Abstract
Background
Extubation failure is associated with mortality and morbidity in the intensive care unit. Ventilator weaning protocols have been introduced, and extubation is conducted based on the results of a spontaneous breathing trial. Room for improvement still exists in extubation management, and additional objective indices may improve the safety of the weaning and extubation process. Static lung-thorax compliance reflects lung expansion difficulty that is caused by several conditions, such as atelectasis, fibrosis, and pleural effusion. Nevertheless, it is not used commonly in the weaning and extubation process. In this study, we investigated whether lung-thorax compliance is a good index of extubation failure in adults even when patients pass a spontaneous breathing trial.
Methods
In a single-center, retrospective cohort study, patients over 18 years of age were mechanically ventilated, weaned with proportional assist ventilation, and underwent a spontaneous breathing trial process in surgical intensive care units of Kagawa University Hospital from July 2014 to June 2016. Extubation failure was the outcome measure of the study. We defined extubation failures as when patients were reintubated or underwent non-invasive positive-pressure ventilation within 24 h after extubation. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the clinical involvement of several parameters. The area under the curve (AUC) was calculated to assess the discriminative power of the parameters.
Results
We analyzed 173 patients and compared the success and failure groups. Most patients (162, 93.6%) were extubated successfully, and extubation failed in 11 patients (6.4%). The averages of lung-thorax compliance values in the success and failure groups were 71.9 ± 23.0 and 43.3 ± 14.6 mL/cmH2O, respectively, and were significantly different (p < 0.0001). In the ROC curve analysis, the AUC was highest for lung-thorax compliance (0.862), followed by the respiratory rate (0.821), rapid shallow breathing index (0.781), Acute Physiology and Chronic Health Evaluation II score (0.72), heart rate (0.715), and tidal volume (0.695).
Conclusions
Lung-thorax compliance measured during a spontaneous breathing trial is a potential indicator of extubation failure in postoperative patients.
Keywords: Lung and thorax compliance, Spontaneous breathing trial, Extubation failure, Proportional assist ventilation
Background
Extubation failure is associated with mortality and morbidity in the intensive care unit (ICU) [1–6]. Ventilator weaning and extubation were previously based on the experience of the intensivist, but in recent years, ventilator weaning protocols have been introduced, and extubation is conducted based on the results of a spontaneous breathing trial (SBT) [7–9]. SBT is a test to determine whether a patient can tolerate the condition without the support of mechanical ventilation. Close observation and objective judgment contribute to shortening mechanical ventilation duration and reducing the reintubation rate [7–9]; however, the reintubation rate is still 11–19% [1–4, 10–15]. Room for improvement still exists in extubation management, and additional objective indices may increase the safety of the weaning and extubation process.
Static lung-thorax compliance (LTC), which is calculated by the formula: tidal volume (mL)/(pressure measured from the onset of end-inspiratory occlusion − positive end-expiratory pressure) (cmH2O) [16–18], is a candidate for an index that can help to more safely extubate patients. LTC reflects the difficulty of lung expansion that is caused by several conditions such as atelectasis, fibrosis, pleural effusion, intrapulmonary fluid retention, or a decrease in compliance due to obesity [17–19]. Monitoring LTC is useful because intensivists can evaluate the conditions of the lung and respiratory muscles [20, 21]. However, LTC is not commonly used in the weaning and extubation process because the measurement of LTC under spontaneous breathing is possible only under proportional assist ventilation (PAV) [16]. Thus, the relationship between LTC during spontaneous breathing and extubation failure is not clear.
PAV is a mode that assists ventilation in proportion to the instantaneous effort of the patient’s breathing [22]. In this mode, LTC can be measured with less stress for the patients [16]. Because PAV is superior to pressure support ventilation (PSV), which is synchronized with spontaneous breathing [23–25], intensivists can reduce sedative use [26] and judge an SBT more precisely [27–29]. In this study, we investigated whether LTC is a good index of extubation failure among adults who passed the SBT.
Methods
Study population
We conducted a single-center, retrospective cohort study involving patients over 18 years of age who were admitted to the surgical intensive care unit (SICU) in Kagawa University Hospital from July 2014 to June 2016. Patients who were ventilated mechanically, weaned with PAV, and underwent a SBT process were included in the analysis. The following patients were excluded because the SBT process was not conducted: patients who had disorders in their central nervous system, patients who died before extubation, patients who underwent a tracheotomy before SICU admission, patients who were extubated accidentally, and patients with a good postoperative condition who were extubated without SBT (Fast track extubation). In addition, patients who were subjected to ventilation modes other than PAV for staff education were also excluded (Fig. 1).
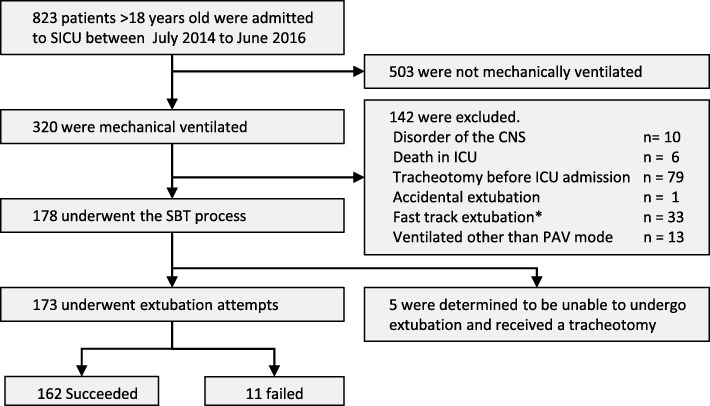
Fig. 1.
Number of patients included and excluded from the study. A total of 823 patients older than 18 years of age were admitted to the SICU. Among them, 503 patients were not mechanically ventilated during the SICU stay. According to the exclusion criteria, 142 patients were excluded. A total of 178 patients underwent the SBT process; however, 5 patients underwent a tracheotomy. Finally, we analyzed 173 patients and compared the success and failure groups. Most patients (162, 93.6%) were extubated successfully, and 11 (6.4%) failed the extubation. *Fast track extubation: extubation without SBT for patients with good postoperative condition; SICU: surgical intensive care unit; CNS: central nervous system; ICU: intensive care unit; PAV: proportional assist ventilation; SBT: spontaneous breathing trial
SBT process
Intensivists assessed the spontaneous breathing ability of the patient before starting the SBT process (Fig. 2). The ventilation mode was changed to synchronized intermittent mandatory ventilation (SIMV) at the time of ICU admission. We used propofol and dexmedetomidine hydrochloride for sedation and maintained the Richmond Agitation-Sedation Scale (RASS) between − 3 and − 1 [30]. We also used a continuous infusion of fentanyl 10–100 mcg/h, peripheral nerve block (PNB), epidural anesthesia, and nonsteroidal anti-inflammatory drugs (NSAIDs) for analgesia. When the patient exhibited spontaneous breathing of ten times per minute or more, PAV was initiated. We adjusted the support rate (15–30%) so that the patient’s work of breathing (WOB) was maintained in a comfortable range (0.3–0.7 J/L). Under close observation and when the patient met the entry criteria (Table 1), sedative drugs were reduced until the RASS was − 2 to 0. Intensivists observed the patient for 30–60 min [31] and determined whether to extubate when the patient met the extubation criteria (Table 1) [32]. If patients did not meet the criteria, PAV was continued, and ICU members, including intensivists, attending physicians, anesthesiologists, and ICU nurses, discussed whether to wait until the patient’s status improved or a tracheotomy was performed [33]. The PB 840 ventilator (Covidien, USA) was employed to apply the PAV mode for patients. After extubation, all patients were given a high-flow nasal cannula (HFNC) or oxygen mask. If the patient failed to maintain with HFNC, we decided whether to use non-invasive positive-pressure ventilation (NPPV) or reintubate.
Fig. 2.
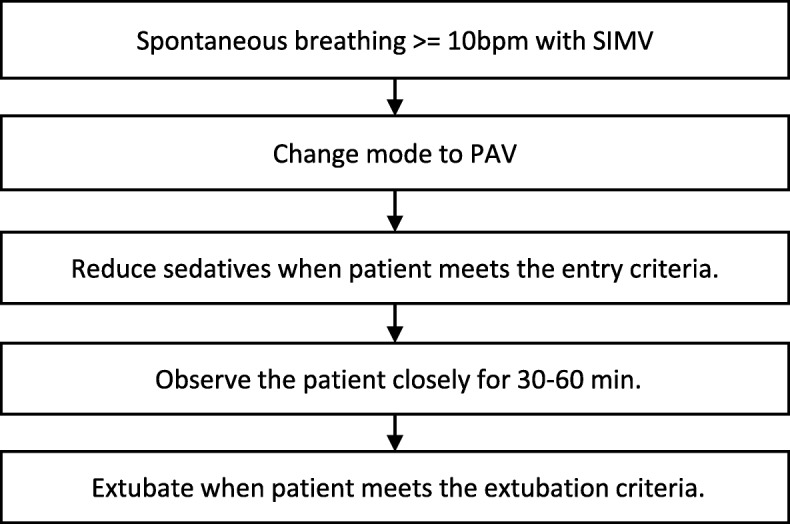
Flowchart of the SBT process. Intensivists assessed the spontaneous breathing ability of the patient before the SBT process. When the patient exhibited spontaneous breathing of 10 times per minute or more, PAV was initiated. Under close observation and when the patient met the entry criteria, sedative drugs were reduced until the RASS was − 2 to 0. Intensivists observed the patient for 30–60 min and determined whether to extubate when the patient met the extubation criteria. SIMV: synchronized intermittent mandatory ventilation; PAV: proportional assist ventilation; SBT: spontaneous breathing trial
Table 1.
Criteria for the SBT process
| Entry criteria | |
| SpO2 | ≥ 94% with FiO2 ≤ 0.5, PEEP ≤ 7 cmH2O, support of WOB ≤ 40% |
| PaO2 | ≥ 70 mmHg |
| Respiratory acidosis | No acidosis |
| Heart rate | ≤ 120 bpm |
| Dopamine | ≤ 5 mcg/kg/min |
| Dobutamine | ≤ 5 mcg/kg/min |
| Noradrenaline | ≤ 0.05 mcg/kg/min |
| Hemoglobin | ≥ 8 g/dl |
| Electrolyte abnormality | No abnormality |
| Extubation criteria | |
| Consciousness | |
| Richmond Agitation-Sedation Scale | − 2 to 0 |
| Confusion assessment method for the ICU | Negative |
| Respiration | |
| Respiratory rate | ≤ 30/min |
| RSBI | < 105 |
| Labored breathing | No |
| Increased WOB | No |
| Gas exchange | |
| SpO2 | ≥ 94% |
| PaO2 | ≥ 70 mmHg |
| pH | ≥ 7.32 |
| PaCO2 | ≤ 45 mmHg |
| Circulation | |
| Heart rate | ≤ 120/min |
| Systolic blood pressure | 80 to 180 mmHg |
SBT spontaneous breathing trial, FiO2 inspired oxygen fraction, PEEP positive end-expiratory pressure, WOB work of breathing, SpO2 arterial oxygen saturation, PaO2 partial pressure of arterial oxygen, RSBI rapid shallow breathing index, PaCO2 partial pressure of arterial carbon dioxide
Outcome and parameters
Extubation failure was the outcome measure of the study. We defined extubation failure as when patients were reintubated or when NPPV was conducted within 24 h after extubation. As potential factors influencing the outcome, sex, age, body mass index (BMI) at admission, type of surgery, emergency surgery, ventilation period, number of SBT, use of HFNC and NPPV, and Acute Physiology and Chronic Health Evaluation II (APACHE II) scores [34] at SICU entrance were collected through the electronic medical record. Parameters that can be monitored during the weaning process, such as the heart rate (HR), respiratory rate (RR), tidal volume (TV), rapid shallow breathing index (RSBI), positive end-expiratory pressure (PEEP), arterial oxygen saturation (SpO2), end-tidal carbon dioxide (EtCO2), WOB, and LTC were also extracted afterward from an ICU electronic medical record system, RPM-7400 (Nihon Koden, Tokyo, Japan). All parameters were measured each minute, and the average of the last 30 min of the observation period was recorded.
Statistical analysis
We calculated that a minimum of eight patients in each group would be required to have 80% power to detect a difference in LTC of 30 mL/cmH2O between the success and failure groups at a significance level of 0.05. In the literature, the LTC in healthy adults is 80–100 mL/cmH2O [35], and in acute respiratory distress syndrome (ARDS) or cardiogenic pulmonary edema, the LTC is 29–42 mL/cmH2O [35–37]. The standard deviations of the LTC are reported to be 7–13 mL/cmH2O [36, 37]. Because LTC data for target patients were not available, we used a difference of 30 mL/cmH2O. Categorical data were analyzed using Fisher’s exact test. For the other parameters, a Mann-Whitney U test was used to compare the success and failure groups. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the clinical implications of parameters. The area under the curve (AUC) was calculated to assess the discriminative power of the parameters. The analysis was performed using JMP Pro version 13.2.1 (SAS Institute Inc., Cary, NC, USA). This study was approved by the Ethical Review Board, Faculty of Medicine, Kagawa University (Heisei 29-049). The authors have no conflicts of interest to declare.
Results
A total of 823 patients older than 18 years of age were admitted to the SICU during the target period. Among them, 503 patients were not mechanically ventilated during the SICU stay. According to the exclusion criteria, 142 patients were excluded. A total of 178 patients underwent the SBT process; however, 5 patients underwent a tracheotomy because they were judged to be unable to meet the extubation criteria by ICU members. Finally, we analyzed 173 patients and compared the success and failure groups. No patient had serious respiratory illness before the surgery or at ICU admission.
The subjects consisted of 65.9% men and 34.1% women, with a mean age of 68.2 years. Most (98.3%) were surgical patients, 90 (52.0%) were cardiac patients, 34 (19.7%) were craniocervical patients, and 16 (9.2%) were gastrointestinal surgery patients. Most patients (162, 93.6%) were extubated successfully, and 11 (6.4%) failed the extubation. In the failure group, three (27.3%) patients used NPPV, and eight (72.3%) were reintubated (Table 2). Four patients failed due to sputum clogging, three had pulmonary edema, two had hypercapnia, and one had aspiration pneumonia, and another was hypoxemic. The ventilation period and SBT number were 1651 ± 3011 min (mean ± SD) and 1 ± 1.7 (median ± SD) in the success group and 2330 ± 3797 min and 2 ± 2.9 in the failure group, respectively. There was no difference between the groups. Only vascular surgery and WOB were significantly associated with extubation failure (Table 2). The mean age and sex ratio was not different between the two groups. Among patient parameters, the APACHE II score, HR, RR, TV, RSBI, and LTC were significantly different. The average LTC values in the success and failure groups were 71.9 ± 23.0 and 43.3 ± 14.6, respectively, which were significantly different (p < 0.0001) (Table 3).
Table 2.
Subject backgrounds
| Total | Success | Failure | p value | |
|---|---|---|---|---|
| n = 173 | n = 162 | n = 11 | ||
| Sex, n (%) | ||||
| Male | 114 (65.9) | 107 (66.1) | 7 (63.6) | 0.553 |
| Female | 59 (34.1) | 55 (34.0) | 4 (36.4) | 0.696 |
| Age (mean ± SD) | 68.2 ± 12.8 | 68.2 ± 12.9 | 69.3 ± 11.7 | 0.854 |
| Surgery, n (%) | 170 (98.3) | 160 (98.8) | 10 (90.9) | 0.179 |
| Cardiac | 90 (52.0) | 86 (53.1) | 4 (36.4) | 0.519 |
| Craniocervical | 34 (19.7) | 34 (21.0) | 0 (0.0) | 0.215 |
| Gastrointestinal | 16 (9.2) | 13 (8.0) | 3 (27.3) | 0.064 |
| Vascular | 14 (8.1) | 11 (6.8) | 3 (27.3) | 0.031 |
| Other surgery | 16 (9.2) | 16 (9.9) | 0 (0.0) | 0.601 |
| Others, n (%) | 3 (1.7) | 2 (1.2) | 1 (9.1) | 0.989 |
| Emergency surgery, n (%) | 14 (8.1) | 13 (8.0) | 1 (9.1) | 0.616 |
| Ventilation period, min (mean ± SD) | 1694 ± 3058 | 1651 ± 3011 | 2330 ± 3797 | 0.516 |
| SBT times (median ± SD) | 1 ± 1.8 | 1 ± 1.7 | 2 ± 2.9 | 0.158 |
| WOB*, J/L (mean ± SD) | 0.77 ± 0.27 | 0.76 ± 0.26 | 0.99 ± 0.41 | 0.015 |
| HFNC, n (%) | 123 (71.1) | 112 (69.1) | 11 (100) | 0.026 |
| NPPV, n (%) | 3 (1.7) | 0 (0.0) | 3 (27.3) | 0.0002 |
The Mann-Whitney U test and Fischer’s exact test were applied. *WOB is a calculated estimate
SBT spontaneous breathing trial, WOB work of breathing, HFNC high-flow nasal cannula, NPPV non-invasive positive-pressure ventilation
Table 3.
Comparison of parameters between the success group and failure group
| Total | Success | Failure | p value | |
|---|---|---|---|---|
| n = 173 | n = 162 | n = 11 | ||
| Female, n (%) | 59 (34.1) | 55 (34.0) | 4 (36.4) | 0.696* |
| Age (years) | 68.2 ± 12.8 | 68.2 ± 12.9 | 69.3 ± 11.7 | 0.854 |
| APACHE II score | 17.6 ± 5.7 | 17.2 ± 5.3 | 23.4 ± 8.7 | 0.015 |
| BMI | 23.9 ± 4.2 | 24.1 ± 4.2 | 22.1 ± 3.8 | 0.075 |
| HR (bpm) | 77 ± 13.5 | 76.3 ± 13.1 | 87.1 ± 15.6 | 0.018 |
| RR (/min) | 16.1 ± 4.7 | 15.7 ± 4.1 | 23.3 ± 7.0 | 0.000 |
| TV (mL) | 457.4 ± 102.3 | 461 ± 98.8 | 405.4 ± 140.6 | 0.031 |
| RSBI | 38.4 ± 18.3 | 36.6 ± 15.7 | 64.3 ± 31.8 | 0.002 |
| PEEP (cmH2O) | 6.1 ± 1.4 | 6.2 ± 1.3 | 5.3 ± 2.4 | 0.065 |
| SpO2 (%) | 99.2 ± 1.0 | 99.2 ± 0.98 | 98.6 ± 1.4 | 0.076 |
| EtCO2 (mmHg) | 39.3 ± 5.2 | 39.4 ± 4.8 | 37.8 ± 9.3 | 0.828 |
| LTC (mL/cmH2O) | 70.1 ± 23.6 | 71.9 ± 23.0 | 43.3 ± 14.6 | < 0.0001 |
Mann-Whitney U Test was applied. *Fischer’s exact test was applied for the comparison of ratios. From age to LTC, data were expressed as mean ± standard deviation
APACHE II score Acute Physiology and Chronic Health Evaluation II score, BMI body mass index, HR heart rate, RR respiratory rate, TV tidal volume, RSBI rapid shallow breathing index, PEEP positive end-expiratory pressure, SpO2 arterial oxygen saturation, EtCO2 end-tidal carbon dioxide, LTC lung-thorax compliance
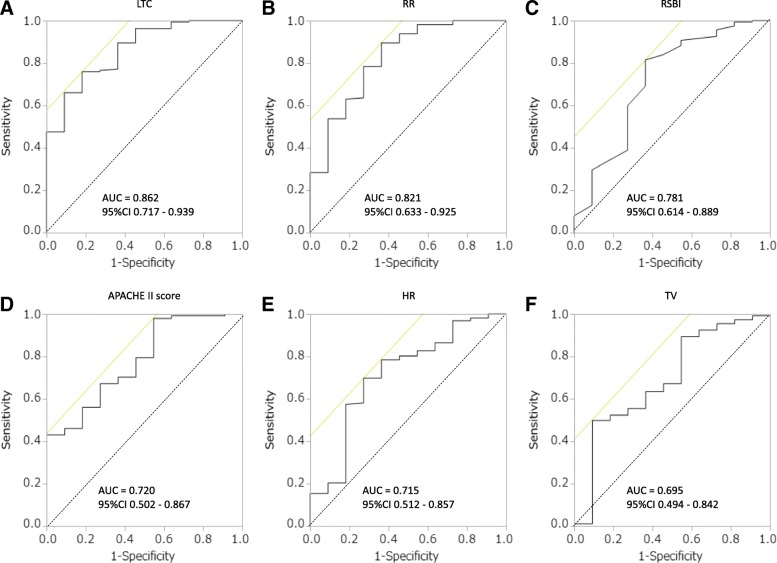
In the ROC curve analysis, the AUC was highest for LTC (0.862), followed by the RR (0.821), RSBI (0.781), APACHE II score (0.720), HR (0.715), and TV (0.695) (Fig. 3).
Fig. 3.
ROC curves for LTC (a), RR (b), RSBI (c), APACHE II score (d), HR (e), and TV (f) used to distinguish the success group from the failure group. In the ROC curve analysis, the AUC was highest for LTC (0.862), followed by the RR (0.821), RSBI (0.781), APACHE II score (0.720), HR (0.715), and TV (0.695). ROC curve: receiver operating characteristic curve; LTC: lung-thorax compliance; RR: respiratory rate; RSBI: rapid shallow breathing index; APACHE II score: Acute Physiology and Chronic Health Evaluation II score; TV: tidal volume; AUC: area under the curve, CI: confidence interval
Discussion
LTC measured during the SBT was highly associated with extubation failure. The AUC of LTC was 0.862, which was highest among the parameters; therefore, LTC may be a good predictor of extubation failure. We compared the sensitivity and specificity of each parameter at the highest value of the Youden index [38], which was defined by the following formula: (sensitivity + specificity − 1). The maximum value of the Youden index was used as a criterion for selecting the optimum cutoff point of diagnostic tests [38]. At a cutoff point of 54, LTC had a moderate degree of sensitivity and a high degree of specificity. Considering patient safety, higher specificity is desirable, which indicates the usefulness of LTC as a predictor of extubation failure (Table 4). The sensitivity, specificity, and success rate of extubation were estimated at the different cutoff points for LTC. At a cutoff of 60, the estimated specificity was greater than 0.9, and the success rate was 96%. However, at a cutoff of 50, the estimated specificity was 0.636, and the success rate was 90%. LTC under 50–60 could indicate an increased probability of extubation failure in the SICU (Table 5).
Table 4.
Sensitivity and specificity at the cutoff of the highest Youden index
| Cutoff | Sensitivity | Specificity | |
|---|---|---|---|
| LTC (mL/cmH2O) | 54 | 0.759 | 0.818 |
| RR (/min) | 21 | 0.895 | 0.636 |
| RSBI | 72 | 0.982 | 0.455 |
| APACHE II | 21 | 0.815 | 0.636 |
| HR (bpm) | 81 | 0.698 | 0.727 |
| TV (mL) | 451 | 0.500 | 0.909 |
Youden index = sensitivity + specificity − 1
LTC lung-thorax compliance, RR respiratory rate, RSBI rapid shallow breathing index, APACHE II score Acute Physiology and Chronic Health Evaluation II score, HR heart rate, TV tidal volume
Table 5.
Estimates of sensitivity, specificity, and success rate at several LTC cutoffs
| Cutoff | Sensitivity | Specificity | Success rate (%) |
|---|---|---|---|
| 35 | 0.994 | 0.364 | 66 |
| 40 | 0.944 | 0.546 | 77 |
| 45 | 0.895 | 0.636 | 84 |
| 50 | 0.840 | 0.636 | 90 |
| 55 | 0.753 | 0.818 | 94 |
| 60 | 0.654 | 0.909 | 96 |
| 65 | 0.562 | 0.909 | 98 |
| 70 | 0.488 | 0.909 | 99 |
LTC lung-thorax compliance
Risk factors for extubation failure are known and include an RSBI greater than 100 [39–42], a PaO2 to FiO2 ratio less than 200 mmHg [40], PaCO2 greater than 44 mmHg during the SBT [43], and others [3, 11, 14, 15, 39–49]. In this study, vascular surgery was also a risk factor. In addition, WOB showed a significant difference between success group (0.76 ± 0.26) and failure group (0.99 ± 0.41). WOB is calculated using the following formula based on LTC: WOB = TV/LTC + (inspiratory flow velocity) × (resistance of the respiratory tract). The normal range of WOB is 0.3–0.7 J/L. The range is narrow, and it is more difficult to judge for determining extubation compared with LTC. In contract, LTC measured during the SBT is a candidate factor for extubation assessment because it is simple and accurate.
We adopted strict criteria in the SBT process because most of the subjects were operable patients and did not have severe respiratory complications. This may be the reason why the failure rate of our study (6.4%) was lower than those of previous reports (11–19%) [1–4, 10–15]. The APACHE II score, HR, RR, TV, and RSBI were significantly different between the two groups even though all subjects met the SBT process criteria. These parameters were recorded as the average of the last 30 min of the observation period. Furthermore, the sensitivities of the RR, RSBI, APACHE II score, and HR were 0.895, 0.982, 0.815, and 0.698, respectively, and the specificities were 0.636, 0.455, 0.636, and 0.727, respectively. The averages of the RR, RSBI, APACHE II score, or HR in the last 30 min of the observation period might be a predictor of extubation failure even when patients meet the SBT process criteria.
During the SBT process, five patients were excluded because they underwent a tracheostomy instead of extubation. The averages of the LTC, RR, RSBI, APACHE II score, and HR in these patients were 45.3, 22.9, 79.7, 18.8, and 75.4, respectively. All data indicated that patients in the failure group were in a worse condition than those in the success group. The results of the analysis were not different even with the inclusion of these patients.
According to Sandy et al., no difference was observed in the rate of extubation failure, duration of mechanical ventilation, or ICU and hospital stays among the SBT using PAV, T-tube, and PSV [50]. Bosma et al. revealed that the SBT using PAV was superior to PSV regarding the duration of mechanical ventilation or ICU stays [51]. Their results suggested that the PAV mode was a valid alternative for use in an SBT. Because PAV shows good synchronization with patients’ spontaneous breathing [23–25, 51], an SBT can be performed when the sedative drugs are reduced, which may allow more accurate measurement of LTC. Moreover, in the PAV mode, LTC can be measured continuously. As a problem of PAV, patients with interstitial pneumonia and ARDS are over-ventilated because the inspiratory flow is fast as a result of restrictive disorders, but the inspiratory time and TV are limited. In patients with severe chronic obstructive pulmonary disease (COPD) and ICU-acquired weakness (ICUAW) [52], respiratory muscle fatigue lowers the work of breathing and inspiratory flow. Ventilation becomes insufficient, and excess CO2 leads to hypercapnia. Conducting an SBT using the PAV mode in these patients with severe respiratory failure is difficult. However, short-term use of PAV is possible for LTC measurement. Further studies on the effectiveness of LTC measurement for patients with severe respiratory disease are necessary.
This study has several limitations. First, the study was conducted in a single center with a small sample size. Therefore, a generalization of the results may not be possible. Second, most of the subjects were surgical patients because the study was conducted in the SICU; few patients had serious respiratory diseases. However, the number of subjects in each group provided sufficient power for adequate statistical calculations, and the study was conducted in a single SICU, resulting in less heterogeneity in patient management and monitoring. Third, because the LTC data were indicated on the monitor of the ventilator, intensivists could view the data. However, the influence of this on our study was low because this study was designed as a retrospective cohort study.
Conclusion
LTC measured during an SBT is a potential indicator of extubation failure in postoperative patients. Even in patients who met the strict SBT process criteria, the average RR, RSBI, APACHE II score, and HR in the last 30 min of the observation period might be the predictors of extubation failure. Further studies are necessary to determine the efficacy of this process for patients with severe respiratory disease.
Acknowledgements
We gratefully acknowledge the work of the members of our laboratory.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- APACHE II score
Acute Physiology and Chronic Health Evaluation II score
- ARDS
Acute respiratory distress syndrome
- AUC
Area under the curve
- BMI
Body mass index
- CNS
Central nervous system
- COPD
Chronic obstructive pulmonary disease
- EtCO2
End-tidal carbon dioxide
- FiO2
Inspired oxygen fraction
- HFNC
High-flow nasal cannula
- HR
Heart rate
- IV-PCA
Intravenous patient-controlled analgesia
- LTC
Lung-thorax compliance
- NPPV
Non-invasive positive-pressure ventilation
- NSAIDs
Nonsteroidal anti-inflammatory drugs
- PaCO2
Partial pressure of arterial carbon dioxide
- PaO2
Partial pressure of arterial oxygen
- PAV
Proportional assist ventilation
- PEEP
Positive end-expiratory pressure
- pH
Power of hydrogen
- PNB
Peripheral nerve block
- RASS
Richmond Agitation-Sedation Scale
- ROC curve
Receiver operating characteristic curve
- RR
Respiratory rate
- RSBI
Rapid shallow breathing index
- SBT
Spontaneous breathing trial
- SICU
Surgical intensive care unit
- SIMV
Synchronized intermittent mandatory ventilation
- SpO2
Arterial oxygen saturation
- TV
Tidal volume
- WOB
Work of breathing
Authors’ contributions
YO contributed to the study conception, research plan creation, data collection, data analysis, and manuscript preparation. TA and SB contributed to the data collection, data analysis confirmation, and chart creation. HS contributed to the data collection, data analysis confirmation, and manuscript preparation. KK contributed to the data collection, manuscript preparation, and chart creation. TY and GS contributed to the data collection, data analysis confirmation, and manuscript proofreading. TH contributed to the research plan creation, data collection, data analysis confirmation, and manuscript proofreading. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Ethical Review Board, Faculty of Medicine, Kagawa University (Heisei 29-049). The consent form was acquired when anesthesia was explained or at the admission to the intensive care unit.
Consent for publication
The consent form was acquired at the time of the explanation of anesthesia or admission to the intensive care units.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yugo Okabe, Phone: +81-087-891-2223, Email: m95018@med.kagawa-u.ac.jp.
Takehiko Asaga, Phone: +81-087-891-2223, Email: asagat@med.kagawa-u.ac.jp.
Sayuri Bekku, Phone: +81-087-891-2223, Email: sayuri@med.kagawa-u.ac.jp.
Hiromi Suzuki, Phone: +81-087-891-2465, Email: tanzuki@med.kagawa-u.ac.jp.
Kanae Kanda, Phone: +81-087-891-2133, Email: oda@med.kagawa-u.ac.jp.
Takeshi Yoda, Phone: +81-086-462-1111, Email: tyoda@med.kawasaki-m.ac.jp.
Tomohiro Hirao, Phone: +81-087-891-2133, Email: sharks@med.kagawa-u.ac.jp.
Gotaro Shirakami, Phone: +81-087-891-2223, Email: gshi@med.kagawa-u.ac.jp.
References
- 1.Frutos-Vivar F, Esteban A, Apezteguia C, Gonzalez M, Arabi Y, Restrepo MI, Gordo F, Santos C, Alhashemi JA, Perez F, et al. Outcome of reintubated patients after scheduled extubation. J Crit Care. 2011;26(5):502–509. doi: 10.1016/j.jcrc.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 2.Thille AW, Cortes-Puch I, Esteban A. Weaning from the ventilator and extubation in ICU. Curr Opin Crit Care. 2013;19(1):57–64. doi: 10.1097/MCC.0b013e32835c5095. [DOI] [PubMed] [Google Scholar]
- 3.Thille AW, Harrois A, Schortgen F, Brun-Buisson C, Brochard L. Outcomes of extubation failure in medical intensive care unit patients. Crit Care Med. 2011;39(12):2612–2618. doi: 10.1097/CCM.0b013e3182282a5a. [DOI] [PubMed] [Google Scholar]
- 4.Thille AW, Richard JC, Brochard L. The decision to extubate in the intensive care unit. Am J Respir Crit Care Med. 2013;187(12):1294–1302. doi: 10.1164/rccm.201208-1523CI. [DOI] [PubMed] [Google Scholar]
- 5.Esteban A, Anzueto A, Frutos F, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002;287(3):345–355. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- 6.Torres A, Gatell JM, Aznar E, El-Ebiary M, de la Bellacasa JP, González J, Ferrer M, Rodriguez-Roisin R. Re-intubation increases the risk of nosocomial pneumonia in patients needing mechanical ventilation. Am J Respir Crit Care Med. 1995;152(1):137–141. doi: 10.1164/ajrccm.152.1.7599812. [DOI] [PubMed] [Google Scholar]
- 7.Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, Pearl R, Silverman H, Stanchina M, Vieillard-Baron A, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29(5):1033–1056. doi: 10.1183/09031936.00010206. [DOI] [PubMed] [Google Scholar]
- 8.Ely EW, Baker AM, Dunagan DP, Burke HL, Smith AC, Kelly PT, Johnson MM, Browder RW, Bowton DL, Haponik EF. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996;335(25):1864–1869. doi: 10.1056/NEJM199612193352502. [DOI] [PubMed] [Google Scholar]
- 9.Robertson TE, Mann HJ, Hyzy R, Rogers A, Douglas I, Waxman AB, Weinert C, Alapat P, Guntupalli KK, Buchman TG. Multicenter implementation of a consensus-developed, evidence-based, spontaneous breathing trial protocol*. Crit Care Med. 2008;36(10):2753–2762. doi: 10.1097/CCM.0b013e3181872833. [DOI] [PubMed] [Google Scholar]
- 10.Burns KEA, Soliman I, Adhikari NKJ, Zwein A, Wong JTY, Gomez-Builes C, Pellegrini JA, Chen L, Rittayamai N, Sklar M, et al. Trials directly comparing alternative spontaneous breathing trial techniques: a systematic review and meta-analysis. Crit Care. 2017;21(1):127. doi: 10.1186/s13054-017-1698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epstein SK, Ciubotaru RL, Wong JB. Effect of failed extubation on the outcome of mechanical ventilation. Chest. 1997;112(1):186–192. doi: 10.1378/chest.112.1.186. [DOI] [PubMed] [Google Scholar]
- 12.Esteban A, Alía I, Tobin MJ, Gil A, Gordo F, Vallverdú I, Blanch L, Bonet A, Vázquez A, Pablo RD, et al. Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. Am J Respir Crit Care Med. 1999;159(2):512–518. doi: 10.1164/ajrccm.159.2.9803106. [DOI] [PubMed] [Google Scholar]
- 13.Esteban A, Alía I, Gordo F, Fernández R, Solsona JF, Vallverdú I, Macías S, Allegue JM, Blanco J, Carriedo D, et al. Extubation outcome after spontaneous breathing trials with T-tube or pressure support ventilation. Am J Respir Crit Care Med. 1997;156(2):459–465. doi: 10.1164/ajrccm.156.2.9610109. [DOI] [PubMed] [Google Scholar]
- 14.Epstein SK. Noninvasive ventilation to shorten the duration of mechanical ventilation. Respir Care. 2009;54:198–211. [PubMed] [Google Scholar]
- 15.Miu T, Joffe AM, Yanez ND, Khandelwal N, Dagal AH, Deem S, Treggiari MM. Predictors of reintubation in critically ill patients. Respir Care. 2014;59(2):178–185. doi: 10.4187/respcare.02527. [DOI] [PubMed] [Google Scholar]
- 16.Younes M, Webster K, Kun J, Roberts D, Masiowski B. A method for measuring passive elastance during proportional assist ventilation. Am J Respir Crit Care Med. 2001;164(1):50–60. doi: 10.1164/ajrccm.164.1.2010068. [DOI] [PubMed] [Google Scholar]
- 17.Ranieri VM, Eissa NT, Corbeil C, Chassé M, Braidy J, Matar N, Milic-Emili J. Effects of positive end-expiratory pressure on alveolar recruitment and gas exchange in patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1991;144(3_pt_1):544–551. doi: 10.1164/ajrccm/144.3_Pt_1.544. [DOI] [PubMed] [Google Scholar]
- 18.Ranieri VM, Giuliani R, Fiore T, Dambrosio M, Milic-Emili J. Volume-pressure curve of the respiratory system predicts effects of PEEP in ARDS: “occlusion” versus “constant flow” technique. Am J Respir Crit Care Med. 1994;149(1):19–27. doi: 10.1164/ajrccm.149.1.8111581. [DOI] [PubMed] [Google Scholar]
- 19.Amato MB, Barbas CS, Medeiros DM, Schettino GD, Filho GL, Kairalla RA, Deheinzelin D, Morais C, Fernandes EO, Takagaki TY. Beneficial effects of the “open lung approach” with low distending pressures in acute respiratory distress syndrome. A prospective randomized study on mechanical ventilation. Am J Respir Crit Care Med. 1995;152(6):1835–1846. doi: 10.1164/ajrccm.152.6.8520744. [DOI] [PubMed] [Google Scholar]
- 20.Tobin MJ. Respiratory monitoring in the intensive care unit. Am Rev Respir Dis. 1988;138(6):1625–1642. doi: 10.1164/ajrccm/138.6.1625. [DOI] [PubMed] [Google Scholar]
- 21.Yokoyama H, Kondou M, Morio Y, Hiraki K. Usefulness of pulmonary compliance determination in patients undergoing artifical respiration: relationship of pulmonary compliance to tidal volume and weaning from mechanical ventilation. Rigakuryoho Kagaku. 2007;22(3):373–378. doi: 10.1589/rika.22.373. [DOI] [Google Scholar]
- 22.Younes M, Riddle W, Polacheck J. A model for the relation between respiratory neural and mechanical outputs. III. Validation. J Appl Physiol. 1981;51(4):990–1001. doi: 10.1152/jappl.1981.51.4.990. [DOI] [PubMed] [Google Scholar]
- 23.Grasso S, Puntillo F, Mascia L, Ancona G, Fiore T, Bruno F, Slutsky AS, Ranieri VM. Compensation for increase in respiratory workload during mechanical ventilation. Am J Respir Crit Care Med. 2000;161(3):819–826. doi: 10.1164/ajrccm.161.3.9902065. [DOI] [PubMed] [Google Scholar]
- 24.Mitrouska J, Xirouchaki N, Patakas D, Siafakas N, Georgopoulos D. Effects of chemical feedback on respiratory motor and ventilatory output during different modes of assisted mechanical ventilation. Eur Respir J. 1999;13:873–882. doi: 10.1034/j.1399-3003.1999.13d30.x. [DOI] [PubMed] [Google Scholar]
- 25.Ranieri VM, Giuliani R, Mascia L, Grasso S, Petruzzelli V, Puntillo N, Perchiazzi G, Fiore T, Brienza A. Patient-ventilator interaction during acute hypercapnia: pressure-support vs. proportional-assist ventilation. J Appl Physiol. 1996;81(1):426–436. doi: 10.1152/jappl.1996.81.1.426. [DOI] [PubMed] [Google Scholar]
- 26.Xirouchaki N, Kondili E, Klimathianaki M, Georgopoulos D. Is proportional-assist ventilation with load-adjustable gain factors a user-friendly mode? Intensive Care Med. 2009;35(9):1599–1603. doi: 10.1007/s00134-009-1524-2. [DOI] [PubMed] [Google Scholar]
- 27.Girard TD, Kress JP, Fuchs BD, Thomason JWW, Schweickert WD, Pun BT, Taichman DB, Dunn JG, Pohlman AS, Kinniry PA, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (awakening and breathing controlled trial): a randomised controlled trial. Lancet. 2008;371(9607):126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 28.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342(20):1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 29.Treggiari MM, Romand J-A, Yanez ND, Deem SA, Goldberg J, Hudson L, Heidegger C-P, Weiss NS. Randomized trial of light versus deep sedation on mental health after critical illness*. Crit Care Med. 2009;37(9):2527–2534. doi: 10.1097/CCM.0b013e3181a5689f. [DOI] [PubMed] [Google Scholar]
- 30.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond Agitation–Sedation Scale. Am J Respir Crit Care Med. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 31.Perren A, Domenighetti G, Mauri S, Genini F, Vizzardi N. Protocol-directed weaning from mechanical ventilation: clinical outcome in patients randomized for a 30-min or 120-min trial with pressure support ventilation. Intensive Care Med. 2002;28(8):1058–1063. doi: 10.1007/s00134-002-1353-z. [DOI] [PubMed] [Google Scholar]
- 32.MacIntyre NR. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest. 2001;120(6, Supplement):375S–395S. doi: 10.1378/chest.120.6_suppl.375S. [DOI] [PubMed] [Google Scholar]
- 33.Keeping A. Early versus late tracheostomy for critically ill patients: a clinical evidence synopsis of a recent Cochrane Review. Canadian Journal of Respiratory Therapy: CJRT = Revue Canadienne de la Thérapie Respiratoire : RCTR. 2016;52(1):27–28. [PMC free article] [PubMed] [Google Scholar]
- 34.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Haenel JB, Johnson JL. Anesthesia secrets. 4. Philadelphia: Mosby; 2011. Chapter 21 - mechanical ventilation in critical illness; pp. 149–156. [Google Scholar]
- 36.Suter PM, Fairley HB, Isenberg MD. Effect of tidal volume and positive end-expiratory pressure on compliance during mechanical ventilation. Chest. 1978;73(2):158–162. doi: 10.1378/chest.73.2.158. [DOI] [PubMed] [Google Scholar]
- 37.Nassar BS, Collett ND, Schmidt GA. The flow-time waveform predicts respiratory system resistance and compliance. J Crit Care. 2012;27(4):418. doi: 10.1016/j.jcrc.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 38.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 39.Frutos-Vivar F, Ferguson ND, Esteban A, Epstein SK, Arabi Y, Apezteguía C, González M, Hill NS, Nava S, D’Empaire G, et al. Risk factors for extubation failure in patients following a successful spontaneous breathing trial. Chest. 2006;130(6):1664–1671. doi: 10.1378/chest.130.6.1664. [DOI] [PubMed] [Google Scholar]
- 40.Namen AM, Ely EW, Tatter SB, Case LD, Lucia MA, Smith A, Landry S, Wilson JA, Glazier SS, Branch CL, et al. Predictors of successful extubation in neurosurgical patients. Am J Respir Crit Care Med. 2001;163(3):658–664. doi: 10.1164/ajrccm.163.3.2003060. [DOI] [PubMed] [Google Scholar]
- 41.Smina M, Salam A, Khamiees M, Gada P, Amoateng-Adjepong Y, Manthous CA. Cough peak flows and extubation outcomes. Chest. 2003;124(1):262–268. doi: 10.1378/chest.124.1.262. [DOI] [PubMed] [Google Scholar]
- 42.Thille AW, Boissier F, Ben Ghezala H, Razazi K, Mekontso-Dessap A, Brun-Buisson C. Risk factors for and prediction by caregivers of extubation failure in ICU patients: a prospective study. Crit Care Med. 2015;43(3):613–620. doi: 10.1097/CCM.0000000000000748. [DOI] [PubMed] [Google Scholar]
- 43.Mokhlesi B, Tulaimat A, Gluckman TJ, Wang Y, Evans AT, Corbridge TC. Predicting extubation failure after successful completion of a spontaneous breathing trial. Respir Care. 2007;52:1710–1717. [PubMed] [Google Scholar]
- 44.Chien J-Y, Lin M-S, Huang Y-CT, Chien Y-F, Yu C-J, Yang P-C. Changes in B-type natriuretic peptide improve weaning outcome predicted by spontaneous breathing trial. Crit Care Med. 2008;36(5):1421–1426. doi: 10.1097/CCM.0b013e31816f49ac. [DOI] [PubMed] [Google Scholar]
- 45.Lemaire F, Teboul JL, Cinotti L, Giotto G, Abrouk F, Steg G, Macquin-Mavier I, Zapol WM. Acute left ventricular dysfunction during unsuccessful weaning from mechanical ventilation. Anesthesiology. 1988;69(2):171–179. doi: 10.1097/00000542-198808000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Su W-L, Chen Y-H, Chen C-W, Yang S-H, Su C-L, Perng W-C, Wu C-P, Chen J-H. Involuntary cough strength and extubation outcomes for patients in an ICU. Chest. 2010;137(4):777–782. doi: 10.1378/chest.07-2808. [DOI] [PubMed] [Google Scholar]
- 47.Teixeira C, da Silva NB, Savi A, Vieira SRR, Nasi LA, Friedman G, Oliveira RP, Cremonese RV, Tonietto TF, Bressel MAB, et al. Central venous saturation is a predictor of reintubation in difficult-to-wean patients*. Crit Care Med. 2010;38(2):491–496. doi: 10.1097/CCM.0b013e3181bc81ec. [DOI] [PubMed] [Google Scholar]
- 48.Tillquist MN, Gabriel RA, Dutton RP, Urman RD. Incidence and risk factors for early postoperative reintubations. J Clin Anesth. 2016;31:80–89. doi: 10.1016/j.jclinane.2015.12.038. [DOI] [PubMed] [Google Scholar]
- 49.Vallverdú I, Calaf N, Subirana M, Net A, Benito S, Mancebo J. Clinical characteristics, respiratory functional parameters, and outcome of a two-hour T-piece trial in patients weaning from mechanical ventilation. Am J Respir Crit Care Med. 1998;158(6):1855–1862. doi: 10.1164/ajrccm.158.6.9712135. [DOI] [PubMed] [Google Scholar]
- 50.Teixeira SN, Osaku EF, Costa CR, Toccolini BF, Costa NL, Candia MF, Leite MA, Jorge AC, Duarte PA. Comparison of proportional assist ventilation plus, T-tube ventilation, and pressure support ventilation as spontaneous breathing trials for extubation: a randomized study. Respir Care. 2015;60(11):1527–1535. doi: 10.4187/respcare.03915. [DOI] [PubMed] [Google Scholar]
- 51.Bosma KJ, Read BA, Bahrgard Nikoo MJ, Jones PM, Priestap FA, Lewis JF. A pilot randomized trial comparing weaning from mechanical ventilation on pressure support versus proportional assist ventilation. Crit Care Med. 2016;44(6):1098–1108. doi: 10.1097/CCM.0000000000001600. [DOI] [PubMed] [Google Scholar]
- 52.Levine S, Nguyen T, Taylor N, Friscia ME, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358(13):1327–1335. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.