Abstract
Objectives
Nivolumab is used at 3 mg/kg or fixed doses of 240 mg every 2 weeks. There was no dose–response/toxicity relationship of nivolumab. This study evaluated the efficacy of low-dose nivolumab as an alternative to the financial toxicity of standard-dose nivolumab in treatment of non-small cell lung cancer (NSCLC).
Methods
Outcomes of patients with NSCLC treated with nivolumab as a routine practice at two tertiary hospitals in Korea were retrospectively analysed. Patients who could not afford standard nivolumab treatment received low-dose nivolumab (20 or 100 mg fixed dose every 3 weeks). Others received standard dose of 3 mg/kg every 2 weeks. Progression-free survival (PFS) and overall survival (OS) were measured and compared between low-dose and standard-dose groups in overall and stratified analyses according to programmed death-ligand 1 (PD-L1) status.
Results
Among the 47 patients with NSCLC, 18 received low-dose nivolumab. PD-L1 positivity was observed in 13 (27.7%) patients and did not differ between the groups. During 5.2 months of follow-up, the objective response rate was 13.8% in the standard-dose group and 16.7% in the low-dose group (p=0.788). Dosing of nivolumab or PD-L1 expression did not significantly affect PFS or OS.
Conclusion
Low-dose nivolumab can be effective in NSCLC and is worth considering as an alternative option to reducing financial toxicity. The efficacy of low-dose nivolumab requires study.
Keywords: low dose, nivolumab, lung cancer, financial toxicity
Key questions.
What is already known about this subject?
Nivolumab is used at 3 mg/kg or fixed doses of 240 mg every 2 weeks.
There has been no dose–response/toxicity relationship of nivolumab.
Immune checkpoint inhibitor (ICI) is hampered by its extremely high cost, which is termed financial toxicity.
What does this study add?
Among patients with non-small cell lung cancer (NSCLC) treated with nivolumab as a routine practice at two tertiary hospitals in Korea, patients who received low-dose nivolumab (20 or 100 mg every 3 weeks) showed similar survival outcomes and response rates to those who received standard-dose nivolumab.
Programmed death-ligand 1 expression did not significantly affect progression-free survival or overall survival.
How might this impact on clinical practice?
Low-dose nivolumab can be effective in NSCLC and is worth considering as an alternative option to reducing financial toxicity.
Patients or country that cannot afford high cost of ICIs might consider low-dose nivolumab rather than standard-dose nivolumab.
The efficacy of low-dose nivolumab requires further well-designed prospective studies, such as a non-inferiority phase III trial with sufficient sample size.
Introduction
Immune checkpoint inhibitor (ICI) that targets either programmed death 1 (PD-1)/programmed death-ligand 1 (PD-L1) is a novel approach for treating certain cancers.1 Anti-PD-1 antibody, including nivolumab or pembrolizumab, has become the standard treatment for non-small cell lung cancer (NSCLC). The CheckMate-017 and 057 studies2 3 confirmed the superiority of nivolumab compared with docetaxel in second-line NSCLC, and the KEYNOTE-010 study4 confirmed pembrolizumab as a standard treatment in second-line NSCLC.
ICI is uniquely different from cytotoxic chemotherapy and molecular-targeted agents. ICI changes T cells so that they selectively recognise cancer cells and indirectly enhances the T cell attack on cancer cells. The efficacy and safety of ICI differs from cytotoxic chemotherapy or molecular-targeted agents.5 No correlation has been observed between dose and efficacy in phase I clinical trials with ICIs, and no dose–toxicity relationship has been evident. The maximal tolerated dose (MTD) for nivolumab has not been identified, and a similar safety profile has been demonstrated across tumour types and dose levels (0.1–10 mg/kg).6 7 Recommended phase II dose and optimal interval were defined without considering MTD. In case of pembrolizumab, antitumour activity was observed at all doses (1–10 mg/kg) and schedules (every 2 or 3 weeks).8 9 There was no difference in efficacy between 10 and 2 mg/kg in the KEYNOTE-010 study.4 Based on that study, the Food and Drug Administration (FDA) deemed the higher dose unnecessary and approved the pembrolizumab dose of 2 mg/kg. More recently a 200 mg fixed dose of pembrolizumab was approved, and the FDA has approved both 3 mg/kg nivolumab and 240 mg fixed dose based on pharmacokinetic data that suggested no difference between these regimens.10
ICI is hampered by its extremely high cost, which is termed financial toxicity.11 The cost per year for an average patient weighing 70 kg is about US$157 000.12 The cost to treat patients with metastatic disease for 1 year in the USA is about US$174 billion.13 Dosing and scheduling are cost drivers. To reduce financial toxicity, the low-dose regimen might be an alternative option. The purpose of this study was to evaluate the efficacy of low-dose nivolumab comparing with standard-dose nivolumab in NSCLC.
Methods
Patients and treatment
We retrospectively analysed a database of patients with NSCLC treated with nivolumab at Seoul National University Hospital and Seoul National University Bundang Hospital between 1 October 2015 and 30 September 2017. The inclusion criteria were pathologically confirmed NSCLC, initial stage IIIB or IV or recurrence after curative surgery and treatment with nivolumab as palliative therapy in routine practice.
At the time of analysis in July 2017, nivolumab use was not a reimbursable expense in the sole, government-run medical insurance system in Korea. Patients with NSCLC were required to pay all the cost of nivolumab treatment. This non-reimbursement policy remained until September 2017. In Korea, the cost of on phial of nivolumab 100 mg and 20 mg was approximately US$1500 and US$350, respectively.
In the current study, patients who cannot afford the high cost of nivolumab but highly desired this treatment were treated on consent with the low dose. We carefully examined the patients’ economic status. We gave low-dose nivolumab only to patients who eagerly want to receive nivolumab but cannot pay the cost of standard-dose nivolumab with full agreement. Patients who afford the high cost received the standard-dose nivolumab. Nivolumab was prescribed regardless of PD-L1 immunohistochemistry (IHC) status.
The standard-dose group received nivolumab 3 mg/kg every 2 weeks, and the low-dose group received a fixed dose of nivolumab 100 or 20 mg every 3 weeks. As nivolumab is available as 100 mg phial and 20 mg phial formula, patients received a fixed dose of nivolumab 100 or 20 mg every 3 weeks by their affordable economic status. We assumed that low-dose nivolumab 100 mg or 20 mg flat dose can work based on the phase I studies of nivolumab which showed response rate of 29% with nivolumab 0.1 mg/kg6 and response rate of 33.3% with nivolumab 1.0 mg/kg.7
Treatment continued until disease progression, unacceptable toxicity that precluded continuing drug treatment or death. Patients were allowed to continue treatment despite disease progression if they were deriving a clinical benefit according to an investigator’s assessment.
Response evaluation
Chest and/or abdominopelvic CT scans were performed every 8–12 weeks as a routine clinical procedure, and additionally as needed to confirm patient response and to assess disease progression. A systemic response to nivolumab was measured by standard Response Evaluation Criteria in Solid Tumors (V.1.1).14 Objective responses were confirmed by at least one sequential tumour assessment. Progression-free survival (PFS) was measured from the date of nivolumab until either progression or death due to any cause. Overall survival (OS) was measured from the date of nivolumab until either death due to any cause or the last follow-up date.
IHC of PD-L1
For patients available for IHC analysis, PD-L1 expression was analysed using rabbit anti-PD-L1 (E1L3N) XP monoclonal antibody (Cell Signaling Technology, Danvers, Massachusetts, USA). The details of IHC methods have been previously described.15 16 PD-L1 IHC was evaluated based on the intensity and proportion of membranous staining, with or without cytoplasmic staining, in tumour cells and was scored as 0+ (no appreciable staining above background), 1+ (weak membranous staining and/or cytoplasmic staining), 2+ (moderate membranous staining and/or cytoplasmic staining) and 3+ (strong membranous staining and/or cytoplasmic staining).15 16 PD-L1 positivity was defined as membranous staining for PD-L1 in >1% of tumour cells. All slides were blinded with respect to clinical characteristics and outcomes and were reviewed and scored by two experienced pathologists.
Statistical analyses
The baseline characteristics of patients and clinicopathological findings according to dose of nivolumab were evaluated using the Χ2 test or Fisher’s exact test where appropriate. Survival analyses were performed using the Kaplan-Meier method and were compared using a log-rank test. Cox proportional hazard regression model was applied to determine the HR for specific variables with respect to survival. Two-sided p<0.05 were considered statistically significant. All statistical tests were two-sided and were performed using STATA, V.12 software (StataCorp LP).
Ethics approval and consent to participate
This study was approved by the SNUH and SNUBH Institutional Review Board (approval nos H-1709-039-883 and B-1710-425-401) and was conducted in accordance with the Declaration of Helsinki provisions. Patients’ consent to participate was waived according to the Institutional Review Board because of the retrospective design of this study.
Results
Patient characteristics
The clinical characteristics of the 47 patients are shown in table 1. The median age of the patients was 62 years, and 40 (85.1%) were men. Four (8.5%) patients had epidermal growth factor receptor-activating mutations, and 2 (4.3%) had anaplastic lymphoma kinase translocation. Thirteen (27.7%) patients were PD-L1-positive by IHC. In the low-dose group, 15 patients received the 100 mg fixed dose, and 3 patients received the 20 mg fixed dose. PD-L1 positivity was not different between two groups (22.2% and 31.0% in the low-dose and high-dose group, respectively; p=0.142). Nivolumab was prescribed as second-line therapy in 18 patients, third-line in 16 patients and fourth-line or more in 13 patients.
Table 1.
Baseline demographic and clinical characteristics by nivolumab dose
| Total (n=47) |
Standard-dose group (n=29) |
Low-dose group (n=18) |
P values | |
| Age at diagnosis | ||||
| Median (range) | 62 (41–81) | 59 (47–81) | 63.5 (41–77) | 0.982 |
| Sex | ||||
| Male | 40 (85.1) | 26 (89.7) | 14 (77.8) | 0.266 |
| Female | 7 (14.9) | 3 (10.3) | 4 (22.2) | |
| Histological subtype | ||||
| ADC | 29 (61.7) | 16 (55.2) | 13 (72.2) | 0.624 |
| SqCC | 7 (14.9) | 5 (17.2) | 2 (11.1) | |
| Other* | 11 (23.4) | 8 (27.6) | 3 (16.7) | |
| Smoking history | ||||
| Current or ex-smoker | 28 (59.6) | 16 (55.2) | 12 (66.7) | 0.435 |
| Never smoker | 19 (40.4) | 13 (44.8) | 6 (33.3) | |
| ECOG PS | ||||
| 0–1 | 34 (72.3) | 20 (69.0) | 14 (77.8) | 0.511 |
| ≥2 | 13 (27.7) | 9 (31.0) | 4 (22.2) | |
| Palliative reason | ||||
| Initial metastatic | 38 (80.9) | 25 (86.2) | 13 (72.2) | 0.236 |
| Recurred | 9 (19.1) | 4 (13.8) | 5 (27.8) | |
| EGFR mutation | ||||
| Positive | 4 (8.5) | 0 (0.0) | 4 (22.2) | 0.035 |
| Negative | 35 (74.5) | 23 (79.3) | 12 (66.7) | |
| Not tested | 8 (17.0) | 6 (20.7) | 2 (11.1) | |
| ALK IHC | ||||
| Positive | 2 (4.3) | 2 (6.9) | 0 (0.0) | 0.628 |
| Negative | 36 (76.6) | 21 (72.4) | 15 (83.3) | |
| Not tested | 9 (19.1) | 6 (20.7) | 3 (16.7) | |
| PD-L1 by IHC | ||||
| Positive | 13 (27.7) | 9 (31.0) | 4 (22.2) | 0.142 |
| Negative | 11 (23.4) | 4 (13.8) | 7 (38.9) | |
| Not tested | 23 (48.9) | 16 (55.2) | 7 (38.9) | |
| Cycles of nivolumab | ||||
| Median (range) | 3 (1–20) | 2 (1–20) | 3.5 (1–10) | 0.249 |
| No of regimen before nivolumab | ||||
| Median (range) | 2 (1–10) | 2 (1–7) | 2 (1–10) | 0.182 |
| 1 | 18 (38.3) | 13 (44.8) | 5 (27.8) | 0.337 |
| 2 | 16 (34.0) | 10 (34.5) | 6 (33.3) | |
| ≥3 | 13 (27.7) | 6 (20.7) | 7 (28.9) |
*Other histological subtypes consist of three sarcomatoid type, two large cell carcinoma, four poorly differentiated carcinoma and two not otherwise specified type.
ADC, adenocarcinoma; ALK, anaplastic lymphoma kinase; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; IHC, immunohistochemistry; PD-L1, programmed death-ligand 1; PS, performance status; SqCC, squamous cell carcinoma.
Outcomes of nivolumab between standard-dose and low-dose groups
The mean follow-up duration of 47 patients was 5.2 months, ranging from 0.5 to 14.2 months, and did not differ between standard-dose and low-dose groups. Among patients in the low-dose group, the best responses were partial response in 3/18 (16.7%) patients, stable disease in 4/18 (22.2%), progressive disease (PD) in 7/18 (38.9%) and mixed response (MR) in 3/18 (16.7%). Although the standard-dose group included more patients with PD (n=20/29, 69%) than the low-dose group, there were neither statistically significant differences of objective response rate (ORR) (p=0.206) or disease control rates (p=0.282) (table 2).
Table 2.
Outcomes of nivolumab by dose
| Total (n=47) |
Standard-dose group (n=29) |
Low-dose group (n=18) |
P values | |
| Best response | ||||
| CR | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.206 |
| PR | 7 (14.9) | 4 (13.8) | 3 (16.7) | |
| SD | 7 (14.9) | 3 (10.3) | 4 (22.2) | |
| PD | 27 (57.5) | 20 (69.0) | 7 (38.9) | |
| MR | 4 (8.5) | 1 (3.4) | 3 (16.7) | |
| NE | 2 (4.2) | 1 (3.4) | 1 (5.5) | |
| Best ORR* | 14.9 | 13.8 | 16.7 | 0.788 |
| Best DCR† | 29.8 | 24.1 | 38.9 | 0.282 |
| Progression-free survival | ||||
| Median months (95% CI) | 1.1 (0.8 to 3.0) | 1.0 (0.6 to 1.7) | 3.0 (0.8 to nr) | 0.242 |
| Overall survival | ||||
| Median months (95% CI) | 12.5 (6.5 to nr) | 8.2 (3.1 to nr) | 12.5 (7.0 to nr) | 0.305 |
| Treatment duration | ||||
| Mean±SD (range) | 2.7±3.1 (0.1–11.9) | 2.8±3.7 (0.1–11.9) | 2.5±1.8 (0.5–6.0) | 0.463 |
| Follow-up duration | ||||
| Mean±SD (range) | 5.2±3.9 (0.5–14.2) | 5.6±4.1 (0.5–14.2) | 4.7±3.5 (0.5–12.7) | 0.622 |
*ORR was calculated as summation of CRs and PRs over number of patients multiplied by 100.
†DCR was calculated as summation of CRs, PRs and SDs over number of patients multiplied by 100.
CR, complete response; DCR, disease control rate; MR, mixed response; NE, not evaluable; ORR, objective response rate; PD, progressive disease; PR, partial response.
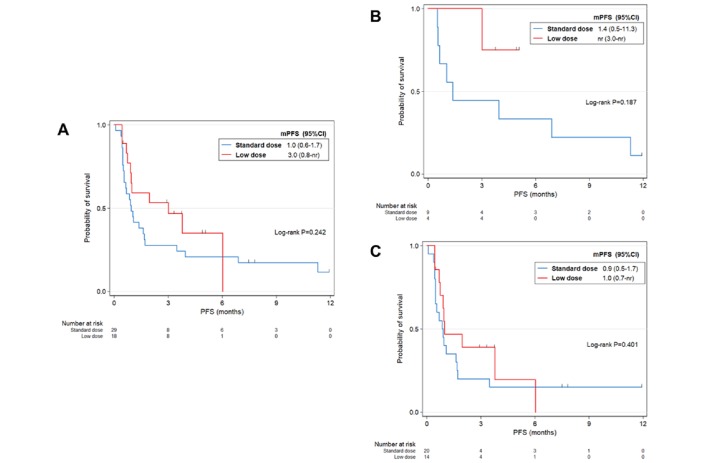
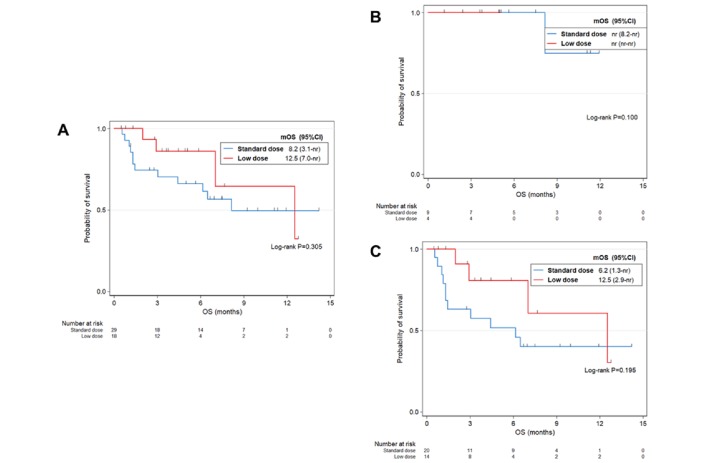
During the study, 25/29 (86.2%) patients in the standard-dose group and 11/18 (61.1%) in the low-dose group had first progression. The median PFS of the low-dose group was 3.0 months (0.8 month to not reached), which was not significantly different from that of the standard-dose groups (p=0.242; figure 1A). Among 16 patients who died, 12/16 (41.4%) received standard dose, and 4/16 (22.2%) received low-dose nivolumab. The median OS was 12.5 months in the total patients, 8.2 months in the standard-dose group and 12.5 months in the low-dose group. We did not observe significant difference between two groups (p=0.305; figure 2A).
Figure 1.
PFS by dose of nivolumab for (A) all patients (n=47), (B) PD-L1-positive patients (n=13) and (C) PD-L1-negative or unknown patients (n=34). mPFS, median progression-free survival; nr, not reached; PD-L1, programmed death-ligand 1; PFS, progression-free survival.
Figure 2.
Kaplan-Meier curves of OS by dose of nivolumab for (A) all patients (n=47), (B) PD-L1-positive patients (n=13) and (C) PD-L1-negative or unknown patients (n=34). mOS, median overall survival; nr, not reached; PD-L1, programmed death-ligand 1; OS, overall survival.
To study the survival of standard-dose and low-dose groups according to PD-L1 expression, we performed stratified survival analysis (PD-L1-positive vs PD-L1-negative or unknown). Regardless of PD-L1 expression, PFS was not statistically different between standard-dose and low-dose groups (p=0.187 in PD-L1-positive population; p=0.969 in PD-L1-negative or unknown population; figure 1B,C). Similarly, these two groups showed non-significant difference of OS in both PD-L1-positive and PD-L1-negative or unknown population (p=1.000 in the PD-L1-positive group; p=0.343 in the PD-L1-negative or unknown group; figure 2B,C).
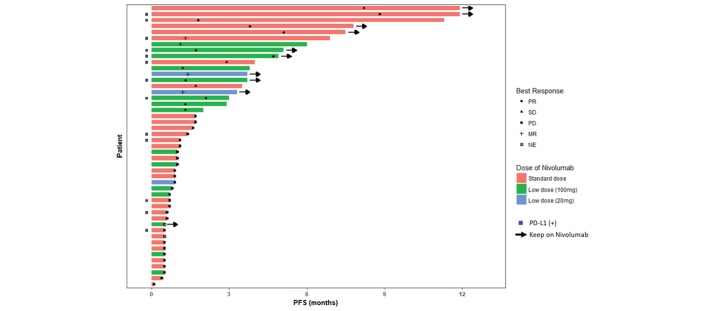
We included patients who received 20 mg fixed dose based on the results of phase 1 trials showing that antitumour activities were observed at 0.1 mg/kg and 0.3 mg/kg dose levels.6 17 Among three patients, one PD and three MR were recorded. A patient who had heavily treated lung adenocarcinoma with pleural metastasis was given nivolumab 20 mg fixed dose every 2 weeks, but presented with mechanical ileus due to colon metastases and progressed after 2 cycles with 0.9 month of PFS. Another patient with adenocarcinoma with lung-to-lung metastasis received 3 cycles of nivolumab with MR of lung lesions and has been on nivolumab until 8 cycles. The last patient with MR after 2 cycles of nivolumab who had disease burden in thorax, abdominal lymph nodes and sternum showed 3.3 months of PFS and OS. Best response, PFS and dose of nivolumab among 47 patients are summarised in figure 3.
Figure 3.
Swimmer plot of 47 patients who received nivolumab as a routine practice. MR, mixed response; NE, not evaluable; PD, progressive disease; PD -L1, programmed death-ligand 1; PFS, progression-free survival; PR, partial response.
Discussion
In this study, low-dose nivolumab (20 or 100 mg fixed dose) was effective in the real-world setting for patients with NSCLC compared with the currently reported dosing of 3 mg/kg every 3 weeks in key landmark trials. ORR, OS and PFS among the patients appear to be lower than those from the trials, being not different between standard-dose and low-dose groups. Based on the assumption that lower dose requires lower costs, our finding suggests that low-dose nivolumab can be both clinically and economically worth considering option for patients with NSCLC.
Dose selection for ICI treatments presents challenges because of the failure to prove a dose–response relationship in several phase I trials with nivolumab or pembrolizumab. In a dose–escalation study,6 1, 3 and 10 mg/kg nivolumab used in NSCLC treatment were safe and tolerable without confirmation of MTD. Antitumour activity was also observed in all the dose levels with a plateau at 3 mg/kg of nivolumab. The ORR was 17% in all and was lower in the 1 mg/kg group than in the 3 or 10 mg/kg dose group regardless of histological type.18 However, OS was higher at 3 mg/kg of dose with 1 year OS rate of 56% than at other doses tested, which was comparable with the 54.2% from our study. These non-linear relationships between dose and clinical outcomes were also observed in another integrative analysis from phase III trials of nivolumab across different cancer types including melanoma, NSCLC and renal cell carcinoma.19
In 2017, based on population pharmacokinetic analyses and dose–efficacy analyses,20 240 mg nivolumab flat dosing showed similar safety and efficacy compared with those of 3 mg/kg nivolumab every 2 weeks. This flat dose regimen simplified dosing and administration of nivolumab and received FDA approval. This unified flat dose provides several advantages in terms of preparation time and variability according to body weight change. Although 240 mg flat dose was selected with consideration of body weight range and nivolumab exposure, a lower nivolumab flat dose, such as 100 mg or even 20 mg, might show comparable effectiveness with a relatively flat dose–response relationship. In addition, despite the absence of a cost-effectiveness analysis, financial toxicity would likely be decreased if the dose of nivolumab could be reduced without compromised efficacy.
Lower doses of ICIs may be as effective as higher doses and considerably less expensive. The KEYNOTE-00121 and KEYNOTE-00222 studies demonstrated that the administration of pembrolizumab at doses ranging from 2 mg/kg every 3 weeks to 10 mg/kg every 2 weeks did not affect outcomes. Thus, the lower dose appears equally effective. The advantage comes in the cost of treatment; the monthly costs for an average-sized patient have been estimated to be US$9000 for 2 mg/kg every 3 weeks, US$46 000 for 10 mg/kg every 3 weeks and US$69 000 for 10 mg/kg every 2 weeks13. An unaffordable price of the drug limits the patient with cancer access and decreases the financial quality of life. Under the current system of insurance, many patients have to pay large sums out of pocket.23 24 In Korea, for a person who weighs 60 kg, the 100 mg fixed dose of nivolumab (KRW 1 340 068/100 mg one phial) every 3 weeks represents a cost saving of about KRW 3 484 176 each month compared with 3 mg/kg nivolumab administered every 2 weeks (KRW 4 824 244/360 mg). The correct patient selection is more important than the correct dose. The lowest dose level that still exhibits antitumour activity needs to be determined.
Our study has several limitations. First, it is a retrospective analysis. Second, selection of the low-dose group was based on economic status of patients. Those who strongly desired nivolumab treatment but who could not afford the high cost of the treatment were given the low-dose nivolumab. Patient consent was based on the lack of knowledge of the dose–efficacy relationship with a prior phase I trial findings of antitumour activity at doses of 0.1 or 1 mg/kg. Patients unable to pay for high cost of standard-dose nivolumab received low-dose nivolumab, which is a potential bias. Third, the small sample size produced low statistical power. Because of small sample size, it is difficult to conclude that there is no statistically significant difference.
Despite these limitations, to our knowledge, our study is the first to suggest the effectiveness of low-dose nivolumab. This concept might be applied to another cancer types and another ICI. In conclusion, low-dose nivolumab can be effective in NSCLC and could be an alternative option for reducing financial toxicity. Patients or country that cannot afford high cost of ICI might consider low-dose nivolumab rather than standard-dose nivolumab. Further well-designed prospective studies, such as a non-inferiority phase III trial with sufficient sample size, are needed to confirm the efficacy of low-dose nivolumab. Such a non-inferiority prospective trial cannot be performed as sponsor initiated trial, academic society should play a role with independent viewpoint.
Acknowledgments
The authors thank the patients and their families for their trust and participation to our studies. The authors thank Juyoun Kim, a data manager at Seoul National University Hospital, who managed the database.
Footnotes
Contributors: All authors have contributed to this article.
Funding: This study was supported by a grant from the Korea Health Technology R&D Project ‘Strategic Center of Cell and Bio Therapy for Heart, Diabetes & Cancer’ through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare (MHW), Republic of Korea (grant no HI17C2085).
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: SNUH and SNUBH Institutional Review Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015;27:450–61. 10.1016/j.ccell.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627–39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123–35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540–50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 5. Keam B, Jung H, Nam BH. Optimal design and endpoint of clinical trials using immune checkpoint blocking agents. Expert Rev Anticancer Ther 2016;16:1217–8. 10.1080/14737140.2016.1248945 [DOI] [PubMed] [Google Scholar]
- 6. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yamamoto N, Nokihara H, Yamada Y, et al. Phase I study of Nivolumab, an anti-PD-1 antibody, in patients with malignant solid tumors. Invest New Drugs 2017;35:207–16. 10.1007/s10637-016-0411-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chatterjee M, Turner DC, Felip E, et al. Systematic evaluation of pembrolizumab dosing in patients with advanced non-small-cell lung cancer. Ann Oncol 2016;27:1291–8. 10.1093/annonc/mdw174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patnaik A, Kang SP, Rasco D, et al. Phase I Study of Pembrolizumab (MK-3475; Anti-PD-1 Monoclonal Antibody) in Patients with Advanced Solid Tumors. Clin Cancer Res 2015;21:4286–93. 10.1158/1078-0432.CCR-14-2607 [DOI] [PubMed] [Google Scholar]
- 10. Freshwater T, Kondic A, Ahamadi M, et al. Evaluation of dosing strategy for pembrolizumab for oncology indications. J Immunother Cancer 2017;5:43 10.1186/s40425-017-0242-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zafar SY. Financial Toxicity of Cancer Care: It’s Time to Intervene. J Natl Cancer Inst 2016;108 10.1093/jnci/djv370 [DOI] [PubMed] [Google Scholar]
- 12. Buist S. New cancer drugs cost more than $10,000 each month: Hamilt Spect. 2016. https://www.thespec.com/news-story/6367581-new-cancer-drugs-cost-more-than-10-000-each-month/ (cited 27 Aug 2017).
- 13. Andrews A. Treating with Checkpoint Inhibitors-Figure $1 Million per Patient. Am Health Drug Benefits 2015;8:9. [PMC free article] [PubMed] [Google Scholar]
- 14. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 15. Kim S, Kim MY, Koh J, et al. Programmed death-1 ligand 1 and 2 are highly expressed in pleomorphic carcinomas of the lung: Comparison of sarcomatous and carcinomatous areas. Eur J Cancer 2015;51:2698–707. 10.1016/j.ejca.2015.08.013 [DOI] [PubMed] [Google Scholar]
- 16. Koh J, Go H, Keam B, et al. Clinicopathologic analysis of programmed cell death-1 and programmed cell death-ligand 1 and 2 expressions in pulmonary adenocarcinoma: comparison with histology and driver oncogenic alteration status. Mod Pathol 2015;28:1154–66. 10.1038/modpathol.2015.63 [DOI] [PubMed] [Google Scholar]
- 17. Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167–75. 10.1200/JCO.2009.26.7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2004–12. 10.1200/JCO.2014.58.3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Agrawal S, Feng Y, Roy A, et al. Nivolumab dose selection: challenges, opportunities, and lessons learned for cancer immunotherapy. J Immunother Cancer 2016;4:72 10.1186/s40425-016-0177-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao X, Suryawanshi S, Hruska M, et al. Assessment of nivolumab benefit-risk profile of a 240-mg flat dose relative to a 3-mg/kg dosing regimen in patients with advanced tumors. Ann Oncol 2017;28:2002–8. 10.1093/annonc/mdx235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018–28. 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 22. Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908–18. 10.1016/S1470-2045(15)00083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bach PB. New Math on Drug Cost-Effectiveness. N Engl J Med 2015;373:1797–9. 10.1056/NEJMp1512750 [DOI] [PubMed] [Google Scholar]
- 24. Dusetzina SB, Winn AN, Abel GA, et al. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol 2014;32:306–11. 10.1200/JCO.2013.52.9123 [DOI] [PubMed] [Google Scholar]