Abstract
We report a case series of three idiopathic unilateral facial nerve palsies in neonates with no identified risk factors. Neuroimaging done was normal. All the neonates had complete spontaneous recovery within a month, with no residual deficits. As per our knowledge, there are very few case reports of facial palsy in a neonate reported in literature and are often labelled as idiopathic.
Keywords: neonatal health, materno-fetal medicine, neonatal and paediatric intensive care
Background
Bell’s palsy is an idiopathic acute-onset, usually unilateral lower motor neuron facial nerve palsy. It is not a common phenomenon in newborns. Facial palsy can be congenital or developmental. Congenital palsy occurs due to perinatal trauma. Developmental palsy occurs due to error in development, for example, aplasia or hypoplasia of the cranial nerve nuclei, nuclear agenesis, and aplasia or hypoplasia of the facial nerve.1 This can be associated with syndromes like Mobius, coloboma, heart defects, atresia choanae (also known as choanal atresia), growth retardation, genital abnormalities, and ear abnormalities. (CHARGE syndrome), Goldenhar and hemifacial macrosomia.2 Congenital facial palsy usually resolves with time; in contrast, developmental facial palsy usually does not resolve with time. Some of the usual causes in newborn period are traumatic delivery, congenital malformations and ear infections. Here, we present a case of idiopathic unilateral lower motor neuron palsy with spontaneous recovery.
Case presentation
Patient 1
A male baby was born at 34+3 weeks with a birth weight of 2064 g to a G4P3A3L0 mother (with previous history of three spontaneous abortions). Antenatally, the mother had intrahepatic cholestasis of pregnancy and hypothyroidism. Antenatal ultrasounds (USGs) were normal. The delivery was done by an emergency caesarean section in view of leaking per vaginum with fetal distress. No forceps were used during delivery. The baby cried immediately after birth and had Apgar scores of 8 and 8 at 1 and 5 min, respectively. He had respiratory distress for which delivery room continuous positive airway pressure (CPAP) was given and also required one dose of surfactant. CPAP was discontinued after 50 hours of life. On day 3 of life, the baby was observed to be unable to close his right eye and there was a deviation of the mouth to the left side with crying (figure 1). There was no forehead wrinkling on the right side. The nasolabial fold was also less prominent on the right side (figure 1). However, during sleep, there was no deviation of face. There was no dysmorphism. No other focal neurological deficit was noted. There was no ear discharge. Clinical diagnosis of right-sided lower motor neuron facial nerve palsy was made. Baby had no difficulty in accepting feeds. USG cranium was normal. Contrast MRI brain was also reported to be normal. Lumbar puncture was done to rule out any meningitis but was normal. Hearing evaluation by otoacoustic emissions (OAEs) and automated auditory brainstem response (AABR) was normal. Later CT brain was also done to rule out any osteomyelitis of temporal bone, but there were no abnormal findings noted.
Figure 1.
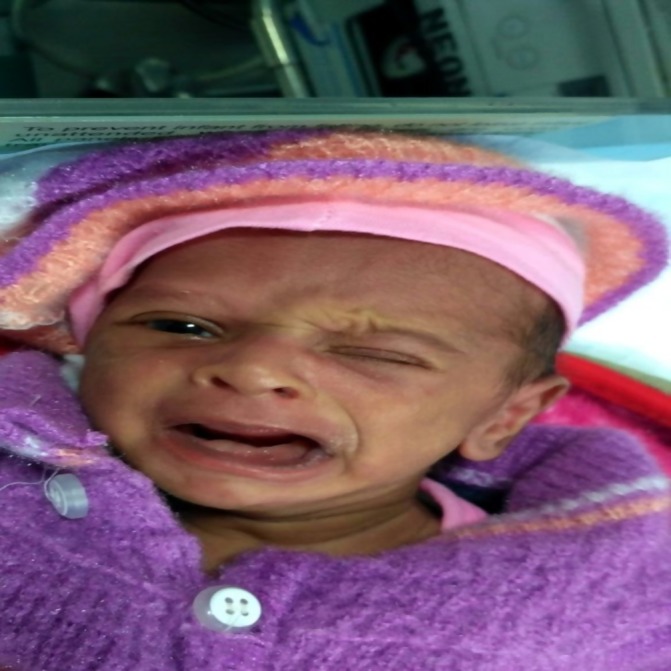
Right-sided lower motor neuron facial palsy.
Patient 2
A male baby was born at 37 weeks with a birth weight of 3380 g to a primigravida mother. Antenatally, the mother had intrahepatic cholestasis of pregnancy and the antenatal USG was suggestive of polyhydramnios. The delivery was done by an emergency caesarean section in view of fetal distress. The baby cried immediately after birth and had Apgar scores of 7 and 9 at 1 and 5 min, respectively. Soon after birth, the baby developed respiratory distress for which the baby was given oxygen. The feed was started on day 4, following which he developed vomiting episodes and abdominal distention, after which he was kept nil per oral. The infant also had seizure episodes for which he was started on antibiotics and anticonvulsants. The baby was referred to our centre on day 8 of life in view of abdominal distension and seizures. At admission, the baby had respiratory distress with Downe’s score of 6 and was having active seizures. The baby was started on nasal CPAP and the seizures were managed as per unit protocol. The X-ray chest was suggestive of right upper lobe pneumonia. On day 3 of admission (day 11), the baby had to be started on mechanical ventilation in view of increasing respiratory distress. The baby could be extubated after 4 days of ventilation to nasal CPAP, which was discontinued after 48 hours. The baby received antibiotics for 21 days in view of meningitis. The baby also required a red blood transfusion for anaemia. On day 18 of life after weaning him from CPAP, the baby was observed to be unable to close his left eye and there was a deviation of the mouth to the right side during crying. There was also loss of forehead wrinkling on the left side. The nasolabial fold was also less prominent on the left side (figure 2). There was no dysmorphism and no other focal neurological deficit was noted. Furthermore, there was no ear discharge. At discharge, palsy was improving.
Figure 2.
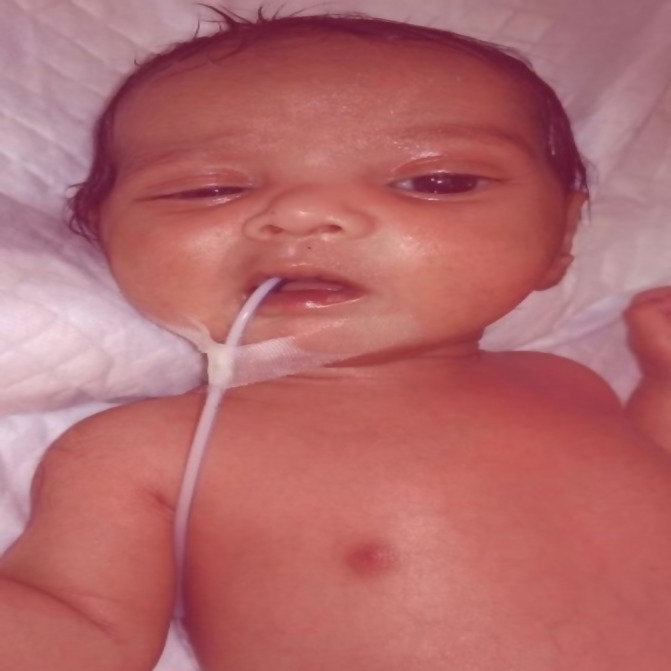
Left-sided lower motor neuron facial palsy.
Patient 3
A male baby was born at 33+2 weeks with a birth weight of 2400 g to a G2P1A1L0 mother. Antenatally, the mother had hypothyroidism and all her antenatal USGs were normal. The baby was born by an emergency caesarean section in view of deranged Doppler and fetal distress. Baby cried immediately after birth and had Apgar scores of 6 and 8 at 1 and 5 min, respectively. He had respiratory distress for which delivery room CPAP and one dose of surfactant were administered. CPAP was discontinued after 28 hours of life. On day 2 of life, the baby was observed to be unable to close his right eye and there was a deviation of the mouth to the left side with crying. There was no forehead wrinkling on the right side. The nasolabial fold was also less prominent on the right side. There was no dysmorphism. No other focal neurological deficit was noted. There was no ear discharge. Clinical diagnosis of right-sided lower motor neuron facial nerve palsy was made. The baby had no difficulty in accepting feeds. USG cranium was normal. Contrast MRI brain was also reported to be normal. Hearing evaluation by OAE and AABR was normal. Later CT brain was also done to rule out any osteomyelitis of temporal bone, but there were no abnormal findings noted. His MRI was also reported to be normal (table 1).
Table 1.
Investigation panel of patients
| Investigation | Patient 1 | Patient 2 | Patient 3 |
| CRP (mg/L) | 4 | 15 | 10 |
| Blood culture | Sterile | Klebsiella | Sterile |
| Serum electrolytes | Normal | Normal | Normal |
| CSF | Normal, glucose—35 mg/dl (against blood glucose—63 mg/dl), protein—99.4 mg/dL, cells—20 (all lymphocytes) | Glucose—51mg/dl (against blood glucose—84mg/dl), protein—85.3 mg/dl, cells—35 (polymorph—30%, lymphocyte—70%) | Normal, glucose—65 mg/dl (against blood glucose—93 mg/dl), protein—59 mg/dL, cells—02 (all lymphocytes) |
| CBC | Haematocrit—41.9%, platelet count—233×109/L, total leucocyte count—8.2×109/L | Haematocrit—30%, platelet count— 102×109/L, total leucocyte count— 18.5×109/L |
Haematocrit—46.5%, platelet count— 182×109/L Total leucocyte count— 11.5×109/L |
| USG cranium | Normal | Normal | Normal |
| CT head | Normal | Normal | Normal |
| MRI brain | Normal | Normal | Normal |
CBC, complete blood count; CRP, C reactive protein; CSF, cerebrospinal fluid; USG, ultrasonography.
Investigations
Differential diagnosis
Birth trauma.
Otitis media.
Cardiofacial syndrome
Craniofacial microsomia
Treatment
Nasal continuous positive airway pressure in all the three patients.
Surfactant in patients 1 and 3.
Antibiotics.
Outcome and follow-up
On follow-up at 6 months, all the three neonates are thriving well and are gaining weight, and facial nerve palsy has completely recovered (figure 3).
Figure 3.

Complete recovery on follow-up.
Discussion
Facial palsy in newborn occurs in 0.23% to 1.8% of live births. Out of these, 78% to 90% cases are associated with birth trauma.3 Neonatal idiopathic facial palsy is even rarer. Some of the risk factors for birth trauma are a particular position of fetus where the face is compressed against the mother’s sacral promontory or fetus’s shoulder, application of forceps, primigravida mother and baby’s birth weight more than 3500 g. However, Laing et al in a retrospective case–control study found no association between permanent congenital facial palsy and presence of risk factors for trauma during delivery. They emphasised that it being a serious medicolegal issue for obstetricians, care should be taken before announcing that facial palsy is due to birth trauma.4 Bell’s palsy was first described by Sir Charles Bell in 1821. He described it as an acute-onset, idiopathic facial paralysis resulting from a dysfunction anywhere along the peripheral part of the facial nerve from the level of pons distally. Facial nerve is the seventh cranial nerve with muscles of the upper face represented bilaterally in the facial nerve nucleus located in the pons. Therefore, upper motor neuron palsy results in the contralateral weakness of facial muscles of the lower part of the face sparing the upper face. However, lower motor palsy results in ipsilateral palsy of the entire face.5 Some of the findings reported in MRI are contrast enhancement of geniculate ganglion within labyrinthine segment of the facial nerve.6 7 In our present case seriesy, MRI brain were normal. In neonates, the mastoid process is not fully developed, thus making facial nerve vulnerable to injury as it exits through the stylomastoid foramen. Compression injury can also occur in mandibular nerve (one of the branches of the facial nerve as it courses just above the lower edge of the mandible).8 We propose the compression of the facial nerve near its exit from the stylomastoid foramen due to application of tight CPAP interface as the cause in all of our patients. Akin to Bell’s palsy, we have named it as Pandita’s palsy (figure 4). Falco and Eriksson found complete resolution in 89% of babies with congenital facial nerve palsy by 7 months.3 Full recovery is possible because of good regenerative capacity of tissues in newborns. Saini et al reported beneficial use of 6 weeks of corticosteroids in newborns with Bell’s palsy.9 We did not give any steroids to the newborn because of concerns of long-term neurological deficit. In cases with permanent facial palsy, plastic surgeons have a role in reconstructing the facial muscles. Surgical exploration should be done only in highly specialised centres and only after a reasonable amount of time has been given for spontaneous recovery. Electrophysiological studies of the nerve may be required to identify the level and degree of nerve injury before doing any surgery. Clinical guidelines of the Nottingham Neonatal Service mention that persistent residual weakness at 3 months should warrant referral to plastic surgery clinic.10 Facial palsy needs to be differentiated from Cayler syndrome, also known as ‘asymmetric crying faces’ or hypoplasia of the depressor. The latter is associated with congenital heart defects and absence or hypoplasia of depressor angular oris muscle that controls the movements of the lower lip. The asymmetry is usually noted by the mother when the newborn cries or smiles and is not seen during sleep.11 The other condition that needs to be differentiated is craniofacial microsomia. It is characterised by facial asymmetry because of maxillary and/or mandibular hypoplasia, usually right-sided hypoplasia, preauricular or facial tags; ear malformations such as microtia, anotia or aural atresia; frontal bossing; diminished or absent parotid secretion; and tongue anomaly in the form of malfunction of taste sensation and hearing loss. It has a variety of presentations, which can range from subtle facial asymmetry with a small skin tag in front of a normal ear to rare bilateral involvement, microtia/anotia with atresia of the ear canals, microphthalmia and respiratory compromise from severe mandibular hypoplasia.
Figure 4.
Continuous positive airway pressure interface causing pressure over the facial nerve near its exit from stylomastoid foramen.
Learning points.
Idiopathic facial nerve palsy is usually a self-limiting condition in newborns.
Ear examination is vital in facial nerve palsy.
Parents should be properly counselled to allay their fear as the site of facial asymmetry in their baby can be frightening for them.
Footnotes
Contributors: NM and AP wrote the manuscript. GG and AS helped in corrections. AP and GG did the critical appraisal.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Song MR. Moving cell bodies: understanding the migratory mechanism of facial motor neurons. Arch Pharm Res 2007;30:1273–82. 10.1007/BF02980268 [DOI] [PubMed] [Google Scholar]
- 2.Sharma D, Kandraju H, Pandita A, et al. Craniofacial microsomia: an unusual finding in newborn. J Neonatal Biol 2014;103 10.4172/2167-0897.1000I.103 [DOI] [Google Scholar]
- 3.Falco NA, Eriksson E. Facial nerve palsy in the newborn: incidence and outcome. Plast Reconstr Surg 1990;85:1–4. [DOI] [PubMed] [Google Scholar]
- 4.Laing JH, Harrison DH, Jones BM, et al. Is permanent congenital facial palsy caused by birth trauma? Arch Dis Child 1996;74:56–8. 10.1136/adc.74.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpenter MB. Core text of neuroanatomy. 2nd edn Baltimore: Williams and Wilkins Co, 1978:126–8. [Google Scholar]
- 6.Swartz JD, Harnsberger HR, Mukherji SK. The temporal bone. Contemporary diagnostic dilemmas. Radiol Clin North Am 1998;36:819–53. 7. [DOI] [PubMed] [Google Scholar]
- 7.Finsterer J. Management of peripheral facial nerve palsy. Eur Arch Otorhinolaryngol 2008;265:743–52. 10.1007/s00405-008-0646-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavlou E, Gkampeta A, Arampatzi M. Facial nerve palsy in childhood. Brain Dev 2011;33:644–50. 10.1016/j.braindev.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 9.Saini A, Singhi P, Sodhi KS, et al. Bell palsy in a neonate with rapid response to oral corticosteroids: a case report. J Child Neurol 2013;28:506–8. 10.1177/0883073812444734 [DOI] [PubMed] [Google Scholar]
- 10.Dushyant B. Management of facial nerve palsy in newborn period. UK: Nottingham Neonatal Service—Clinical Guidelines, 2013:16. [Google Scholar]
- 11.Pawar SJ, Sharma DK, Srilakshmi S, et al. Cayler cardio-facial syndrome: an uncommon condition in newborns. Iran J Pediatr 2015;25:e502 10.5812/ijp.502 [DOI] [PMC free article] [PubMed] [Google Scholar]


