Abstract
Background
Oesophageal cancer (OC) survival rates have improved since the widespread adoption of neoadjuvant chemoradiation therapy (NACRT) followed by oesophagectomy (trimodality therapy). Unfortunately, the overall prognosis for patients with locally advanced disease remains poor. In this study, we sought to assess the effect of adjuvant chemotherapy (AC) in patients treated with trimodality therapy.
Methods
Using the National Cancer Database we retrospectively identified 6785 patients with locally advanced (cT1b-T4a, N0-N+, M0) OC who were treated with trimodality therapy from 2006 to 2014. Patients were separated based on receipt of AC (n=463), as well as clinical and pathological lymph node involvement. Overall survival (OS) between groups was compared using the Kaplan-Meier method and Cox proportional hazard modelling.
Results
Based on multivariate analysis, AC was associated with a statistically significantly reduced risk of death (HR 0.77, p<0.001). Subgroup analysis revealed that AC was associated with reduced risk of death compared with NACRT alone in the cN+/pN0 (median OS 64 vs 43 months; p=0.019) and the cN+/pN+ (median OS 27 vs 22 months; p=0.010) groups, but not in the cN0/pN0 (median OS 48 vs 49 months; p=0.253) or cN0/pN+ (median OS 31 vs 24 months; p=0.077) groups.
Conclusion
AC following trimodality therapy may improve survival in patients with locally advanced OC. Patients who undergo lymph node downstaging may be the most likely to benefit from AC. Prospective studies are needed to confirm this finding.
Keywords: esophageal cancer, trimodality therapy, adjuvant chemotherapy, downstaging, national cancer database
Key questions.
What is already known about this subject?
Retrospective analyses have suggested that adjuvant chemotherapy may improve survival in locally advanced oesophageal cancer following trimodality therapy.
Currently, published data are inconsistent with regard to which patients may be most likely to benefit from adjuvant chemotherapy, specifically patients with node-negative disease following oesophagectomy.
What does this study add?
Our retrospective analysis of the National Cancer Database suggests that patients with clinically positive nodes who are pathologically node-negative following surgery may be the most likely to benefit from adjuvant chemotherapy.
How might this impact on clinical practice?
An assessment of both clinical and pathological lymph node status may help determine the likelihood that a patient will benefit from adjuvant chemotherapy in locally advanced oesophageal cancer and should be considered in future clinical trials.
Introduction
In 2018, there will be an estimated 17 290 new cases of oesophageal cancer (OC) diagnosed in the USA. While this number represents only 1% of all newly diagnosed cancers in the USA, OC will account for >2.5% of all cancer-related deaths, with 5-year overall survival (OS) <20%.1 The poor OS is partially attributed to a large proportion of patients who have either locally advanced or metastatic disease at the time of diagnosis.
With regard to those who present with locally advanced disease, numerous trials have compared various combinations of surgical resection, radiation therapy and chemotherapy.2 3 While the conclusions of these studies are not all in agreement, neoadjuvant chemoradiotherapy (NACRT) followed by oesophagectomy (trimodality therapy), as validated in the 2012 phase III ChemoRadiotherapy for Oesophageal cancer followed by Surgery Study (CROSS) trial,4 5 has been widely adopted as the standard of care in Western countries.6
The adoption of trimodality therapy has appeared to improve survival rates over the past decade.7 However, the overall prognosis for patients with locally advanced OC remains poor. Disease recurrence is common in patients with positive lymph nodes at the time of resection and is possible even in the setting of a pathological complete response to chemoradiation therapy.8 9 This has led to increased interest in the role of postoperative therapies.
In this study, we used a large multicentre database to evaluate the effect of adjuvant chemotherapy (AC) on survival in patients with locally advanced OC who are initially treated with trimodality therapy. We also performed a subgroup analysis based on a patient’s nodal response to NACRT in an attempt to further identify which patient populations may benefit most from AC. We hypothesised that patients who are downstaged by nodal status after NACRT may have more chemotherapy-sensitive disease and thus may be more likely to benefit from AC.
Methods
Data source
Data were obtained by retrospectively reviewing the 2014 OC participant user file provided by the National Cancer Database (NCDB). The NCDB is a joint project of the American Cancer Society and the Commission on Cancer of the American College of Surgeons. Over 1500 Commission-accredited cancer programmes submit reports to the NCDB, which include data on approximately 70% of all new cases of cancer diagnosed in the USA each year.
Study cohort
Using the NCDB, we identified all patients diagnosed with locally advanced (cT1b-T4a, N0-N+, M0) OC who underwent NACRT followed by oesophagectomy from 2006 to 2014. Patients diagnosed before 2006 were excluded as this was the first year that the NCDB included data on the sequence of systemic therapy, thus allowing identification of patients who received both neoadjuvant and adjuvant therapy. Only patients with adenocarcinoma or squamous cell carcinoma histology were included. In an attempt to exclude patients who received non-curative intent therapy, we included patients who received multiagent chemotherapy and neoadjuvant radiation therapy between 40 and 60 Gray (Gy) given in 1.8 or 2.0 Gy fractions delivered to the neck, chest, oesophagus, stomach, abdomen, lymph nodes or unknown site. Patients with incomplete follow-up data or who died within 90 days of diagnosis were excluded. The study CONSORT (Consolidated Standards of Reporting Trials) diagram with the inclusion criteria is shown in figure 1.
Figure 1.
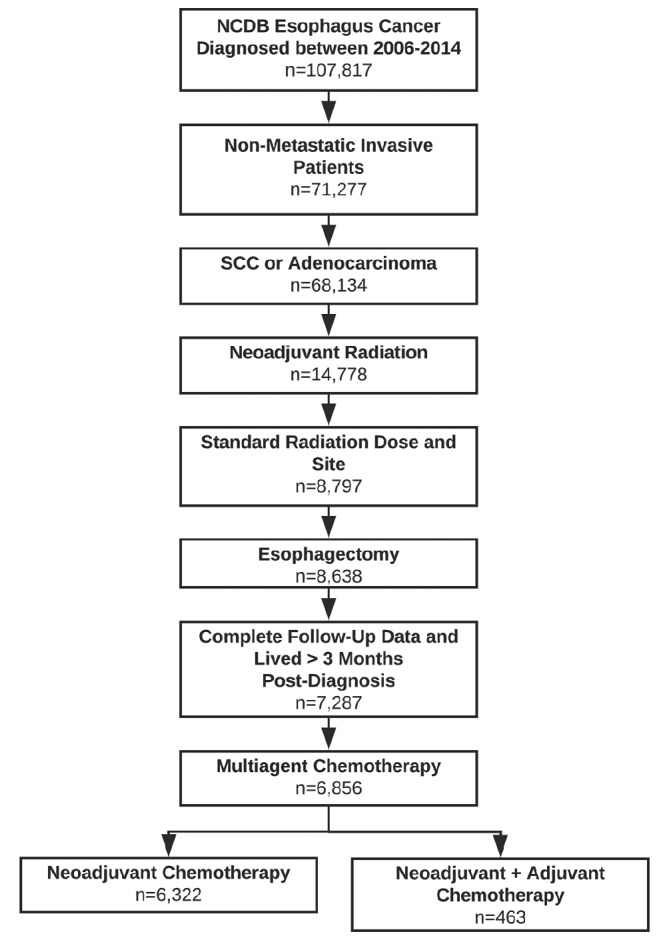
CONSORT diagram. CONSORT, Consolidated Standards of Reporting Trials; NCDB, National Cancer Database; SCC, squamous cell carcinoma.
Statistical analysis
χ2 analysis was used to compare categorical demographic and tumour characteristics between the NACRT and the neoadjuvant plus adjuvant therapy (NACRT+AC) groups. Student’s t-test was used to compare continuous variables between groups. The primary outcome of interest for all comparisons was OS. Univariable and multivariable (MVA) Cox proportional hazard modelling was used to identify factors associated with OS, reported as HRs. Multivariate models were created using a reverse stepwise approach by initially including all covariates and then removing each covariate with a p value >0.2, starting with the covariate with the largest p value. Kaplan-Meier survival analysis with log-rank testing was also employed. Statistical analysis was carried out using STATA V.14.2.
Results
Study cohort characteristics
A total of 107 817 patients diagnosed with OC were identified from 2006 to 2014. Based on our inclusion criteria, 6785 patients were included for analysis. Of these, 463 patients received NACRT+AC (figure 1). The characteristics of the patients are shown in table 1.
Table 1.
Baseline patient demographics
| NACRT | NACRT + AC | Total | P-value | ||||
| N | (%) | N | (%) | N | (%) | ||
| Age, years | |||||||
| Mean (SD) | 61.9 (9.3) | 58.5 (9.0) | 61.7 (9.3) | 0.000 | |||
| Sex | 0.001 | ||||||
| Male | 5311 | (84) | 416 | (90) | 5727 | (84) | |
| Female | 1011 | (16) | 47 | (10) | 1058 | (16) | |
| Total | 6322 | (100) | 463 | (100) | 6785 | (100) | |
| Charlson Comorbidity Score | 0.407 | ||||||
| 0 | 4690 | (74) | 353 | (76) | 5043 | (74) | |
| 1 | 1336 | (21) | 94 | (20) | 1430 | (21) | |
| ≥ 2 | 296 | (5) | 16 | (3) | 312 | (5) | |
| Total | 6322 | (100) | 463 | (100) | 6785 | (100) | |
| Histology | 0.000 | ||||||
| Adenocarcinoma | 5184 | (83) | 421 | (91) | 5605 | (83) | |
| SCC | 1068 | (17) | 40 | (9) | 1108 | (17) | |
| Total | 6252 | (100) | 461 | (100) | 6713 | (100) | |
| Grade | 0.010 | ||||||
| Well-Differentiated | 289 | (5) | 14 | (3) | 303 | (5) | |
| Moderately-Differentiated | 2376 | (44) | 159 | (38) | 2535 | (43) | |
| Poorly-Differentiated | 2695 | (49) | 238 | (57) | 2933 | (50) | |
| Undifferentiated | 94 | (2) | 4 | (1) | 98 | (2) | |
| Total | 5454 | (100) | 415 | (100) | 5869 | (100) | |
| LVSI | 0.038 | ||||||
| Negative | 2051 | (32) | 133 | (29) | 2184 | (32) | |
| Positive | 406 | (6) | 42 | (9) | 448 | (7) | |
| Unknown | 3865 | (61) | 288 | (62) | 4153 | (61) | |
| Total | 6322 | (100) | 463 | (100) | 6785 | (100) | |
| Clinical T-Stage | 0.531 | ||||||
| T0 | 5 | (0) | 0 | (0) | 5 | (0) | |
| T1 | 331 | (6) | 19 | (5) | 350 | (6) | |
| T2 | 1168 | (20) | 80 | (19) | 1248 | (20) | |
| T3 | 4077 | (71) | 311 | (74) | 4388 | (71) | |
| T4 | 170 | (3) | 9 | (2) | 179 | (3) | |
| Total | 5751 | (100) | 419 | (100) | 6170 | (100) | |
| Clinical N-Stage | 0.017 | ||||||
| N0 | 2264 | (38) | 138 | (31) | 2402 | (38) | |
| N1 | 3174 | (53) | 265 | (60) | 3439 | (54) | |
| N2 | 450 | (8) | 30 | (7) | 480 | (8) | |
| N3 | 59 | (1) | 7 | (2) | 66 | (1) | |
| Total | 5947 | (100) | 440 | (100) | 6387 | (100) | |
| Pathologic T-Stage | 0.000 | ||||||
| ypT0 | 1021 | (21) | 49 | (12) | 1070 | (20) | |
| ypT1 | 924 | (19) | 51 | (13) | 975 | (19) | |
| ypT2 | 962 | (20) | 89 | (22) | 1051 | (20) | |
| ypT3 | 1897 | (39) | 212 | (53) | 2109 | (40) | |
| ypT4 | 57 | (1) | 1 | (0) | 58 | (1) | |
| Total | 4861 | (100) | 402 | (100) | 5263 | (100) | |
| Pathologic N-Stage | 0.000 | ||||||
| ypN0 | 3309 | (65) | 123 | (30) | 3432 | (63) | |
| ypN1 | 1345 | (27) | 205 | (50) | 1550 | (28) | |
| ypN2 | 316 | (6) | 54 | (13) | 370 | (7) | |
| ypN3 | 94 | (2) | 31 | (8) | 125 | (2) | |
| Total | 5064 | (100) | 413 | (100) | 5477 | (100) | |
| Surgery Type | 0.032 | ||||||
| Partial Esophagectomy | 949 | (15) | 57 | (12) | 1006 | (15) | |
| Total Esophagectomy | 646 | (10) | 48 | (10) | 694 | (10) | |
| Esophagectomy & Laryngectomy or Gastrectomy | 4294 | (68) | 339 | (73) | 4633 | (68) | |
| Esophagectomy, NOS | 433 | (7) | 19 | (4) | 452 | (7) | |
| Total | 6322 | (100) | 463 | (100) | 6785 | (100) | |
| Margin Status | 0.001 | ||||||
| Negative Margins | 5765 | (97) | 411 | (94) | 6176 | (97) | |
| Positive Margins | 186 | (3) | 27 | (6) | 213 | (3) | |
| Total | 5951 | (100) | 438 | (100) | 6389 | (100) | |
| Radiation Dose, Gy | 0.012 | ||||||
| Median (IQR) | 50.40 (46.80 – 50.40) | 50.40 (45.00 – 50.40) | 50.40 (46.00 – 50.40) | ||||
AC, adjuvant chemotherapy; Gy, Gray; IQR, interquartile range; LVSI, lymphovascular space invasion; NACRT, neoadjuvant chemoradiotherapy; No., number; NOS, not otherwise specified; SCC, squamous cell carcinoma; SD, standard deviation.
There was no difference in clinical T-stage between those who received NACRT or NACRT+AC. However, a larger percentage of pT0-2 tumours were identified at the time of resection in the NACRT-only group (60%) compared with the NACRT+AC group (47%). Additionally, 69% of those who received NACRT+AC had clinically positive nodes compared with 62% of those who received NACRT alone (p=0.017). Following resection, 71% of the NACRT+AC group and 35% of the NACRT-alone group were pN+ (p<0.001). Overall, this suggests that lymph node downstaging after NACRT was more frequently found in the subset of patients who received NACRT alone.
Survival analyses
With a median follow-up of 25.7 months, patients in the NACRT group had a median OS of 36.5 months (95% CI 35.3 to 38.3 months), compared with the NACRT+AC group, which had a median follow-up of 28.7 months and a median OS of 35.5 months (95% CI 31.1 to 43.0 months; p=0.38) (figure 2). The 5-year OS of the NACRT group was 37.8% and for the NACRT+AC group was 36.3%. However, after accounting for the fact that the NACRT+AC group included more adenocarcinoma histology patients, more patients with a poorly differentiated grade of tumour, more patients with evidence of lymphovascular space invasion, and more advanced clinical N-stage as well as pathology T-stage and N-stage, our MVA revealed a significant association between receiving AC and reduced risk of death (HR 0.77, p<0.001) (online supplementary table 1).
Figure 2.
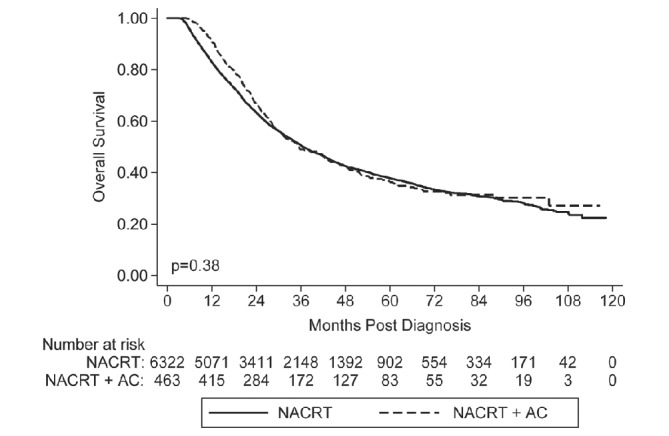
Kaplan-Meier survival curve for the overall cohort. AC, adjuvant chemotherapy; NACRT, neoadjuvant chemoradiotherapy.
esmoopen-2018-000386supp001.pdf (296KB, pdf)
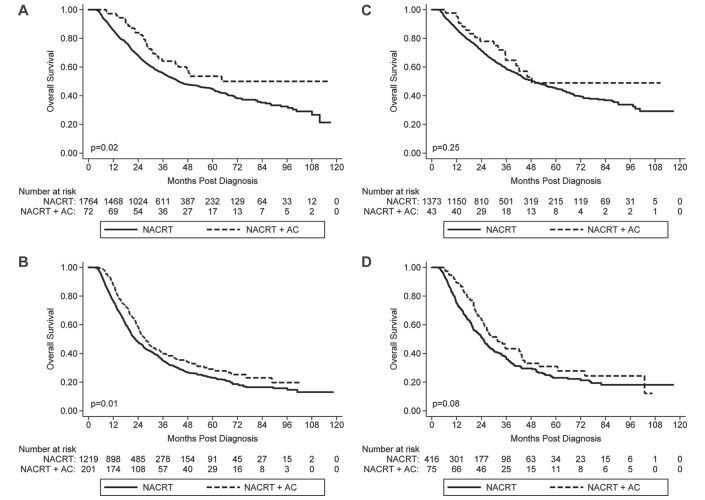
On subgroup analysis based on pathological nodal status following surgery, MVA showed a significantly reduced risk of death with NACRT+AC compared with NACRT alone for both the pN0 group (HR 0.65, p=0.007) and the pN+ group (HR 0.80, p=0.008) (online supplementary table 2). Additional subgroup analysis considering both clinical and pathological nodal status revealed that NACRT+AC was associated with a significantly reduced risk of death compared with NACRT alone for the cN+/pN0 group (median OS 64 vs 43 months; p=0.019) and the cN+/pN+ group (median OS 27 vs 22 months; p=0.010), but not in the cN0/pN0 group (median OS 48 vs 49 months; p=0.253) or cN0/pN+ group (median OS 31 vs 24 months; p=0.077) (figure 3). These findings were confirmed on MVA (online supplementary tables 3 and 4). The key results are summarised in table 2.
Figure 3.
Kaplan-Meier survival curves for the (A) cN+/pN0, (B) cN+/pN+, (C) cN0/pN0 and (D) cN0pN+ groups. AC, adjuvant chemotherapy; NACRT, neoadjuvant chemoradiotherapy.
esmoopen-2018-000386supp002.pdf (328.2KB, pdf)
Table 2.
Median overall survival separated based on receipt of adjuvant chemotherapy. Hazard ratios showing risk of death with receipt of adjuvant chemotherapy compared to postoperative observation
| Median Overall Survival (Months) | Multivariate analysis | ||||
| Group | NACRT | NACRT + AC | P | Hazard Ratio | P |
| Overall Cohort | 36.5 (35.3 - 38.3) | 35.5 (31.1 - 43.0) | 0.380 | 0.77 (0.66 – 0.89) | <0.001 |
| cN+/pN+ | 22.7 (20.9 - 24.6) | 27.8 (24.2 - 32.6) | 0.010 | 0.82 (0.67 - 1.00) | 0.048 |
| cN+/pN0 | 43.7 (40.5 - 50.7) | 64.4 (41.9 - NR) | 0.019 | 0.59 (0.39 - 0.88) | 0.009 |
| cN0/pN+ | 24.9 (21.7 - 27.3) | 31.4 (24.5 - 43.5) | 0.077 | 0.76 (0.54 - 1.06) | 0.105 |
| cN0/pN0 | 49.1 (43.6 - 55.4) | 48.3 (35.7 - NR) | 0.253 | 0.73 (0.41-1.30) | 0.253 |
AC, adjuvant chemotherapy; HR, hazard ratio; NACRT, neoadjuvant chemoradiotherapy; NR, not reached.
Discussion
The standard of care for locally advanced OC in patients with surgically resectable disease is an area of active debate. In the USA, standard treatment often includes NACRT followed by oesophagectomy (trimodality therapy).4 10 11 Alternatively, at some institutions, patients with gastro-oesophageal junction cancers are now offered treatment with perioperative chemotherapy based on the fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) trial data.12 Importantly, this trial has also shown that the administration of postoperative chemotherapy remains a challenge in many patients as only 50% of patients were able to complete postoperative FLOT per protocol. With regard to radiation therapy, the pathological complete response rates seem to be higher in neoadjuvant studies including chemoradiation when compared with chemotherapy alone.13–16 The ongoing perioperative chemotherapy compared to neoadjuvant chemoradiation in patients with adenocarcinoma of the esophagus (ESOPEC) trial comparing perioperative FLOT versus NACRT per the CROSS schedule will help to further elucidate the best treatment strategy in these patients.17 At present, the optimal postoperative management strategy remains unknown.
In this study, we found that the patients with locally advanced OC who are treated with NACRT+AC often have worse tumour characteristics when compared with patients who do not receive AC. After adjusting for this imbalance, we found that the addition of AC was associated with improved OS. The use of AC following trimodality therapy was investigated in two recently published retrospective analyses of the NCDB by Burt et al and Mokdad et al.18 19 Both studies concluded that AC appears to improve OS in patients who are found to have positive nodes following oesophagectomy. However, these studies provide conflicting evidence when the subgroup of patients with negative lymph nodes at the time of resection was analysed. In the study by Burt et al,18 a benefit from AC was not seen in patients with pathological complete response or residual non-nodal disease (pN0). In contrast, Mokdad et al 19 showed that AC improved survival regardless of lymph node status at the time of resection, with a 32% decrease in the risk of death i patients with negative lymph nodes and a 16% decrease in those who had positive lymph nodes.
Our multivariate analysis is in agreement with the conclusions by Mokdad et al 19 in that AC appears to be associated with a survival benefit regardless of a patient’s pathological node status. We also went a step further with our analysis to identify a subset of patients without residual nodal disease that may benefit from AC. Thus, in an analysis not previously reported, we performed a subgroup analysis which incorporated both clinical and pathological node status. We found that patients who underwent nodal downstaging (cN+/pN0) received the greatest survival benefit with the addition of AC (median OS 64 vs 43 months; p=0.019). Patients who were node-positive at the time of diagnosis and following surgery (cN+/pN+) had a smaller, yet still significant, improvement in survival with the addition of AC (median OS 27 vs 22 months; p=0.010). Interestingly, we found that AC was not associated with a significant increase in OS in patients who were clinically node-negative at the time of diagnosis, regardless of whether or not they had positive lymph nodes following surgery (cN0/pN0 and cN0/pN+).
It is believed that patients who have clinically positive nodes at the time of diagnosis are more likely to have widespread, yet clinically undetectable, micrometastases. These micrometastases are likely responsible for the predominantly distant disease recurrence pattern observed in patients following trimodality therapy.20 21 At least some of the survival benefit of AC we observed in the cN+ groups may be attributed to preventing progression of these distant micrometastases. We hypothesise that patients who underwent nodal downstaging (cN+/pN0) likely had a more favourable disease biology (ie, more chemosensitive malignancy), and the effect of AC on distant micrometastases in these patients was accentuated.
cN0/pN0 patients are unlikely to have distant micrometastases. Thus our finding that AC in these patients was not associated with a significant survival benefit was not unexpected. In fact, in this population, AC may be harmful as evidenced by a slightly, although statistically insignificant, decreased median OS in the AC group. Also, taking into account the effect of chemotherapy toxicities on the overall quality of life, a fairly strong case could be made against using AC in these patients.
Finally, patients who have nodal progression of the disease despite trimodality therapy (cN0/pN+) likely have unfavourable disease biology. Thus, while these patients likely have distant micrometastatic disease following surgery, the fact that they responded poorly to initial chemotherapy may make it less likely that they will respond to AC. However, given a slight trend towards a survival benefit in this group, it is possible that there may still be some individuals who could benefit from additional therapy. While the NCDB data do not record the specific chemotherapy used, oncologists who choose to treat individuals in this group may find benefit in using agents that were not used in the initial chemoradiotherapy regimen.
Of course, it should be noted that our conclusions are subject to the limitations inherent to any retrospective database study. Our analysis included a large number of patients; however, only a small fraction received AC, creating the possibility for selection bias. While we attempted to control for overall health by excluding patients who died within 90 days of diagnosis and included comorbidities based on the Charlson/Deyo Scores in our analyses, it is possible that some of the benefits we observed with AC could be attributed to a preference for healthier patients to be selected for adjuvant treatment. However, patients in the NACRT-alone group were more likely to undergo nodal downstaging than the NACRT+AC group. Thus there may have been bias towards more favourable disease biology in the NACRT-alone group. Lastly, while we included only patients who received multiagent chemotherapy (excluding those who received non-curative intent single-agent chemotherapy), the exact chemotherapy agents and dosing regimen are not available in the NCDB data.
Conclusion
AC following trimodality therapy is associated with improved survival in patients with locally advanced OC. Patients who undergo downstaging with regard to nodal status following NACRT may be the most likely to benefit from AC. Prospective trials are needed to verify these findings.
esmoopen-2018-000386supp003.pdf (328.9KB, pdf)
esmoopen-2018-000386supp004.pdf (328KB, pdf)
Footnotes
Contributors: CN-P: conceptualisation, visualisation, writing - original draft, and writing - review and editing. SF: conceptualisation, data curation, formal analysis, visualisation, writing - original draft, and writing - review and editing. CC: writing - review and editing. RT: writing - review and editing. JW: writing - review and editing. RG: writing - review and editing. CS: writing - review and editing. SL: supervision and writing - review and editing. IG-L: supervision and writing - review and editing.
Funding: This work was supported by a Cancer Center Support Grant (5P30CA042014-23).
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1. Siegel RL, Miller KD, Jemal A, et al. Cancer statistics, 2018. CA: A Cancer Journal for Clinicians 2018;68:7–30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2. Altorki N, Harrison S. What is the role of neoadjuvant chemotherapy, radiation, and adjuvant treatment in resectable esophageal cancer? Ann Cardiothorac Surg 2017;6:167–74. 10.21037/acs.2017.03.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. So B, Marcu L, Olver I, et al. Oesophageal cancer: Which treatment is the easiest to swallow? A review of combined modality treatments for resectable carcinomas. Crit Rev Oncol Hematol 2017;113:135–50. 10.1016/j.critrevonc.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 4. van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074–84. 10.1056/NEJMoa1112088 [DOI] [PubMed] [Google Scholar]
- 5. Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090–8. 10.1016/S1470-2045(15)00040-6 [DOI] [PubMed] [Google Scholar]
- 6. Merkow RP, Bilimoria KY, McCarter MD, et al. Use of multimodality neoadjuvant therapy for esophageal cancer in the United States: assessment of 987 hospitals. Ann Surg Oncol 2012;19:357–64. 10.1245/s10434-011-1945-3 [DOI] [PubMed] [Google Scholar]
- 7. Makowiec F, Baier P, Kulemann B, et al. Improved long-term survival after esophagectomy for esophageal cancer: influence of epidemiologic shift and neoadjuvant therapy. J Gastrointest Surg 2013;17:1193–201. 10.1007/s11605-013-2212-7 [DOI] [PubMed] [Google Scholar]
- 8. Meguid RA, Hooker CM, Taylor JT, et al. Recurrence after neoadjuvant chemoradiation and surgery for esophageal cancer: does the pattern of recurrence differ for patients with complete response and those with partial or no response? J Thorac Cardiovasc Surg 2009;138:1309–17. 10.1016/j.jtcvs.2009.07.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luc G, Gronnier C, Lebreton G, et al. Predictive factors of recurrence in patients with pathological complete response after esophagectomy following neoadjuvant chemoradiotherapy for esophageal cancer: a multicenter study. Ann Surg Oncol 2015;22(Suppl 3):1357–64. 10.1245/s10434-015-4619-8 [DOI] [PubMed] [Google Scholar]
- 10. Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086–92. 10.1200/JCO.2007.12.9593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462–7. 10.1056/NEJM199608153350702 [DOI] [PubMed] [Google Scholar]
- 12. Al-Batran S-E, Homann N, Schmalenberg H, et al. Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): A multicenter, randomized phase 3 trial. Journal of Clinical Oncology 2017;35:4004. [Google Scholar]
- 13. Klevebro F, Alexandersson von Döbeln G, Wang N, et al. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann Oncol 2016;27:660–7. 10.1093/annonc/mdw010 [DOI] [PubMed] [Google Scholar]
- 14. Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009;27:851–6. 10.1200/JCO.2008.17.0506 [DOI] [PubMed] [Google Scholar]
- 15. Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11–20. 10.1056/NEJMoa055531 [DOI] [PubMed] [Google Scholar]
- 16. Samson P, Robinson C, Bradley J, et al. Neoadjuvant chemotherapy versus chemoradiation prior to esophagectomy: impact on rate of complete pathologic response and survival in esophageal cancer patients. J Thorac Oncol 2016;11:2227–37. 10.1016/j.jtho.2016.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoeppner J, Lordick F, Brunner T, et al. ESOPEC: prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (NCT02509286). BMC Cancer 2016;16:503 10.1186/s12885-016-2564-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burt BM, Groth SS, Sada YH, et al. Utility of adjuvant chemotherapy after neoadjuvant chemoradiation and esophagectomy for esophageal cancer. Ann Surg 2017;266:297–304. 10.1097/SLA.0000000000001954 [DOI] [PubMed] [Google Scholar]
- 19. Mokdad AA, Yopp AC, Polanco PM, et al. Adjuvant chemotherapy vs postoperative observation following preoperative chemoradiotherapy and resection in gastroesophageal cancer: a propensity score-matched analysis. JAMA Oncol 2018;4:31-38 10.1001/jamaoncol.2017.2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shaikh T, Zaki MA, Dominello MM, et al. Patterns and predictors of failure following tri-modality therapy for locally advanced esophageal cancer. Acta Oncol 2016;55:303–8. 10.3109/0284186X.2015.1110252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oppedijk V, van der Gaast A, van Lanschot JJ, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol 2014;32:385–91. 10.1200/JCO.2013.51.2186 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2018-000386supp001.pdf (296KB, pdf)
esmoopen-2018-000386supp002.pdf (328.2KB, pdf)
esmoopen-2018-000386supp003.pdf (328.9KB, pdf)
esmoopen-2018-000386supp004.pdf (328KB, pdf)