °C, followed by incubation with fluorescence-conjugated secondary antibodies (1:500, Invitrogen, Life Technologies, Carlsbad, CA,USA) for 2 h, and then whole-mounted. Retinas were washed with PBS and mounted on microscope slides in mounting medium. Retinas were examined by Laser Scanning Microscope (Zeiss 510; Carl Zeiss). Areas of vaso-obliteration and vitreoretinal neovascular tufts were quantified by using Adobe Photoshop CS 5 software. Eight non-overlapping and randomly selected microscopic fields per retina were imaged by confocal scanning laser microscopy (LSM 710; Carl Zeiss, Oberkochen, Germany) to assess the formation of endothelial tip cells;four non-overlapping and randomly selected microscopic fields in the avascular area per retina were imaged by confocal scanning laser microscopy to assess astrocyte function.
To assess normal postnatal development of retinal angiogenesis, C57BL/6 J mice littermates received vehicle (breeding in room air) were sacrificed and the eyes were harvested at P17, respectively. Whole-mount retinas were stained with isolectin B4. Eight non-overlapping and randomly selected microscopic fields per retina and whole-mounted retina were assessed for morphology and distribution of retinal vessels at P17. Vessels in the three layers (superficial, intermediate and deep) were skeletonized (Kornfield and Newman 2014), and the total vessel length in each microscopic field was calculated using Image-Pro Plus software. Vessel density was quantified as the ratio of the total vessel length to microscopic field.
Neovascular quantification
Quantification of neovascularization was performed according to the procedure described previously (Liu et al. 2010; Smith et al. 1994). The extent of neo-vascularization was evaluated by counting the number of neovascular nuclei, which were defined as the nuclei of cells extended beyond the inner limiting membrane of the retina into the vitreous body. In this study, eyes of 10 mice from each group were examined and analyzed. For each eye, 20 retinal sections (excluding the optic nerve) were evaluated. All neovascular nuclei were counted under 400× magnifications with hematoxylin and eosin–stained retinal sections by an investigator who was blind to the specific group assignment.
Semi-quantification of astrocyte in retinal Vaso-obliteration areas
After OIR exposure, mouse retinas were harvested on P17 to assess the astrocyte. GFAP staining of whole-mounted retinas were examined by fluorescence microscopy. The extent of astrocyte persistence in the obliterated zones were scored by the blind observer using the semi-quantitation method on a 1–6 scale as described previously (Dorrell et al. 2010; Liang et al. 2012). As indicated in Fig. 3e, the score of 1–2 indicated retinas with a large number of astrocytes in the vascular obliterated areas which formed a nearly normal astrocytic template. The score of 3–4 indicated retinas with substantial numbers of astrocytes remaining in the vascular obliterated areas (fewer than normal), but failed to form normal astrocytic template. The score of 5–6 indicated there were very few astrocytes in the vascular obliterated zone.
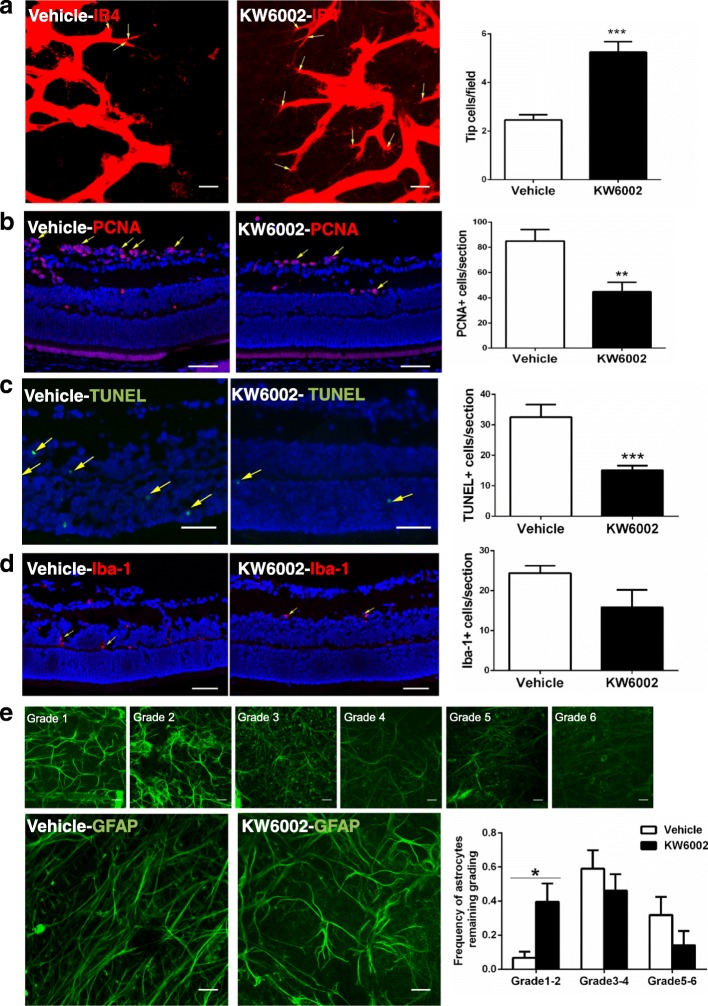
Fig. 3.
In OIR, the effect of KW6002 treatment on cellular proliferation, tip cell, astrocytes and microglial numbers, and apoptosis in retina at P17. a Endothelial tip cells in retina at P17 of OIR were stained with isolectin B4 (red). Scale bar: 20 μm. Quantitative analysis shows that KW6002 treatment increased tip cell number compared to the vehicle-treated pups (***p < 0.001, Student’s t-test, n = 11–13/group). b Cell proliferation in retina was assessed by immunohistochemistry of PCNA at P17 of OIR. PCNA+ cells (yellow arrow) were quantified. (**p < 0.01, Student’s t-test, n = 8/group). Scale bar: 50 μm. c Apoptotic cells in retina were analyzed by TUNEL (green) staining and individual cells were visualized by DAPI (blue) staining at P17 of OIR. Retinal TUNEL-positive cells (yellow arrow) were quantified and analyzed. KW6002 treatment reduced cellular apoptosis in (**p < 0.01, Mann-Whitney U test, n = 8–9/group). Scale bar: 50 μm. d Microglial activation in retinas was assessed by immunofluorescence staining of Iba-1 at P17 of OIR. Retinal Iba-1-positive cells (yellow arrow) were quantified and analyzed. (p > 0.01, Student’s t-test, n = 8/group). Scale bar: 50 μm. e Representative images show GFAP-positive cells in the avascular areas by anti-GFAP (green) staining at P17 of OIR. Scale bar: 20 μm. GFAP staining in the avascular area was graded on a scale from 1 to 6 as described in the Methods section. The grades of astrocytes from each treatment group were analyzed at P17 of OIR. KW6002 treatment enhanced GFAP staining (with the reduced grade) (*p < 0.05, Mann-Whitney U test, n = 9–12/group), Scale bar: 20 μm
Terminal deoxynucleotidyl transferase biotin-dUTP nick end labelling (TUNEL) assay
Retinal cell apoptosis was evaluated at P12 or P17. TUNEL staining was performed with the Roche In Situ Cell Death Detection kit (Roche Diagnostic, Basel, Switzerland) following the manufacturer’s instructions. Ten-micrometer–thick cryostat sections with optic nerve head were permeabilized and antigen retrieval was performed by 0.1% sodium citrate buffer solution with 0.5% Triton X-100 for 5 min. After washing 3 times, the sections were incubated in TUNEL reaction solutions for 1 h in 37 °C, then washed and stained for another 5 min using DAPI (1:2000; Beyotime Biotechnology). TUNEL-positive cells were evaluated in three sections crossing the optic nerve per retina under fluorescent microscopy (Zeiss 510; Carl Zeiss).
Immunohistochemistry
Mouse eyes were dissected and embedded in paraffin at P17 of OIR. For anti–proliferating cell nuclear antigen (PCNA) staining, after being deparaffinized and heated in 10 mM sodium citrate for antigen repairing, 3 retinal paraffin sections were blocked and permeabilized, then incubated with PCNA rabbit polyclonal antibody (1:200; Santa Cruz Biotechnology, SantaCruz, CA, USA). Fluorescence-conjugated secondary Abs (1:500; Thermo Fisher Scientific) were applied to detect positive signals.
For microglial activation by immunostaining with anti-Iba-1 antibody at P12/P17 of OIR, mouse eyes were dissected and embedded in optimum cutting temperature compound. Three cryostat sections with optic nerve head per retina were blocked and permeabilized, then incubated with the anti-Iba-1 rabbit polyclonal antibody (1:100; Wako, Osaka, Japan) overnight at 4 °C. Fluorescence-conjugated second antibodies (1:500; Invitrogen, Life Technologies) were applied for detecting the positive signal.
Quantitative real-time reverse transcription PCR
The mRNA level of A1R, A2AR, VEGFA, and eNOS in retina harvested at P12 was done by the quantitative real-time polymerase chain reaction (qPCR) procedure as we have described previously (Zhang et al. 2017) using the following forward and reverse primers: A2AR: forward,5’-CCGAATTCCACTCCGGTACA-3′; reverse, 5’-CAGTTGTTCCAGCCCAGCAT-3′; A1R: forward, 5’-ATCCCTCTCCGGTACAAGACAGT-3′; reverse, 5’-ACTCAGGTTGTTCCAGCCAAAC-3′; VEGFA: forward, 5’-GAAAGGGTCAAAAACGAAAGC-3′; reverse, 5’-CGCTCTGAACAAGGCTCAC-3′; eNOS: forward, 5’-TGTGACCCTCACCGCTACAA-3′; reverse, 5’-GCACAATCCAGGCCCAATC-3′. GAPDH: forward, 5′- AGGTCGGTGTGAACGGATTTG-3′; reverse, 5’-TGTAGACCATGTAGTTGA GGTCA-3′.
Statistical analysis
The data were presented as the mean ± standard error (SE). The effect of KW6002 versus vehicle was analyzed by independent Student’s t-test or Mann-Whitney U test. The effects of multiple factors were analyzed by two-way ANOVA followed by Bonferroni post-hoc test or Kruskal-Wallis test. These statistical analyses were performed with commercial analytical software (SPSS 25.0) with p value < 0.05 being considered as statistically significant.
Results
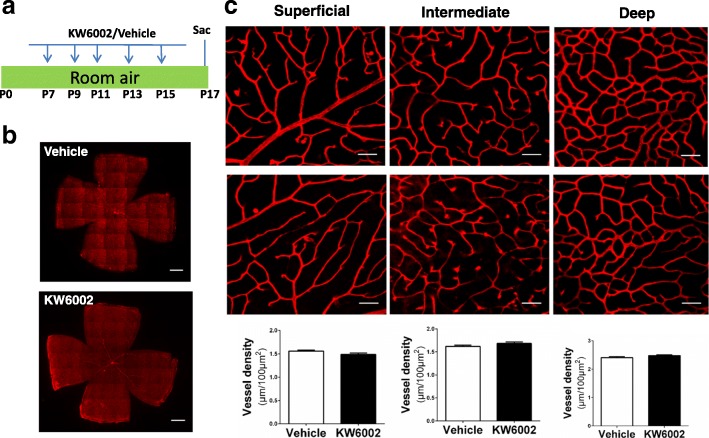
Repeated KW6002 treatment did not affect normal postnatal development of retinal vasculature
To address whether KW6002 affects normal retinal vasculature during postnatal development, we analyzed development of the retinal vascular networks at P17 of C57BL/6 mice after repeated treatment with KW6002 (from P7 to P17, 10 mg/kg, i.p. every other day, Fig. 1a), by fluorescent staining of whole-mounted retinas under room air conditions (Fig. 1b). After exposure to room air from P0 to P21, the mice displayed normal development of the retinal vasculature, the superficial layer reaching near completion at P12. Both the superficial and the deep vascular layers from the optic disc to the periphery were well developed at P12-P17, and the intermediate layer was almost finished at P21. KW6002 treatment did not affect retinal vasculature in the superficial, intermediate and deep vascular plexus, as revealed by isolectin B4 analysis in whole-mounts retina scanned with laser scanning microscope (Fig. 1c). No avascular areas were detected at P17 in mice treated either with vehicle or KW6002 (Fig. 1b), indicating that the superficial retinal vasculature grew with similar rates and indistinguishable patterns of the vessels distribution between mice treated with vehicle or KW6002. Furthermore, morphology, distribution and density of three retinal vessels layers (superficial, intermediate and deep) were indistinguishable between mice treated with the vehicle and KW6002 when analyzed by confocal scanning laser microscopy at P17 (Fig. 1C). These analyses demonstrated that repeated KW6002 treatment did not affect normal postnatal development of retinal vasculature in mice.
Fig. 1.
KW6002 treatment did not affect normal postnatal development of retinal vasculature. a Schematic of the experimental design: KW6002 was administered by intraperitoneal injection at volume of 10 mg/kg from P7 to P15 every other day. Mice were euthanized at P17.(Sac:Sacrifice). b Mouse whole-mount retinas from KW6002- and vehicle-treated mice were harvested and immune-stained with isolectin B4. The retinal vasculature morphologies were indistinguishable between KW6002- or vehicle-treated pups at P17 in room-air. Scale bar: 500 μm. c The retinal vasculatures of the superficial, intermediate and deep vascular layers were examined at P17 by isolectin B4 staining of whole-mount retinas. The distributions of three retinal vascular layers were displayed in distinct confocal planes. Vessel density was quantified as the ratio of the total vessel length to microscopic field. The vessel densities of the superficial, intermediate and deep plexuses were indistinguishable between KW6002-treated and vehicle-treated pups. Scale bar: 50 μm
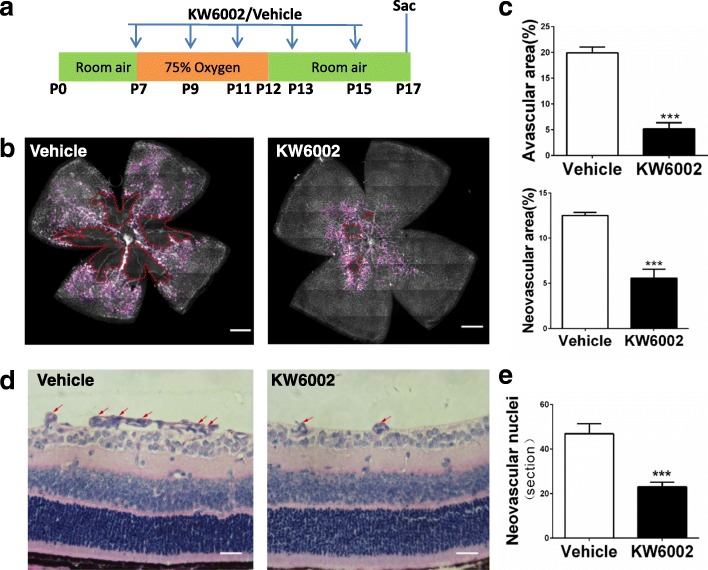
KW6002 treatment selectively and effectively reduced retinal avascular area and pathological angiogenesis at P17 in OIR
We first evaluated the effect of repeated KW6002 treatment for 10 days (from P7 to P17, 10 mg/kg, i.p. every other day, Fig. 2a) on vaso-obliteration and neovascularization by analyzing the avascular area and neovascular area with isolectin B4 staining in whole-mounted retina (Fig. 2b). Repeated treatment with KW6002 largely prevented hypoxia-induced retinopathy. Quantitative analysis demonstrated that KW6002 treatment reduced avascular area by 74.2% (P < 0.001) and neovascular area by 55.0% (P < 0.001) compared to the vehicle-treated mice (Fig. 2c). Independent and quantitative analysis of neovascular nuclei numbers in cross sections demonstrated that KW6002 treatment for 10 days markedly reduced pathological angiogenesis with reduced neovascular nuclei in retina of OIR (46.85 ± 4.53) compared to vehicle-treated mice (23.03 ± 2.105) (P < 0.001, Fig. 2d, e). These studies demonstrated that repeated KW6002 treatment protects against oxygen-induced vaso-obliteration and pathological angiogenesis.
Fig. 2.
KW6002 treatment attenuated retinal avascular area and pathological angiogenesis at P17 in OIR. a Schematic of the experimental design: KW6002 was administered by intraperitoneal injection at volume of 10 mg/kg from P7 to P15 every other day. OIR mice were euthanized at P17. b Following the OIR, retinal vasculatures from KW6002- and vehicle-treated mice were visualized by whole-mount isolectin B4 at P17. Avascular areas are indicated by red dotted line. Neovascularization tufts are indicated by purple line. Scale bar: 500 μm. c Quantitative analysis of avascular areas by isolectin B4 and the neovascular tufts area showed that KW6002 treatment reduced avascular areas(Student’s t-test) and neovascular tufts area(Mann-Whitney U test)compared to the vehicle-treated pups. ***P < 0.001, n = 11–13/group. d Representative H-E staining images showing the neovascular nuclei numbers (red arrow) on the vitreal side of the inner limiting membrane. Scale bar: 20 μm. e Quantitative histochemical analysis showed that KW6002 treatment (P7–17) reduced neovascular nuclei numbers as compared to the vehicle-treated pups. ***P < 0.001, Student’s t-test, n = 11–13 /group
KW6002-induced protection against OIR was associated with increased astrocyte and endothelial tip cell functions and decreased cellular proliferation and apoptosis at P17
We further investigated the cellular basis underlying the KW6002 effects on pathological angiogenesis by analyzing function of astrocytes and endothelial tip cells in retina of mice treated with KW6002 or vehicle at P17 stage of OIR. Consistent with the previous study (Weidemann et al. 2010), GFAP-positive cells with normal morphology were mainly detected in the peripheral vascular areas while GFAP-positive cells with abnormal morphology were detected in the avascular area of retina by immunofluorescence staining. Using the semi-quantitative method described by Dorrell et al. (Dorrell et al. 2010), we estimated GFAP expression in the avascular area. Analysis showed that OIR markedly reduced GFAP-positive cells, as expected, but KW6002 partially reversed this reduction of astrocyte function in retina compared to the vehicle-treated retina (Fig. 3e). Quantitative analysis revealed that the number of endothelial tip cells in retina was significantly increased by KW6002 treatment compared to the vehicle group (Fig. 3a). Because pathologic angiogenesis is characterized by proliferation of endothelial cells in the hypoxic phase, we analyzed expression of PCNA, a marker for cellular proliferation, at P17 of OIR (Fig. 3b). KW6002 treatment decreased the number of PCNA-positive cells in the retina. TUNEL assay showed that most TUNEL-positive signals were found within the outer nuclear layer, in which mainly photoreceptor cells were distributed (Fig. 3c). Quantitative analysis showed that KW6002 treatment decreased TUNEL-positive signals in retinas compared with vehicle group. Collectively, KW6002 treatment enhanced function of astrocyte and endothelial tip cells and reduced cellular proliferation and apoptosis to confer protection against oxygen-induced pathological angiogenesis in retina at P17 of OIR. Microglial activation in retinas was assessed by Iba-1 immunohistochemistry antibody at P17. OIR increased microglial activation in retina but KW6002 did not have a major effect on microglial activation in retina at P17 (Fig. 3d).
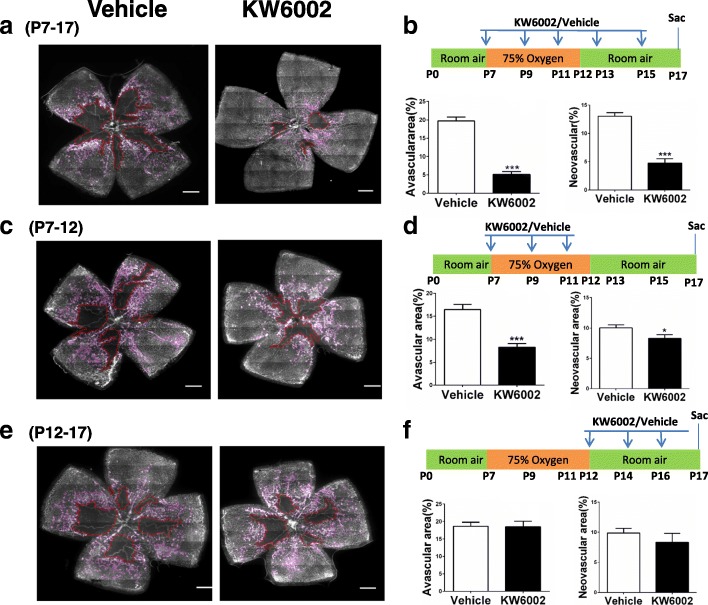
KW6002 treatment at P7–12 (but not P12–17) was required to confer protection against OIR
To define the effective therapeutic window, we treated the mice with KW6002 in three different treatment regimens (i.e. P7-P12, P12-P17 and P7-P17, Fig. 4d, d, f). KW6002 treatment at P7–12 (8.25 ± 0.85%) or 7–17 (5.07 ± 0.82%) was effective in reducing avascular area by 49.9% (p < 0.001) and 74.5% (p < 0.001), respectively, comparing to the vehicle-treated control (Fig. 4a, b, c, d). In addition, quantitative analysis confirmed that KW6002 treatment at P7–12 (8.29 ± 0.62%) or 7–17 (4.76 ± 0.76%) was effective in neovascularization tufts towards the vitreous body by 17.6% (p < 0.05) and 63.44% (p < 0.001), respectively, comparing to the vehicle-treated control (Fig. 4a, b, c, d). By contrast, repeated KW6002 treatment at P12–17 was not effective in reducing vaso-obliteration or neovascularization (Fig. 4 e, f). Thus, the administration of KW6002 at P7–12 (i.e. the hyperoxic phase) is required to confer protection against OIR, whereas the injection of KW6002 at P12–17 (i.e. the hypoxic phase) is not sufficient to confer the protection.
Fig. 4.
Effective therapeutic windows for KW6002 to confer protection against OIR. a, c, e Pups were treated with KW6002 (10 mg/kg) in different developmental stages (P7–17, P7–12, and P12–17) and retinal vasculatures were analyzed by whole-mount isolectin B4 staining at P17 of OIR. Avascular areas are shown by red dotted line. Neovascularization tufts are indicated by purple line. Scale bar: 500 μm. b, d, f Schematic of the experimental design: KW6002 was administered by intraperitoneal injection at volume of 10 mg/kg at different postnatal developmental stages (P7 or P12) and for different period (P7-P17, P7-P12 or P12-P17) every other day. OIR mice were euthanized at P17. b, d, f Avascular area (%) was quantified as a percentage of the whole retinal surface (n = 7–9/group). The neovascularization tufts area (%) was quantified as a percentage of whole retinal area (n = 7–9/group). ***p < 0.001, *p < 0.05, Student’s t-test or Mann-Whitney U test, n = 7–9/group
KW6002 treatment reduced hyperoxia-induced retinal vascular regression at P9 and 12 and cellular apoptosis at P12
Next, we further examined the protective effect of KW6002 treatment at the hyperoxic phase by analyzing hyperoxia-induced avascular area at P9 and P12 in whole-mounted retinas with isolectin B4 staining (Fig. 5a,c). Analysis revealed that KW6002 treatment reduced avascular areas in the central retina at P9 and P12. Quantitative analysis of retinal vaso-obliteration demonstrated that KW6002 treatment reduced avascular areas by 27.0% at P9 and 27.5% at P12, respectively, compared to the vehicle-treated mice (Fig. 5d, d).
Fig. 5.
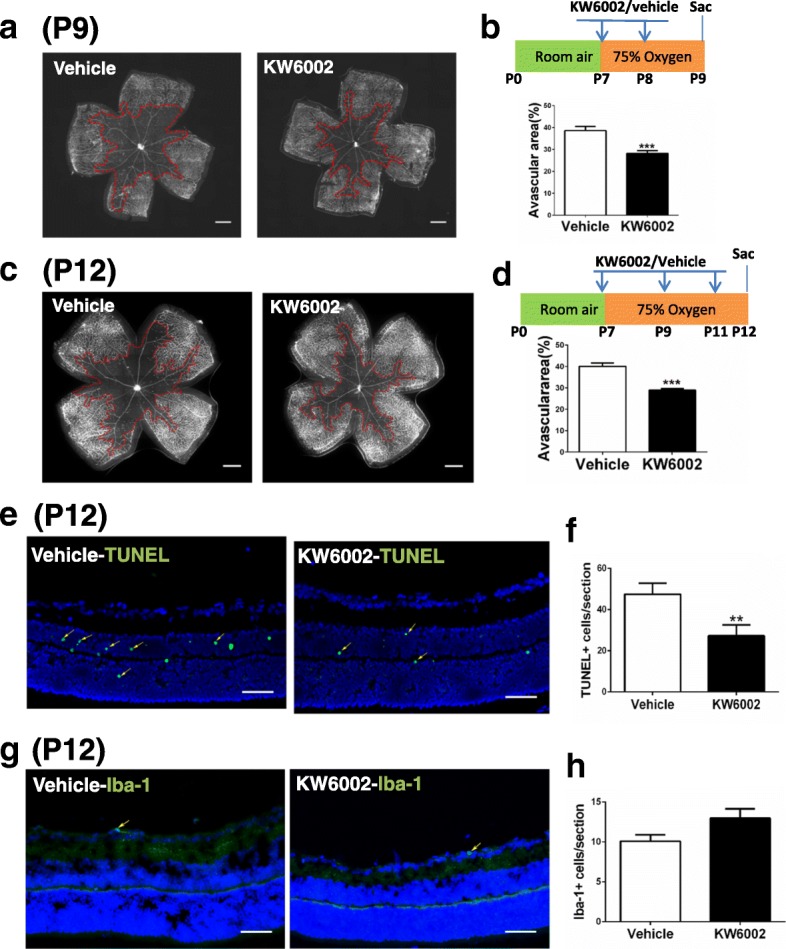
KW6002 treatment reduced hyperoxia-induced retinal vascular regression at P9 and P12 and cellular apoptosis at P12, does not affect the activation of microglia. Whole-mount retinas (a, c) or retinal cross-section (e, g) from the vehicle-OIR and KW6002 groups were harvested on P9 (a) or P12 (c, e, g) and examined by immunostaining with isolectin B4, or histological analysis by TUNEL or Iba-1. Representative images show the avascular areas (indicated by red dotted line) at P9 (a) and P12 (c) (Scale bar: 500 μm) and TUNEL+ cells (indicated by yellow arrow) (e) or Iba-1+ cells (indicated by yellow arrow) (g) in retinal sections at P12 (Scale bar: 50 μm). Areas of vaso-obliteration (b, d) and TUNEL+ cells (f) or Iba-1+ cells (h) were quantified and analyzed **P < 0.01, ***P < 0.001, Student’s t-test or Mann-Whitney U testn = 8 /group
Consistent with our previously (Zhang et al. 2015) and other studies (Duan et al. 2011; Ludewig et al. 2014; Narayanan et al. 2014), hyperoxia induced TUNEL-positive signals mainly within the inner nuclear layer (mainly neurons such as amacrine cells and bipolar cells) of the avascular area of OIR, in both KW6002- and vehicle-treated groups (Fig. 5e). Quantitative analysis showed that TUNEL-positive cells were lower in mice treated with KW6002 than in mice treated with vehicle at P12 (Fig. 5f). These results confirmed that cellular apoptosis pattern after KW6002 treatment in OIR paralleled with the results of the avascular area, suggesting that KW6002 may reduce avascular areas partly by reducing hyperoxia-induced neuronal apoptosis in retina. We have performed analysis for microglial activation in retinas, and found that KW6002 treatment did not affect microglial activation at P12 (Fig. 5g, h).
KW6002 treatment reversed hyperoxia-induced reduction of the mRNAs for A2AR and eNOS at P12
We further explored the possible mechanism underlying the A2AR-mediated protection at the hyperoxic phase and found that in agreement with the early finding (Taomoto et al. 2000) A2AR mRNA was reduced by hyperoxic exposure at P12 (n = 6, p < 0.01,Fig. 6b). This reduction of the A2AR mRNA was reversed by KW6002 treatment. By contrast, A1R mRNA in retina of OIR harvested at P12 was affected by neither to hyperoxia or KW6002 treatment (n = 6, p > 0.05, Fig. 6a). Moreover, as eNOS and VEGF play important role for in the vaso-obliterative phase of ROP (Hartnett et al. 2008), we also examined the expression of eNOS and VEGF at P12 in eye exposed to hyperoxia and after KW6002 treatment (P7–12). Consistent with the previous study (Wang et al. 2013; Abdelsaid et al. 2014), both VEGF mRNA and eNOS mRNA levels were reduced after exposure to hyperoxia compared to the room air condition (n = 6, p < 0.01) (Fig. 6c, d). Importantly, KW6002 treatment reversed the reduction of eNOS but not VEGF mRNA in eye, suggesting the possible involvement of eNOS in A2AR modulation of OIR (independent of VEGF) at the hyperoxia phase.
Fig. 6.
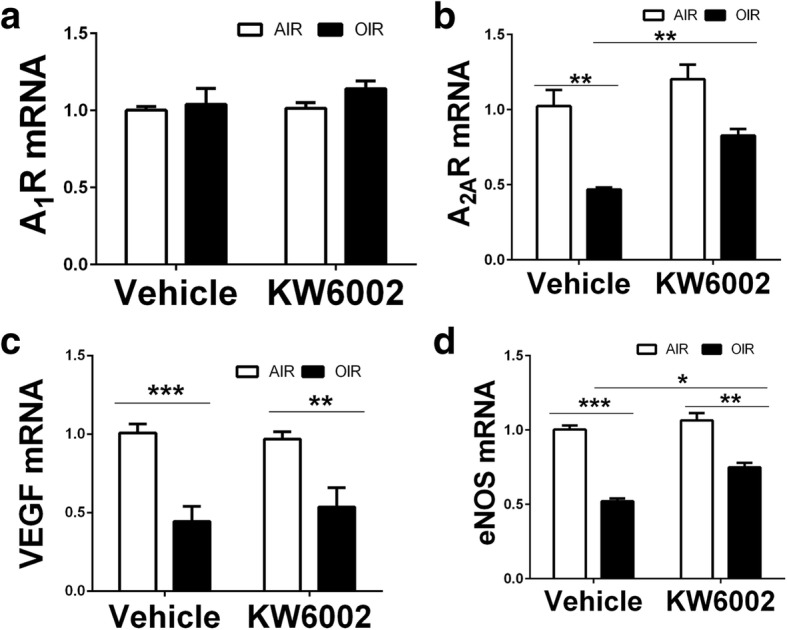
KW6002 treatment reversed hyperoxia-induced reduction of the mRNAs for A2AR and eNOS at P12. Retina of OIR and room air was dissected and harvested at P12 in retina after KW6002 or vehicle treatment (P7–12) and A2AR and A1R mRNAs as well as the mRNAs for VEGF and eNOS were determined by qPCR analysis. Compared to the room air group, A1R was affected by neither to hyperoxia or KW6002 treatment (a) whereas the mRNAs for A2AR, VEGF and eNOS were reduced by hyperoxic exposure. KW6002 treatment reversed the reduction of A2AR mRNA and eNOS by hyperoxia (b, c, d). n = 6/group *p < 0.05, **p < 0.01, ***p < 0.001, two-way ANOVA followed by Bonferroni post-hoc test or Kruskal-Wallis test n = 6/group
Discussion
A2AR antagonists act at the hyperoxic phase to confer its protection against OIR
Current research efforts (such as anti-VEGF and other strategies) have largely focused on the hypoxic phase for proliferative angiogenesis is a key feature of ROP, driven largely by HIF-1α signaling and VEGF pathway. Because hypoxia is associated with huge surge of adenosine signaling as result of increased expression of CD73/CD39 and A2AR mRNA and of hypoxia-induced inhibition of ADK and ENT (the enzyme responsible for adenosine degradation), A2AR antagonists are expected to act mainly at the hypoxic phase to blunt hypoxia-induced pathological angiogenesis. To our surprise, KW6002 treatment at P12–17 was in fact ineffective, whereas KW6002 treatment at P7–12 was effective in protecting against OIR. KW6002 treatment just at P7–8 was sufficient to confer protection against the damage to the developing retinal vessels (as indicated by reduced avascular area). Lack of protective effect of KW6002 upon administration at P12–17 may be interpreted as follows: direct, acute effect of KW6002 at the hypoxic phase alone is not sufficient and the combined effect of KW6002 at both phases is needed to confer its protection against OIR. The reduced pathological angiogenesis by A2AR KO at P17 could be attributed to the action at the hyperoxic phase (in addition to the hypoxic phase) as the A2AR KO conceives at the early embryonic stage and throughout life. The finding of the required treatment of KW6002 at P7–12 is consistent with the protection against OIR by caffeine observed at P12 (Zhang et al. 2017), and with the finding of the importance of the hyperoxic phase in A1R-mediated modulation of OIR (Zhang et al. 2015). Developmental factors may also contribute to the selective effect of A2AR antagonists at the hyperoxic phase. Retinal vasculature development at postnatal day 7 might be particularly sensitive to pharmacological interference, since from P7 onward the superficial capillaries start sprouting vertically in the retina to form, firstly, the deep, then the intermediated vascular plexus in the vitreous body of C57BL/6 mice (Smith et al. 1994; Stahl et al. 2010). In addition, KW6002 concentration may be higher at P7-P12 than P12-P17 following the same dose regime because of different metabolism of drugs during postnatal development, as evidenced by caffeine treatment at different postnatal stages producing different steady-state levels of caffeine in human and rodents (Parsons and Neims 1981; Pearlman et al. 1989).
Intriguingly, despite clear vaso-obliteration at the retina center, the hyperoxic phase is associated with lack of “hypoxia” in the retina by in vivo labelling of hypoxic tissues/cells with nitroimidazole EF5 (Smith et al. 2012) and with the reduced expression of ecto-5′ nucleotidase (CD73) and A2AR (Lutty and McLeod 2003) and, presumably, adenosine level. Yet, the hyperoxic phase (P7–12 in OIR) is most critical to confer protection against OIR by KW6002, caffeine (Zhang et al. 2017) and A1R KO (Zhang et al. 2015). Where did adenosine level come from at the hyperoxic phase? This may be due to the fact that adenosine production is intrinsically linked to energy metabolism and biosynthetic processes (Chen et al. 2013), generating basal levels of adenosine intracellularly and extracellularly through effective bidirectional ENT activity. Consequently, there is always a finite amount of extracellular adenosine that is likely sufficient to activate the A2AR (with Kd value at 1–10 nM) (Chen et al. 2013). Thus, despite low but nonetheless sufficient concentration of adenosine, KW6002 treatment primarily acts at the early stage of ROP to confer its protection.
The biphasic disease of ROP suggests that hyperoxic damage to developing retinal vasculatures is the primary and root cause of pathological angiogenesis in ROP despite the fact that pathological angiogenesis, the hallmark of ROP pathology, is most evident at the hypoxic phase of ROP (Sapieha et al. 2010). Thus, therapeutic strategies targeting the initial obliteration at the hyperoxia phase can preserve retinal endothelial and neuronal functions and to prevent progression to the proliferative phase of the disease. Our demonstration of the effective therapeutic window (i.e. P7–12) of KW6002 treatment argues that KW6002 treatment is more effective when administered during the early stage of ROP (i.e. when premature infants receive oxygen support) to prevent the devastating latter stage (the hypoxic phase) of the disease. Currently, caffeine treatment for sleep apnea of prematurity is used during the first 10 days after birth in preterm infants subjected to oxygen treatment (Schoen et al. 2014). A2AR antagonists and caffeine may be developed as prophylactic measures for ROP by targeting the hyperoxic and hypoxic phases to achieve maximal therapeutic benefits.
A2AR antagonists protect against OIR by acting at the distinct mechanisms at the hyperoxic and hypoxic phases
The mechanisms underlying A2AR antagonist-induced protection at the hyperoxic phase are not clear. Hyperoxia-induced reactive oxygen species (ROS) can promote apoptosis in ganglia cells and developing endothelial cells, and can inhibit endothelial cells proliferation and migration, leading to vaso-obliteration (Aiello et al. 1994; Alon et al. 1995). Accordingly, attenuation of the hyperoxia-induced avascular area by the A2AR antagonist is associated with the reduced neuronal apoptosis in the inner nuclear layer of the retina (i.e. TUNEL-positive cells). This is consistent with the previous finding that A2AR antagonists and A2AR KO exert protection against OIR (Liu et al. 2010) and seizure susceptibility (Georgiev et al. 1993) in neonates. In keep with this, caffeine treatment during the first 7 days after birth affected neonates sensitivity to ischemic brain injury (Bona et al. 1995), reduced white matter injury by preventing early differentiation of oligodendrocytes (Rivkees and Wendler 2011) and reduced the effects of NMDA on seizure susceptibility in neonates (Georgiev et al. 1993). As the A2AR activation can increase Nox2-dependent generation of ROS in some cell types (Ribe et al. 2008; Thakur et al. 2010), A2AR antagonists may attenuate cellular apoptosis in the inner ganglion cells by reducing ROS production by hyperoxic exposure. Furthermore, hyperoxic exposure has been shown to impair endothelium function (Garcia-Quintans et al. 2016) with depletion of the eNOS cofactor (6R)-5,6,7,8-tetrahydrobiopterin (BH4) (Edgar et al. 2015). Our observations that KW6002 treatment reversed the reduction of hyperoxia-induced of eNOS mRNA at P12 suggests that A2AR antagonists may specifically modulate hyperoxia-induced apoptosis and damage to developing retinal vessels through eNOS-ROS pathway. However, the interplay of the A2AR activity and eNOS in OIR are complex because eNOS can have both beneficial (via vasodilation) (Dimmeler et al. 1999) and detrimental (via production of ROS) (Brooks et al. 2001; Edgar et al. 2012) effects on retinal vascular (Edgar et al. 2012) development and because A2AR activation can either enhance (Carlstrom et al. 2011)or suppress (Dai et al. 2010; Lin et al. 2007) NOS activity depending on cellular type or local environment (e.g. glutamate). On the other hand, KW6002 reduced avascular areas and promoted revascularization by enhancing the functions of astrocytes and endothelial tip cells at the hypoxic phase at P17. Furthermore, a recent study with endothelial cell-specific A2AR knockout elegantly shows that A2AR control pathological neovascularization in the OIR mice by transcriptional modulation of glycolytic enzymes, via ERK- and Akt-dependent translational activation of HIF-1α protein, to promote glycolysis in endothelial cells and endothelial cell proliferation (Liu et al. 2017).
In this regard, we noted that both A2AR antagonist (Fig. 5c, d) and A1R KO (Zhang et al. 2015) reduced the oxygen-induced avascular areas at P12. Thus, adenosine acting at A2AR and A1R corporately controls oxygen-induced damage to developing retinal vasculature at the hyperoxic phase. This is in striking contrast to the fact that A2ARs and A1Rs exert opposite control on angiogenesis at the hypoxic phase in OIR, as indicated by reduced avascular areas in A2AR-KO mice (Liu et al. 2010) and increased avascular areas in A1R-KO mice (Zhang et al. 2015). The different interactions between A2AR and A1R at the hyperoxia versus hypoxic phases likely reflect fundamentally different mechanisms of adenosine signaling action at these two phases. It would be critical to determine the retinal adenosine level at the hypoxic phase and to dissect out the specific mechanism of the A1R and A2AR interactions at P12 versus P17.
Distinct features of A2AR antagonists versus anti-VEGF and caffeine therapeutic strategy in ROP
The present study also revealed additional features of A2AR antagonists in control of pathological angiogenesis with translational implications for ROP. At first, in contrast to anti-VEGF and other therapeutic strategies, A2AR antagonism selectively attenuated retinal pathological vaso-obliteration and neovascularization in OIR, but did not affect postnatal retinal vascularization (with normal morphology, density and distribution of retinal vessels), a feature shared by other adenosine-based manipulations including caffeine treatment (Zhang et al. 2017) and A2AR KO (Liu et al. 2010) and A1R KO (Zhang et al. 2015). However, the lingering concerns on the possible specific effect of the caffeine on embryonic development (Ma et al. 2014; Back et al. 2006) and possible postnatal development and maturation of cortical GABAergic neurons at the microstructural level after perinatal exposure to the caffeine (Favrais et al. 2014) should be taken into consideration carefully. This treatment confers distinct advantages over the currently testing anti-VEGF antibody treatment in ROP. This selectivity has been attributed to the aberrantly enhanced adenosine signaling (Lutty and McLeod 2003), which amplifies A2AR antagonist effect. However, our demonstration of the primary action of A2AR antagonists at the hyperoxic phase (with presumably relatively low adenosine signaling) suggests that additional mechanisms may be critical for preferential control of retinal vascularization by A2AR signaling at the hyperoxic phase. At second, protective effect of the A2AR antagonist against OIR (≈75% avascular area by KW6002 at P17) is stronger/more robust than caffeine treatment (35%) and other experimental manipulations of adenosine signaling, including A2AR KO (~ 60%). The robust protection conferred by the A2AR antagonist may be attributed to the unique feature of A2AR antagonists targeting primarily at the hyperoxic phase. As such, A2AR antagonists are more effective at the hyperoxic phase (i.e. the early stage of ROP), whereas anti-VEGF treatment is more effective at the hypoxic phase (i.e. later stage of ROP, such as ROP stage II-III).
Conclusion
Our identification of the hyperoxic phase as the effective window, together with selective and robust protection against pathological (but not physiological) angiogenesis, provide the preclinical evidence for translating A2AR antagonists as a novel therapeutic strategy for ROP treatment.
Acknowledgments
The authors thank Dr. Sergii Vakal (Wenzhou Medical University) for editing and proof-reading of the manuscript. This study was sponsored by the National Natural Science Foundation of China (Grants 81630040 to J.-F.C., 81600753 to S.Z., and 81100672 to R.Z.), the Start-up Fund from Wenzhou Medical University (Grants 89211010 and 89212012 to J.-F.C.), the Zhejiang Provincial Special Funds (Grant 604161241 to J.-F.C.), Key Laboratory of Vision Science, Ministry of Health, China (Grant 601041241 to J.-F.C.), the Central Government Special Fund for Local Universities’ Development (Grant 474091314 to J.-F.C.), Zhejiang Provincial Natural Science Foundation Grant (Grant LY12H12007 to X.-L.L.; Grant LY17H120006 to R.Z.) and Public welfare science and technology plan project of Wenzhou city (Grant Y20160059 to R.Z.).
Funding
This study was sponsored by the National Natural Science Foundation of China (Grants 81630040 to J.-F.C., 81600753 to S.Z., and 81100672 to R.Z.), the Start-up Fund from Wenzhou Medical University (Grants 89211010 and 89212012 to J.-F.C.), the Zhejiang Provincial Special Funds (Grant 604161241 to J.-F.C.), Key Laboratory of Vision Science, Ministry of Health, China (Grant 601041241 to J.-F.C.), the Central Government Special Fund for Local Universities’ Development (Grant 474091314 to J.-F.C.), Zhejiang Provincial Natural Science Foundation Grant (Grant LY12H12007 to X.-L.L.; Grant LY17H120006 to R.Z.) and Public welfare science and technology plan project of Wenzhou city (Grant Y20160059 to R.Z.).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- A2AR
Adenosine A2A receptor
- eNOS
Endothelial nitric oxide synthase
- HIF-1α
Hypoxia-induced factor-1α
- OIR
Oxygen-induced retinopathy
- ROP
Retinopathy of prematurity
- VEGF
Vascular endothelial growth factor
Authors’ contributions
RZ, SZ, X-LL and J-FC designed the experiment, analyzed the data and wrote the paper; RZ, SZ, XG, YG, DZ, YZ and LT performed the experiments, collected and analyzed the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All procedures with animals in this study were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee of Wenzhou Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiao-Ling Liu, Phone: +86 577 88068855, Email: lxl@mail.eye.ac.cn.
Jiang-Fan Chen, Phone: +86 577 88121352, Email: chenjf555@gmail.com.
References
- Abdelsaid MA, Matragoon S, Ergul A, El-Remessy AB. Deletion of Thioredoxin interacting protein (TXNIP) augments Hyperoxia-induced Vaso-obliteration in a mouse model of oxygen induced-retinopathy. PLoS One. 2014;9 [DOI] [PMC free article] [PubMed]
- Aiello LP, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- Alon T, et al. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- Aranda JV, et al. Pharmacologic synergism of ocular ketorolac and systemic caffeine citrate in rat oxygen-induced retinopathy. Pediatr Res. 2016;80:554–565. doi: 10.1038/pr.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, et al. Protective effects of caffeine on chronic hypoxia-induced perinatal white matter injury. Ann Neurol. 2006;60:696–705. doi: 10.1002/ana.21008. [DOI] [PubMed] [Google Scholar]
- Bona E, Aden U, Fredholm BB, Hagberg H. The effect of long term caffeine treatment on hypoxic-ischemic brain damage in the neonate. Pediatr Res. 1995;38:312–318. doi: 10.1203/00006450-199509000-00007. [DOI] [PubMed] [Google Scholar]
- Brooks SE, et al. Reduced severity of oxygen-induced retinopathy in eNOS-deficient mice. Invest Ophthalmol Vis Sci. 2001;42:222–228. [PubMed] [Google Scholar]
- Campochiaro PA. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog Retin Eye Res. 2015;49:67–81. doi: 10.1016/j.preteyeres.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlstrom M, Wilcox CS, Welch WJ. Adenosine a(2A) receptor activation attenuates tubuloglomerular feedback responses by stimulation of endothelial nitric oxide synthase. Am J Physiol-Renal. 2011;300:F457–F464. doi: 10.1152/ajprenal.00567.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallaro G, et al. The pathophysiology of retinopathy of prematurity: an update of previous and recent knowledge. Acta Ophthalmol. 2014;92:2–20. doi: 10.1111/aos.12049. [DOI] [PubMed] [Google Scholar]
- Charles BA, et al. Variants of the adenosine a(2A) receptor gene are protective against proliferative diabetic retinopathy in patients with type 1 diabetes. Ophthalmic Res. 2011;46:1–8. doi: 10.1159/000317057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets--what are the challenges? Nat Rev Drug Discov. 2013;12:265–286. doi: 10.1038/nrd3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, et al. Neuroprotection by caffeine and a(2A) adenosine receptor inactivation in a model of Parkinson's disease. J Neurosci. 2001;21 art. no.-RC143 [DOI] [PMC free article] [PubMed]
- Chen JF, et al. Adenosine receptors and caffeine in retinopathy of prematurity. Mol Asp Med. 2017;55:118–125. doi: 10.1016/j.mam.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, et al. Risk factors for retinopathy of prematurity in six neonatal intensive care units in Beijing, China. Br J Ophthalmol. 2008;92:326–330. doi: 10.1136/bjo.2007.131813. [DOI] [PubMed] [Google Scholar]
- Connor KM, et al. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009;4:1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai SS, et al. Local glutamate level dictates adenosine a(2A) receptor regulation of Neuroinflammation and traumatic brain injury. J Neurosci. 2010;30:5802–5810. doi: 10.1523/JNEUROSCI.0268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaliwal C, Wright E, Graham C, McIntosh N, Fleck BW. Wide-field digital retinal imaging versus binocular indirect ophthalmoscopy for retinopathy of prematurity screening: a two-observer prospective, randomised comparison. Br J Ophthalmol. 2009;93:355–359. doi: 10.1136/bjo.2008.148908. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Hermann C, Galle J, Zeiher AM. Upregulation of superoxide dismutase and nitric oxide synthase mediates the apoptosis-suppressive effects of shear stress on endothelial cells. Arterioscl Throm Vas. 1999;19:656–664. doi: 10.1161/01.ATV.19.3.656. [DOI] [PubMed] [Google Scholar]
- Dorrell MI, et al. Maintaining retinal astrocytes normalizes revascularization and prevents vascular pathology associated with oxygen-induced retinopathy. Glia. 2010;58:43–54. doi: 10.1002/glia.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan LJ, Takeda K, Fong GH. Prolyl hydroxylase domain protein 2 (PHD2) mediates oxygen-induced retinopathy in neonatal mice. Am J Pathol. 2011;178:1881–1890. doi: 10.1016/j.ajpath.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar K, Gardiner TA, van Haperen R, de Crom R, McDonald DM. eNOS overexpression exacerbates vascular closure in the Obliterative phase of OIR and increases Angiogenic drive in the subsequent proliferative stage. Invest Ophthalmol Vis Sci. 2012;53:6833–6850. doi: 10.1167/iovs.12-9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar KS, Matesanz N, Gardiner TA, Katusic ZS, McDonald DM. Hyperoxia depletes (6R)-5,6,7,8-tetrahydrobiopterin levels in the neonatal retina Implications for Nitric Oxide Synthase Function in Retinopathy. Am J Pathol. 2015;185:1769–1782. doi: 10.1016/j.ajpath.2015.02.021. [DOI] [PubMed] [Google Scholar]
- Elsherbiny NM, et al. Potential roles of adenosine deaminase-2 in diabetic retinopathy. Biochem Biophys Res Commun. 2013;436:355–361. doi: 10.1016/j.bbrc.2013.05.023. [DOI] [PubMed] [Google Scholar]
- Favrais G, et al. Impact of common treatments given in the perinatal period on the developing brain. Neonatology. 2014;106:163–172. doi: 10.1159/000363492. [DOI] [PubMed] [Google Scholar]
- Fleck BW, McIntosh N. Pathogenesis of retinopathy of prematurity and possible preventive strategies. Early Hum Dev. 2008;84:83–88. doi: 10.1016/j.earlhumdev.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Frick JS, et al. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol. 2009;182:4957–4964. doi: 10.4049/jimmunol.0801324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Quintans N, et al. Oxidative stress induces loss of pericyte coverage and vascular instability in PGC-1 alpha-deficient mice. Angiogenesis. 2016;19:217–228. doi: 10.1007/s10456-016-9502-0. [DOI] [PubMed] [Google Scholar]
- Georgiev V, Johansson B, Fredholm BB. Long-term caffeine treatment leads to a decreased susceptibility to NMDA-induced clonic seizures in mice without changes in adenosine A1 receptor number. Brain Res. 1993;612:271–277. doi: 10.1016/0006-8993(93)91672-F. [DOI] [PubMed] [Google Scholar]
- Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84:77–82. doi: 10.1016/j.earlhumdev.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Hartnett ME. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology. 2015;122:200–210. doi: 10.1016/j.ophtha.2014.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartnett ME, Penn JS. Mechanisms and Management of Retinopathy of prematurity. N Engl J Med. 2012;367:2515–2526. doi: 10.1056/NEJMra1208129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartnett ME, et al. Neutralizing VEGF decreases tortuosity and alters endothelial cell division orientation in arterioles and veins in a rat model of ROP: relevance to plus disease. Invest Ophthalmol Vis Sci. 2008;49:3107–3114. doi: 10.1167/iovs.08-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom A, Smith LEH, Dammann O. Retinopathy of prematurity. Lancet. 2013;382:1445–1457. doi: 10.1016/S0140-6736(13)60178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain SM, et al. Relationships between maternal ethnicity, gestational age, birth weight, weight gain, and severe retinopathy of prematurity. J Pediatr. 2013;163:67–72. doi: 10.1016/j.jpeds.2012.12.038. [DOI] [PubMed] [Google Scholar]
- Kornfield TE, Newman EA. Regulation of blood flow in the retinal trilaminar vascular network. J Neurosci. 2014;34:11504–11513. doi: 10.1523/JNEUROSCI.1971-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc KH, Maidment NT, Ostlund SB. Repeated cocaine exposure facilitates the expression of incentive motivation and induces habitual control in rats. PLoS One. 2013;8:e61355. doi: 10.1371/journal.pone.0061355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, et al. TMP prevents retinal neovascularization and imparts neuroprotection in an oxygen-induced retinopathy model. Invest Ophthalmol Vis Sci. 2012;53:2157–2169. doi: 10.1167/iovs.11-9315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CL, et al. Attenuation of experimental subarachnoid hemorrhage-induced cerebral vasospasm by the adenosine a(2A) receptor agonist CGS 21680. J Neurosurg. 2007;106:436–441. doi: 10.3171/jns.2007.106.3.436. [DOI] [PubMed] [Google Scholar]
- Linden J. Regulation of leukocyte function by adenosine receptors. Adv Pharmacol. 2011;61:95–114. doi: 10.1016/B978-0-12-385526-8.00004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XL, et al. Genetic inactivation of the adenosine A2A receptor attenuates pathologic but not developmental angiogenesis in the mouse retina. Invest Ophthalmol Vis Sci. 2010;51:6625–6632. doi: 10.1167/iovs.09-4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZP, et al. Endothelial adenosine A2a receptor-mediated glycolysis is essential for pathological retinal angiogenesis. Nat Commun. 2017;8 [DOI] [PMC free article] [PubMed]
- Ludewig P, et al. CEACAM1 confers resistance toward oxygen-induced vessel damage in a mouse model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2014;55:7950–7960. doi: 10.1167/iovs.13-13403. [DOI] [PubMed] [Google Scholar]
- Lutty GA, McLeod DS. Retinal vascular development and oxygen-induced retinopathy: a role for adenosine. Prog Retin Eye Res. 2003;22:95–111. doi: 10.1016/S1350-9462(02)00058-7. [DOI] [PubMed] [Google Scholar]
- Ma ZL, et al. Excess caffeine exposure impairs eye development during chick embryogenesis. J Cell Mol Med. 2014; [DOI] [PMC free article] [PubMed]
- Mintz-Hittner HA, Kennedy KA, Chuang AZ. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364:603–615. doi: 10.1056/NEJMoa1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan SP, et al. Arginase 2 deficiency reduces hyperoxia-mediated retinal neurodegeneration through the regulation of polyamine metabolism. Cell Death Dis. 2014;5 [DOI] [PMC free article] [PubMed]
- Nishijima K, et al. Vascular endothelial growth factor-a is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171:53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons WD, Neims AH. Prolonged half-life of caffeine in healthy tem newborn infants. J Pediatr. 1981;98:640–641. doi: 10.1016/S0022-3476(81)80784-6. [DOI] [PubMed] [Google Scholar]
- Pearlman SA, Duran C, Wood MA, Maisels MJ, Berlin CM., Jr Caffeine pharmacokinetics in preterm infants older than 2 weeks. Dev Pharmacol Ther. 1989;12:65–69. [PubMed] [Google Scholar]
- Penn JS, et al. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008;27:331–371. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribe D, et al. Adenosine a(2A) receptor signaling regulation of cardiac NADPH oxidase activity. Free Radic Biol Med. 2008;44:1433–1442. doi: 10.1016/j.freeradbiomed.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivkees SA, Wendler CC. Adverse and protective influences of adenosine on the newborn and embryo: implications for preterm white matter injury and embryo protection. Pediatr Res. 2011;69:271–278. doi: 10.1203/PDR.0b013e31820efbcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Geniez M, et al. Endogenous VEGF is required for visual function: evidence for a survival role on muller cells and photoreceptors. PLoS One. 2008;3:e3554. doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapieha P, et al. Retinopathy of prematurity: understanding ischemic retinal vasculopathies at an extreme of life. J Clin Invest. 2010;120:3022–3032. doi: 10.1172/JCI42142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schingnitz U, et al. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J Immunol. 2010;184:5271–5279. doi: 10.4049/jimmunol.0903035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B, et al. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357:1893–1902. doi: 10.1056/NEJMoa073679. [DOI] [PubMed] [Google Scholar]
- Schoen K, Yu T, Stockmann C, Spigarelli MG, Sherwin CM. Use of methylxanthine therapies for the treatment and prevention of apnea of prematurity. Paediatric Drugs. 2014;16:169–177. doi: 10.1007/s40272-013-0063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Virkud A, Deisseroth K, Graybiel AM. Reversible online control of habitual behavior by optogenetic perturbation of medial prefrontal cortex. Proc Natl Acad Sci U S A. 2012;109:18932–18937. doi: 10.1073/pnas.1216264109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LE, Hard AL, Hellstrom A. The Biology of Retinopathy of Prematurity How Knowledge of Pathogenesis Guides Treatment. Clin Perinatol. 2013;40:201−+. doi: 10.1016/j.clp.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LE, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- Stahl A, et al. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci. 2010;51:2813–2826. [DOI] [PMC free article] [PubMed]
- Taomoto M, McLeod DS, Merges C, Lutty GA. Localization of adenosine A2a receptor in retinal development and oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2000;41:230–243. [PubMed] [Google Scholar]
- Thakur S, Du JJ, Hourani S, Ledent C, Li JM. Inactivation of adenosine a(2A) receptor attenuates basal and angiotensin II-induced ROS production by Nox2 in endothelial cells. J Biol Chem. 2010;285:40104–40113. doi: 10.1074/jbc.M110.184606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindeirinho J, Costa GN, Correia MB, Cavadas C, Santos PF. Effect of diabetes/hyperglycemia on the rat retinal Adenosinergic system. PLoS One. 2013;8 [DOI] [PMC free article] [PubMed]
- Wang XM, et al. (2013) LRG1 promotes angiogenesis by modulating endothelial TGF-beta signalling. Nature vol 499, pg 306. [DOI] [PMC free article] [PubMed]
- Weidemann A, et al. Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia. 2010;58:1177–1185. doi: 10.1002/glia.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, et al. Adenosine A1 receptors selectively modulate oxygen-induced retinopathy at the Hyperoxic and hypoxic phases by distinct cellular mechanisms. Invest Ophthalmol Vis Sci. 2015;56:8108–8119. doi: 10.1167/iovs.15-17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SY, et al. Caffeine preferentially protects against oxygen-induced retinopathy. FASEB J. 2017;31:3334–3348. doi: 10.1096/fj.201601285R. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.