Abstract
Background
Lung cancer is the most incident and lethal form of cancer, with late diagnosis as a major determinant of its bad prognosis. Immunotherapies targeting immune checkpoints improve survival, but positive results encompass only 30%–40% of the patients, possibly due to alternative pathways to immunosuppression, including tumour-associated macrophages (TAM). Colony stimulating factor-1 (CSF-1) is implicated in TAM differentiation and recruitment to tumours and in tumour angiogenesis, through a special setting of Tie-2-expressing macrophages, which respond to angiopoietin-2 (Ang-2). We evaluated the role of serum levels of CSF-1 in non-small cell lung cancer (NSCLC) prognosis and whether these could serve as biomarkers for NSCLC detection, along with Ang-2.
Participants and methods
We prospectively studied an unselected cohort of 145 patients with NSCLC and a group of 30 control individuals. Serum levels of Ang-2 and CSF-1 were measured by ELISA prior to treatment.
Results
Serum levels of CSF-1 and Ang-2 are positively correlated (p<0.000001). Individuals with high serum levels of CSF-1 have a 17-fold risk for NSCLC presence and patients with combined High Ang-2/CSF-1 serum levels present a 5-fold increased risk of having NSCLC. High Ang-2/CSF-1 phenotype is also associated with worst prognosis in NSCLC.
Conclusions
Combined expression of CSF-1 and Ang-2 seems to contribute to worst prognosis in NSCLC and it is worthy to understand the basis of this unexplored partnership. Moreover, we think CSF-1 could be included as a biomarker in NSCLC screening protocols that can improve the positive predictive value of the current screening modalities, increase overall cost effectiveness and potentially improve lung cancer survival.
Keywords: non-small cell lung cancer, ANG-2, CSF-1
Key questions.
What is already known about this subject?
CSF-1 has already been studied as a potential target of directed therapies to improve response to anti-angiogenic therapies and immunotherapies in cancer, but this is the first study to evaluate the correlation between serum levels of CSF-1 and Ang-2 in lung cancer patients.
What does this study add?
This study shows that elevated CSF-1 levels are correlated with an increased probability of the presence of lung cancer.
It also shows that when serum Ang-2 and CSF-1 are simultaneously elevated, the prognosis of NSCLC is worst than in patients with low levels of the two molecules combined.
How might this impact on clinical practice?
The results presented in this study may open the door to a new vision of lung cancer screening, with the design of protocols that may predict with higher accuracy which group of individuals should be studied with more attention.
Moreover, it may also shed some light into the mechanisms of non-responders to immunotherapy and CSF-1 serum levels could be measured in patients recruited to future clinical to evaluate whether or not they are influencing the results observed.
Introduction
Lung cancer is the most common incident form of cancer worldwide, with an estimated 1.8 million new cases in 2012, representing 12.9% of all new cancer cases. The number of lung cancer related deaths exceed those from any other type of malignancy, accounting for nearly one in five deaths (1.6 million deaths in total).1 Approximately 85% of those cases are currently classified as non-small cell lung cancers (NSCLCs), with two predominant histological phenotypes, adenocarcinoma (ADC; ~50%) and squamous cell carcinoma (SCC; ~40%).2
Unfortunately, no screening programme is proven consistent for early stage NSCLC detection, when curative surgery is still an option,3 and most patients with NSCLC presents at an advanced stage of disease, when curative treatment is no longer a possibility, resulting in a dismal prognosis and low overall survival (OS).4 5 In the last decade, however, NSCLC treatment has evolved in an overwhelming fashion, mainly due to targeted approaches, which have led to great improvement in outcomes for this disease.6 7 Undoubtedly, new agents targeting immune checkpoints (immune checkpoint inhibitors), namely antibodies that block the programmed death-1 receptor (PD-1) and its ligand 1 (PD-L1) pathway, are among the most powerful therapeutic strategies approved in recent years to treat advanced NSCLC.8 Despite its great impact in lung cancer treatment, with durable responses now observed in patients with previously untreatable tumors,9 objective response rates are somewhat disappointing and complete responses are still rare.6 This limited clinical success can be explained by the fact that inhibition of T cell functions by PD-1 overexpression is just one of the various mechanisms that tumours use to supress the host immune responses and evade elimination by the immune system.6 10 Tumour-associated macrophages (TAM), a major component of the stroma of solid tumours (and the most abundant cell population in many cases), deserve careful attention as main players in this context, owing to its capacity to suppress T-cell responses, inducing regulatory T cells and eliciting immune tolerance.11–13 The recognised importance of TAM in tumour microenvironment boosted research in the immunotherapy field, aiming for strategies to halt TAM recruitment, differentiation and functions.14 TAM derive from circulating monocytes, which are recruited from the bloodstream into tumours and, as they extravasate across the tumour vasculature, begin to differentiate into fully mature macrophages depending on microenvironment stimuli.13 15 Colony stimulating factor-1 (CSF-1), also known as macrophage colony-stimulating factor (M-CSF), is the chemokine that stands out most, for its principal role in TAM biology.9 14 It is a key regulator for monocyte activation, migration and its influx to tumours16–18 and it is the master signal that enhances macrophages survival and polarisation, driving TAM differentiation towards an immunosuppressive, tumour-promoting phenotype.13 15
Apart from promoting tumour escape from immune surveillance, CSF-1-educated TAM have a central role in promoting angiogenesis.11–14 19 A very elegant study conducted by Forget and coworkers showed that CSF-1 is also involved in the differentiation of a subpopulation of macrophages which express endothelial cell tyrosine kinase receptor (Tie-2) (TEMs),20 that directly respond to angiopoietin-2 (Ang-2) and are committed to intrinsic proangiogenic activity.21 22 They established a novel role for CSF-1 in regulating Tie2 receptor expression on macrophages in vitro and in vivo in a model of murine breast cancer and that these cells are more responsive to Ang-2 stimulation on CSF-1 exposure, resulting in increased cell migration and proangiogenic potential.20
Several studies have demonstrated that solid tumours, including lung cancer cells, overexpress CSF-1 and that these correlate with great TAM infiltrate and poorer prognosis.23 The aim of our study was to evaluate the role of serum levels of CSF-1 in NSCLC prognosis and whether these could serve as biomarkers for NSCLC detection, using the same cohort of patients. We previously showed that Ang-2 serum levels are associated with NSCLC prognosis and with the probability of individuals presenting NSCLC.24 Attending to the established role of CSF-1 in TEM differentiation and of Ang-2 in its recruitment to tumours, we also wondered if Ang-2 and CSF-1 might have linked serum expression in patients with NSCLC, since there is no information on this probable partnership in literature, and which, if any, influences it might have in the outcome of this disease. To the best of our knowledge, this is the first study to focus on the simultaneous expression of CSF-1 and Ang-2 in NSCLC.
Methods
Participants
We prospectively recruited a group of 145 Caucasian individuals, admitted to the Portuguese Institute of Oncology of Porto (IPO-Porto), Portugal, between 2006 and 2009, with cytological or histological newly diagnosed and untreated NSCLC, with an Eastern Cooperative Oncology Group (ECOG) performance status ≤2, no prior or concurrent oncological disease and available clinical data (median age 64.0 years and mean age of 63.8 years, SD 10.3). The median follow-up time was 22 months (range 1–63 months).
The studied patients included 33 women (22.8%) and 112 men (77.2%), with 105 (72.4%) smokers/former smokers, 36 (24.8%) non-smokers and 4 (2.8%) not stated.
Patients were evaluated with the TMN staging System and by the time of diagnosis, its distribution according to stage of was as follows: six presented with stage I disease (4.1%), three with stage II (2.1%), 65 with stage III (44.8%) and 71 with stage IV (49.0%). Histologically, we registered 72 ADCs (49.6%), 51 SCCs (35.2%), 19 not other specified NSCLC (13.1), 2 large cells carcinomas (1.4%) and 1 mixed carcinoma (0.7%).
The patients included in this study were treated according to the guidelines at the time of the study, namely in first line with a platin-based doublet chemotherapy in combination with a third-generation cytotoxic compound such as paclitaxel, gemcitabine or pemetrexed. In second and posterior lines, they performed docetaxel, pemetrexed or erlotinib.
The control group consisted of 30 healthy individuals, without clinical history of malign, inflammatory and connective tissue diseases, recruited from the blood donor bank of the Instituto Português de Oncologia–Porto, from the same geographical area as the case group. The gender distribution in this group was six female (20.0%) and 24 male (80.0%) individuals (median age 60.0 years; mean age 58.7 years; SD 4.63).
The study was conducted according to the principles of the Helsinki Declaration and was approved by the local ethics committee at the Instituto Português de Oncologia—Porto (Portugal). A signed written informed consent was obtained for all study participants prior to their inclusion in the study.
Sample collection and serum determination of CSF-1 and Ang-2
A 3 mL sample of venous blood was collected into serum separator tubes from each study participant before any treatment, allowed to coagulate at room temperature for 1 hour and then centrifuged before serum collection. Serums aliquots were stored at −20°C until analyses were carried on.
The measurement of CSF-1 serum concentration was performed through ELISA using the Human M-CSF Quantikine ELISA kit, purchased from R&D Systems (catalogue #DMC00B) and used the values of serum Ang-2 levels previously determined in this study population,24 using the Human Angiopoietin-2 Quantikine ELISA kit, also from R&D Systems (catalogue #DANG20), according to manufacturer’s protocols. We performed the specimens’ assays in duplicate, using the average of the two measurements to data analysis. The minimum detectable levels of Ang-2 and CSF-1 were 1.2 pg/mL and 1.3 pg/mL, respectively.
Statistical analysis
Data analysis was performed using the Statistical Package for Social Science (SPSS) for Windows V.18 (Chicago, Illinois, USA). The level of statistical significance was set at 5% (p≤0.05).
Spearman’s correlation coefficient test was used to analyse associations between CSF-1 and Ang-2 variables. Serum CSF-1 and ang-2 levels were considered as categorical variables using as cut-off points the median of serum levels of CSF-1 and Ang-2 determined in the 30 healthy volunteers of the control group.
To compare categorical variables, we used χ² analysis with a 5% level of significance. We calculated the OR and its 95% CI as a measurement of the association between the amount of the studied molecules and the probability of lung cancer presence. Logistic regression analysis was used to calculate the adjusted OR (aOR) and 95% CI, with adjustment for age, gender and smoking status.
OS was assessed by the Kaplan-Meyer method and compared with a two-sided log-rank test. Multivariate Cox proportional analysis was performed to evaluate Ang-2 and CSF-1 serum levels and NSCLC survival, adjusting by tumour stage, age, gender, histological type and smoking status. HRs estimated from the Cox analysis were reported as relative risks with corresponding 95% CIs. A difference at a p≤0.05 was considered significant.
Results
Serum Ang-2 and CSF-1 in patients with NSCLC and healthy control individuals
Both CSF-1 and Ang-2 showed great differences in median serum levels between controls and lung cancer cases. These differences are illustrated in figures 1 and 2, respectively. Median serum CSF-1 level was more than 3-fold higher in patients with NSCLC, 310.0 pg/mL (values ranged from 7.0 pg/mL to 3600.0 pg/mL) than in the control group, 74.0 pg/mL (ranging from 1.5 pg/mL to 311.0 pg/mL). The median serum level of Ang-2 was already determined in our previous study24 (3432.5 pg/mL in patients with NSCLC and 2710 pg/mL in the control group). In all patients with lung cancer, serum Ang-2 was significantly correlated with serum CSF-1 with a Spearman’s correlation coefficient of r=0.410 (p<0.000001).
Figure 1.
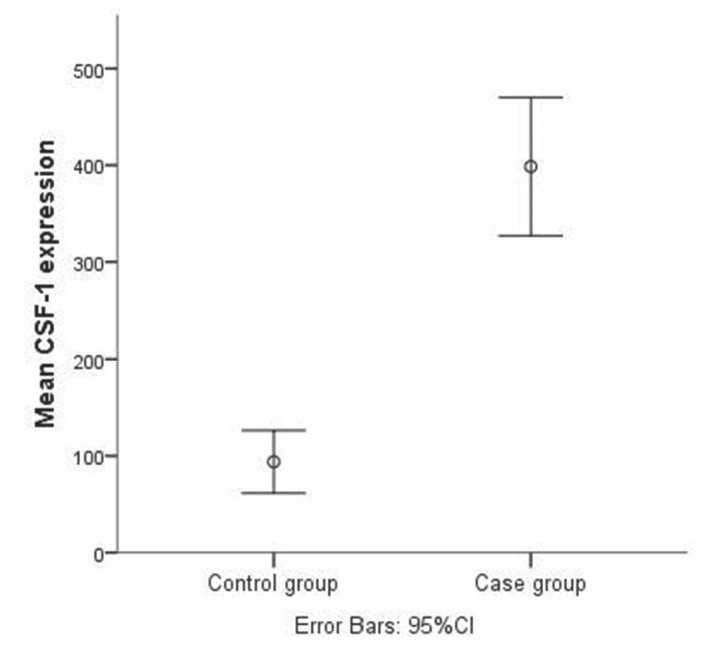
Mean levels of CSF-1 in control and case groups. CSF-1, colony stimulating factor-1.
Figure 2.
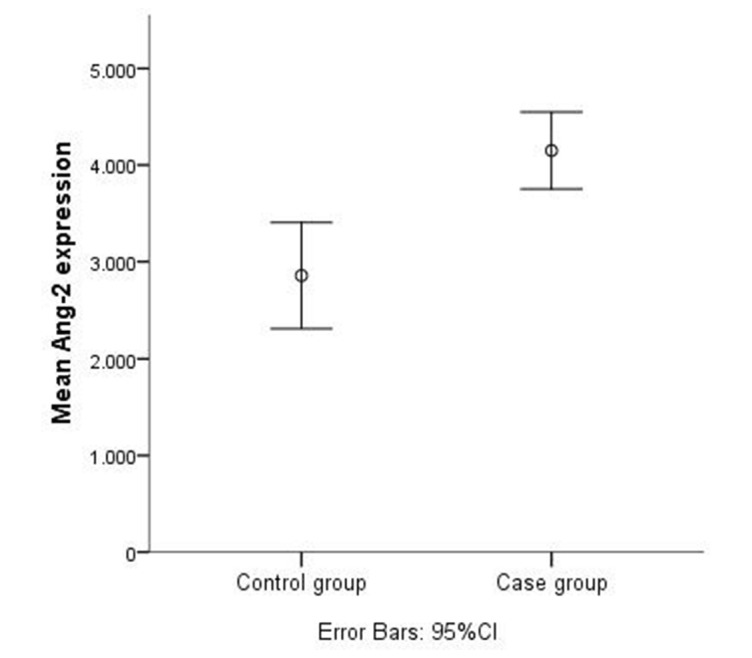
Mean levels of Ang-2 in control and case groups. Ang-2, angiopoietin-2.
Serum Ang-2 and CSF-1 levels and susceptibility for NSCLC
We had previously showed that, in this study population, individuals with high serum levels of Ang-2 had higher probability of developing NSCLC than individuals with low serum levels.24 Therefore, we performed the same study regarding CSF-1 serum levels and calculated the OR with its 95% CI to determine whether serum CSF-1 expression levels are associated with NSCLC development. To perform this calculation, we divided our samples, using the median value of the healthy control group as a cut-off point (74 pg/mL for CSF-1), defining High and Low serum level subgroups (as we previously did with Ang-2). Therefore, we defined a High CSF-1 group (High CSF-1>74 pg/mL) and a low serum level group (Low CSF-1≤74 pg/mL). The results showed that the probability of presenting NSCLC in individuals with High CSF-1 serum levels is 17-fold higher in comparison with individuals with Low CSF-1 serum levels (OR=17.13; 95% CI 6.24 to 47.0; p<0.000001).
Moreover, since we observed that serum Ang-2 expression levels were significantly correlated with serum CSF-1 expression levels, we grouped our High Ang-2 and High CSF-1 samples (>2710 pg/mL and>74 pg/mL, respectively), in a different setting, which we termed HighAng-2/CSF-1 and compared it with all the other samples regarding the probability of presenting NSCLC. We observed that individuals within the HighAng-2/CSF-1 group present a 5-fold increased risk of presenting NSCLC (OR=5.19; 95% CI 2.20 to 12.2, p<0.0001). Multivariate logistic regression analysis shows that this risk remains statistically significant for the three groups defined, regardless of age, gender and smoking status (table 1).
Table 1.
Multivariate logistic regression analysis of serum high expression of Ang-2, CSF-1 and both combined regarding the susceptibility to develop NSCLC
| aOR | 95% CI | P values* | |
| High Ang-2 | 2.59 | 1.13 to 5.93 | 0.025 |
| High CSF-1 | 18.23 | 6.20 to 53.54 | <0.000001 |
| High Ang-2 and CSF-1 (n=109) | 5.12 | 2.12 to 12.35 | 0.0003 |
*aOR, 95% CI and p value using logistic regression analysis, adjusted by age, smoking status and gender.
Ang-2, angiopoietin-2; aOR, adjusted OR; CSF-1, colony stimulating factor-1; NSCLC, non-small cell lung cancer.
NSCLC survival analysis attending to serum Ang-2 and CSF-1 levels
We had previously shown that Ang-2 serum levels were independent prognostic factors in this group of patients with NSCLC.24 Our results demonstrated that patients with High Ang-2 serum levels have diminished OS when compared with those with Low Ang-2 (21.0 months vs 42.6 months, respectively; log-rank test, p=0.001).
To evaluate the role of serum CSF-1 expression levels and combined high expression of Ang-2 and CSF-1 in OS of patients with NSCLC, we used the same subgroups of the above analysis (High CSF-1 and Low CSF-1 serum expression levels) as well as the HighAng-2/CSF-1 setting, plotted against all the other samples (figures 3 and 4).
Figure 3.
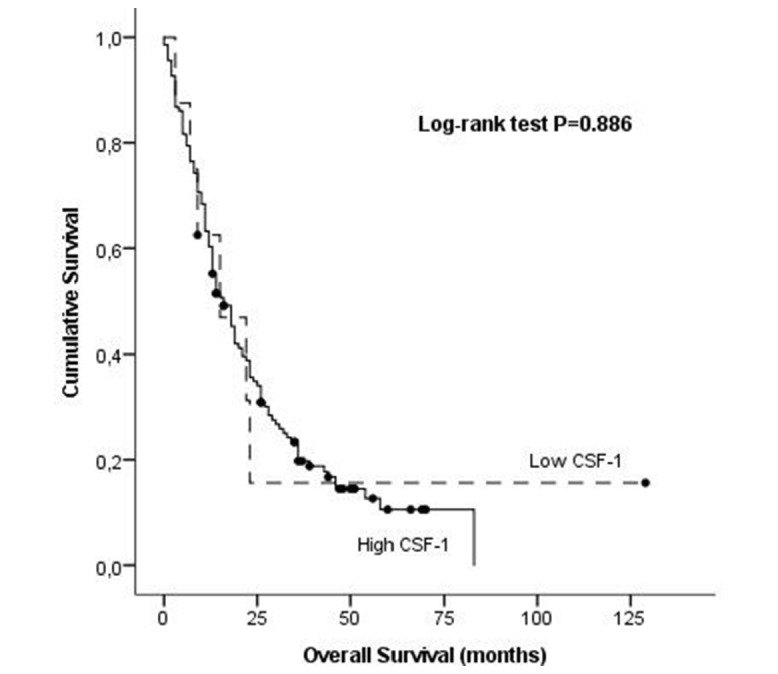
Association of serum levels of CSF-1 overall survival in NSCLC by Kaplan-Meier curves. CSF-1, colony stimulating factor-1; NSCLC, non-small cell lung cancer.
Figure 4.
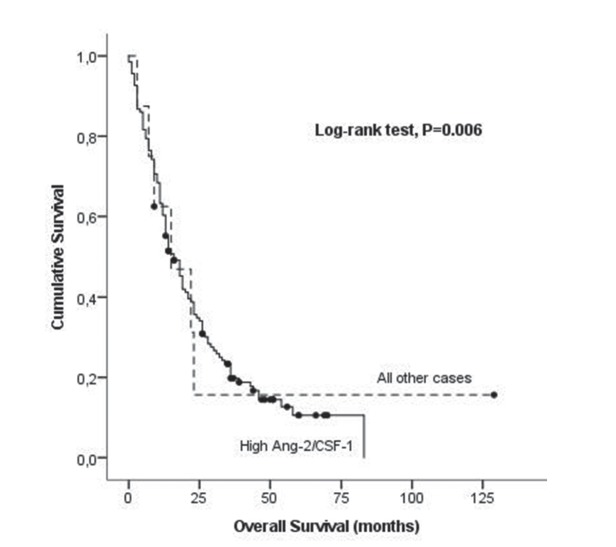
Association of combined serum levels of CSF-1 and Ang-2 with overall survival in NSCLC by Kaplan-Meier curves. Ang-2, angiopoietin-2; CSF-1, colony stimulating factor-1; NSCLC, non-small cell lung cancer.
There is no statistically significant association between CSF-1 High and Low serum expression levels and OS in our group of patients with NSCLC (24.8 months vs 31.9 months, respectively; log-rank test, p=0.886) (figure 3). When comparing the setting of HighAng-2/CSF-1 phenotype against all other samples, we observed a marginal, although significant, decrease in OS in both groups, (19.4 months vs 39.2 months, respectively; log-rank test, p=0.006), (figure 4) suggesting that when both Ang-2 and CSF-1 are elevated in serum, the effect in OS of patients with NSCLC is more pronounced than the elevation of each marker by itself.
To determine the independent prognostic value in OS of serum Ang-2 and CSF-1 expression levels, using the above described approach, a multivariate analysis with Cox proportional hazard model was performed. In the multivariate analysis that included tumour stage, age, gender, histological type, smoking status and serum Ang-2, CSF-1 and the combination of both, we identified tumour stage and High Ang-2 and the HighAng-2/ CSF-1 as independent determinants of poor outcome in patients with NSCLC (table 2).
Table 2.
Multivariate Cox regression model adjusted for predictable determinants of poor outcome in NSCLC
| aHR | 95% CI | P values* | |
| High Ang-2 | 1.88 | 1.23 to 2.88 | 0.003 |
| High CSF-1 | 0.879 | 0.330 to 2.34 | 0.797 |
| HighAng-2/CSF-1 | 1.61 | 1.06 to 2.42 | 0.026 |
*P value, aOR and 95% CI using Cox regression analysis, adjusted by tumour stage, age, gender, histological type and smoking status.
aHR, adjusted Harzard Ratio; Ang-2, angiopoietin-2; CSF-1, colony stimulating factor-1; NSCLC, non-small cell lung cancer.
Discussion
Infiltrating myeloid cells, specially from the monocyte/macrophage lineage, are potent regulators of tumour-associated immune suppression, cell invasion and metastases, and targeting of these innate immune cells may be the key to developing new immunotherapies, aiming the reactivation of T cells and to diminish angiogenesis.9 CSF-1 is found at biologically active concentrations in the circulation and regulates the survival, proliferation and differentiation of mononuclear phagocytes from undifferentiated precursors to fully differentiated, non-dividing macrophages.25 It is also a potent regulator of macrophage motility, acting as a chemokine that recruits TAM to tumour microenvironment.26 TAM are by now established as important regulators of tumour progression by impacting on tumour immunity, angiogenesis and metastasis.13 27–29 Kubota and coworkers described a role for CSF-1 in pathological angiogenesis in mice with osteosarcoma30 and recent studies suggest that CSF-1 plays a key role in the formation of high-density vessel networks and acts as an ‘angiogenic switch’ in a mouse model of mammary tumors.20 Our results seem to be in line with these findings, because we found a significant positive correlation between Ang-2 and CSF-1 serum levels in patients with NSCLC, meaning that when circulating levels of CSF-1 are high, so are the Ang-2 levels, suggesting a combined involvement of these molecules in NSCLC angiogenesis, through a mechanism no yet fully elucidated, that we can hypothesise that involves the Tie-2 expressing monocytes/macrophages differentiation and recruitment. Although some authors have described CSF-1 serum levels as a marker of bad prognosis in NSCLC,31 32 our study does not confirm these results. This might be explained by the different methods used to calculate cut-off points to define high and low levels of the cytokine as well as different settings of patients. However, when evaluating the joint influence of HighAng-2/CSF-1 phenotype in NSCLC prognosis, we verified that there is a slight, yet significant, decline in OS in patients with NSCLC, when compared with our previous findings regarding OS associated with Ang-2 high levels alone.24 Clinical and experimental data have established that targeting CSF-1/CSF-1R axis along with an antiangiogenic strategy results in greater inhibition of tumour angiogenesis than antiangiogenic therapy alone,33 and in view of these findings, we can hypothesise that the Ang-2/CSF-1 partnership is somehow fuelling angiogenesis and the progression of the growing tumour, an idea that should be exploited in future functional experiments.
Given the high prevalence of NSCLC and low survival rates, efforts have been focused in the development of more efficacious targeted therapies and on developing screening protocols for the high-risk population likely to develop NSCLC. Early detection of lung cancer is the best opportunity for decreasing lung cancer mortality, since NSCLC stage remains the most important prognostic factor of recurrence rates and survival times.34 Technologies such as low-dose computed tomography (LDCT) scans have low specificity, only 61%, when screening for lung cancer and for approximately each true positive scan there are 19 false positive scans.35 This constitutes a problem in the generalisation of lung cancer LDCT screening for clinical, psychological and economic reasons, due to exposure to X-rays, unnecessary anxiety induced by a false positive result and fear in the screened individual and his family along with costly unnecessary follow-up interventions, including invasive biopsies or surgical resection.36
We had previously showed that individuals with isolated high Ang-2 serum levels have higher probability of presenting NSCLC than individuals with low serum levels of Ang-2.24 Our current results show that median CSF-1 levels were dramatically higher in patients with NSCLC when compared with healthy individuals and that individuals with high expression of CSF-1 have a 17-fold increased likelihood of presenting NSCLC. Moreover, we also found that the combination of High Ang-2 and High CSF-1 serum levels is associated with a 5-fold higher probability of NSCLC presence. The conclusions are valid regardless of age, gender and smoking status. Our results suggest that individuals with high serum levels of Ang-2 and CSF-1 (alone or in combination) can be useful as easy-to-obtain molecular biomarkers of lung cancer presence that could help to define with more accuracy a subset of individuals at higher risk of having NSCLC. Specifically, these molecules may be targets for further assay refinement, alone or in combination with other non-imaging screening targets under development, including some serum marker panels already proposed by other authors.3 Furthermore, examination in future prospective trials with a larger sample group is necessary to determine if serial serum testing of CSF-1 and Ang-2 can improve the positive predictive value of the current screening modalities, increase overall cost effectiveness and potentially improve lung cancer survival.
Acknowledgments
The authors would like to thank the Liga Portuguesa Contra o Cancro—Centro Regional do Norte (Portuguese League Against Cancer) for the educational grants conceded to Ana Coelho and Mónica Gomes.
Footnotes
Contributors: ALC, AMA and MPG conceived and designed the experiments. ALC and MPG performed the experiments. ALC, RJC and RMM performed the statistical analyses of the data. ALC and RMM coordinated the study. ALC, AMA and RJC participated in the draft of the manuscript. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The study was approved by the local ethics committee at the Instituto Português de Oncologia—Porto (Portugal) and the national research committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2. Chen Z, Fillmore CM, Hammerman PS, et al. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer 2014;14:535–46. 10.1038/nrc3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blanco-Prieto S, De Chiara L, Rodríguez-Girondo M, et al. Highly Sensitive Marker Panel for Guidance in Lung Cancer Rapid Diagnostic Units. Sci Rep 2017;7:41151 10.1038/srep41151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davidson MR, Gazdar AF, Clarke BE. The pivotal role of pathology in the management of lung cancer. J Thorac Dis 2013;5(Suppl 5):S463–78. 10.3978/j.issn.2072-1439.2013.08.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw 2013;11:645–53. 10.6004/jnccn.2013.0084 [DOI] [PubMed] [Google Scholar]
- 6. Assi HI, Kamphorst AO, Moukalled NM, et al. Immune checkpoint inhibitors in advanced non-small cell lung cancer. Cancer 2018;124:248–61. 10.1002/cncr.31105 [DOI] [PubMed] [Google Scholar]
- 7. Bergsma DP, Salama JK, Singh DP, et al. Radiotherapy for Oligometastatic Lung Cancer. Front Oncol 2017;7:210 10.3389/fonc.2017.00210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rolfo C, Caglevic C, Santarpia M, et al. Immunotherapy in NSCLC: A Promising and Revolutionary Weapon. Adv Exp Med Biol 2017;995:97–125. 10.1007/978-3-319-53156-4_5 [DOI] [PubMed] [Google Scholar]
- 9. Elliott LA, Doherty GA, Sheahan K, et al. Human Tumor-Infiltrating Myeloid Cells: Phenotypic and Functional Diversity. Front Immunol 2017;8:86 10.3389/fimmu.2017.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aerts JG, Hegmans JP. Tumor-specific cytotoxic T cells are crucial for efficacy of immunomodulatory antibodies in patients with lung cancer. Cancer Res 2013;73:2381–8. 10.1158/0008-5472.CAN-12-3932 [DOI] [PubMed] [Google Scholar]
- 11. Allavena P, Mantovani A. Immunology in the clinic review series; focus on cancer: tumour-associated macrophages: undisputed stars of the inflammatory tumour microenvironment. Clin Exp Immunol 2012;167:195–205. 10.1111/j.1365-2249.2011.04515.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chanmee T, Ontong P, Konno K, et al. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers 2014;6:1670–90. 10.3390/cancers6031670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res 2006;66:605–12. 10.1158/0008-5472.CAN-05-4005 [DOI] [PubMed] [Google Scholar]
- 14. Mantovani A, Marchesi F, Malesci A, et al. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 2017;14:399–416. 10.1038/nrclinonc.2016.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lewis CE, De Palma M, Naldini L. Tie2-expressing monocytes and tumor angiogenesis: regulation by hypoxia and angiopoietin-2. Cancer Res 2007;67:8429–32. 10.1158/0008-5472.CAN-07-1684 [DOI] [PubMed] [Google Scholar]
- 16. Ugel S, De Sanctis F, Mandruzzato S, et al. Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages. J Clin Invest 2015;125:3365–76. 10.1172/JCI80006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Geissmann F, Manz MG, Jung S, et al. Development of monocytes, macrophages, and dendritic cells. Science 2010;327:656–61. 10.1126/science.1178331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Overmeire E, Stijlemans B, Heymann F, et al. M-CSF and GM-CSF Receptor Signaling Differentially Regulate Monocyte Maturation and Macrophage Polarization in the Tumor Microenvironment. Cancer Res 2016;76:35–42. 10.1158/0008-5472.CAN-15-0869 [DOI] [PubMed] [Google Scholar]
- 19. Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol 2002;196:254–65. 10.1002/path.1027 [DOI] [PubMed] [Google Scholar]
- 20. Forget MA, Voorhees JL, Cole SL, et al. Macrophage colony-stimulating factor augments Tie2-expressing monocyte differentiation, angiogenic function, and recruitment in a mouse model of breast cancer. PLoS One 2014;9:e98623 10.1371/journal.pone.0098623 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21. De Palma M, Venneri MA, Galli R, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 2005;8:211–26. 10.1016/j.ccr.2005.08.002 [DOI] [PubMed] [Google Scholar]
- 22. Murdoch C, Tazzyman S, Webster S, et al. Expression of Tie-2 by human monocytes and their responses to angiopoietin-2. J Immunol 2007;178:7405–11. 10.4049/jimmunol.178.11.7405 [DOI] [PubMed] [Google Scholar]
- 23. Achkova D, Maher J. Role of the colony-stimulating factor (CSF)/CSF-1 receptor axis in cancer. Biochem Soc Trans 2016;44:333–41. 10.1042/BST20150245 [DOI] [PubMed] [Google Scholar]
- 24. Coelho AL, Araújo AM, Gomes MP, et al. Combined Ang-2 and VEGF serum levels: holding hands as a new integral biomarker in non-small-cell lung cancers. Future Oncol 2015;11:3233–42. 10.2217/fon.15.207 [DOI] [PubMed] [Google Scholar]
- 25. Stanley ER, Berg KL, Einstein DB, et al. Biology and action of colony--stimulating factor-1. Mol Reprod Dev 1997;46:4–10. [DOI] [PubMed] [Google Scholar]
- 26. Dwyer AR, Greenland EL, Pixley FJ. Promotion of Tumor Invasion by Tumor-Associated Macrophages: The Role of CSF-1-Activated Phosphatidylinositol 3 Kinase and Src Family Kinase Motility Signaling. Cancers 2017;9:68 10.3390/cancers9060068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell 2013;23:277–86. 10.1016/j.ccr.2013.02.013 [DOI] [PubMed] [Google Scholar]
- 28. Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010;141:39–51. 10.1016/j.cell.2010.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol 2012;33:119–26. 10.1016/j.it.2011.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kubota Y, Takubo K, Shimizu T, et al. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med 2009;206:1089–102. 10.1084/jem.20081605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaminska J, Kowalska M, Kotowicz B, et al. Pretreatment serum levels of cytokines and cytokine receptors in patients with non-small cell lung cancer, and correlations with clinicopathological features and prognosis. M-CSF - an independent prognostic factor. Oncology 2006;70:115–25. 10.1159/000093002 [DOI] [PubMed] [Google Scholar]
- 32. Katsumata N, Eguchi K, Fukuda M, et al. Serum levels of cytokines in patients with untreated primary lung cancer. Clin Cancer Res 1996;2:553–9. [PubMed] [Google Scholar]
- 33. Priceman SJ, Sung JL, Shaposhnik Z, et al. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood 2010;115:1461–71. 10.1182/blood-2009-08-237412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Woodard GA, Jones KD, Jablons DM, et al. Lung Cancer Staging and Prognosis. Cancer Treat Res 2016;170:47–75. 10.1007/978-3-319-40389-2_3 [DOI] [PubMed] [Google Scholar]
- 35. Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 2012;307:2418–29. 10.1001/jama.2012.5521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gyoba J, Shan S, Roa W, et al. Diagnosing Lung Cancers through Examination of Micro-RNA Biomarkers in Blood, Plasma, Serum and Sputum: A Review and Summary of Current Literature. Int J Mol Sci 2016;17:494 10.3390/ijms17040494 [DOI] [PMC free article] [PubMed] [Google Scholar]