Abstract
This is a case of an 86-year-old woman with gradually progressive dyspnoea and hypoxaemia that occurred after a cardiac surgery. It was underdiagnosed for several years, but diagnosis was triggered by the finding of hypoxaemia even during supplemental oxygen administration when in the upright position, such as when taking a shower, that rapidly improved when the patient returned to the supine position. A thorough workup disclosed platypnoea–orthodeoxia syndrome (POS) associated with right-to-left shunting through a patent foramen ovale (PFO). Percutaneous closure of the PFO was performed. After treatment, the patient’s arterial oxygen saturation gradually recovered to 98% on room air while she was in the sitting position and her symptoms disappeared. Reviewing this case retrospectively, we determined that the deviation of the spine with kyphosis progression had apparently proceeded as POS worsened over time. We therefore hypothesised that kyphosis progression had played a major role in the POS progression.
Keywords: cardiovascular medicine, interventional cardiology
Background
Platypnoea–orthodeoxia syndrome (POS) is an uncommon clinical condition first described by Burchell in 19491 that is characterised by dyspnoea and hypoxaemia occurring during a change from a supine to an upright position. POS is caused by an overlap of anatomical features of left and right atrium shunt and functional components, causing the shunt direction to change, thereby increasing the amount of right-to-left shunt due to a deformation of the atrial septum while in the upright position. Here, we present a case of gradually progressing POS in an elderly woman after aortic valve replacement surgery. Kyphosis progression was largely involved in the onset of POS in this case.
Case presentation
An 86-year-old woman had undergone aortic valve replacement surgery (AVR) and ascending aortic replacement surgery for an aortic valve obstruction deficiency and ascending aortic aneurysm 11 years ago. No remarkable events were observed after the surgery, but she began to experience palpitations and dyspnoea 5 years after the surgery. She had hypoxaemia, with oxygen saturation of 80%–90% on room air. Her physicians sought to determine the cause of the hypoxia, but was underdiagnosed, and home oxygen therapy was started. Her hypoxaemia gradually worsened, and the oxygen dose increased to 3 L at rest and 5 L on exertion. When she was in an upright position, such as when taking a shower, her oxygen saturation decreased to lower than 70%, even with administration of 5 L of oxygen. However, her oxygen saturation typically improved promptly when she returned to rest in the supine position. She was admitted to the hospital for reinspection of the causes of the hypoxaemia. She had a medical history of AVR, ascending aortic replacement surgery and lumbar compression fracture before the surgery.
Investigations
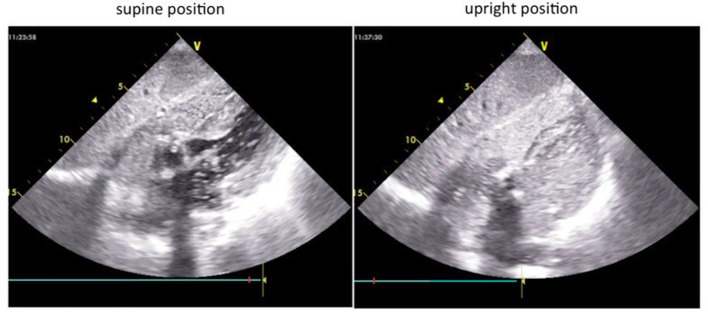
On physical examination, the patient’s blood pressure was 154/87 mm Hg, and her pulse rate was 80 beats per minute. While her oxygen saturation was 95% on room air in the supine position, but 75% with administration of 5 L of oxygen in the sitting position, no apparent cyanosis, peripheral oedema or clubbing of the extremities was observed. The patient was 143 cm tall, and she weighed 33.7 kg. A chest X-ray showed no visible lung congestion, despite the presence of cardiomegaly with a cardiothoracic ratio of 63.7% (figure 1). On electrocardiography, left axis deviation and complete right bundle branch block were detected (figure 2). Transthoracic echocardiography (TTE) revealed that both atria were enlarged; however, no interatrial shunt flow was observed. Laboratory data revealed no remarkable abnormalities. CT revealed a deformity of the right atrium caused by an elongated ascending aorta and kyphosis; however, there were no findings in the lung field to explain the hypoxaemia. Detailed history taking disclosed that the patient’s symptoms worsened on sitting or being in an upright position and that they improved when the patient returned to the supine position. Additionally, oxygen saturation improved from 70% on standing to 98% in the supine position. This finding was also recognised on an arterial blood gas analysis, in which PaO2 was 53 mm Hg while the patient was standing and 61 mm Hg while she was in the supine position (table 1). According to the patient’s history and her physical and laboratory examination findings, a diagnosis of POS was suspected. Transoesophageal echocardiography with the administration of intravenous agitated saline contrast solution was therefore performed, and a right-to-left shunt flow through the patent foramen ovale (PFO) was observed (figure 3). TTE with intravenous agitated saline contrast solution was performed again while the patient was in both the supine and upright positions; it revealed bubbles in the left atrium and ventricle within eight cardiac cycles in both positions. However, the shunt flow and the amount of bubbles appearing in the left atrium and ventricle were remarkably increased in the upright position compared with those in the supine position (figure 4). Her right ventricular outflow tract velocity time integral increased from 7.57 while standing to 10.59 while in the supine position. Cardiac catheterisation revealed normal haemodynamics, but her oxygen saturation measurements supported a right-to-left shunt, with SaO2 of 98.8% in the right pulmonary artery and of 88.2% in the aorta, although these should be nearly the same value; additionally, the shunt rate was 23%. Pulmonary angiography showed no evidence of pulmonary artery venous fistula or hepatic lung syndrome. Based on these findings, the patient was diagnosed with POS.
Figure 1.
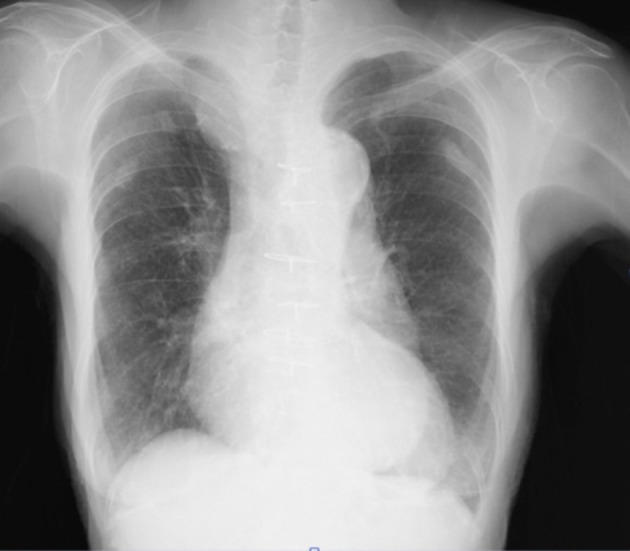
Cardiothoracic ratio was 63.7%. There was no visible lung congestion.
Figure 2.
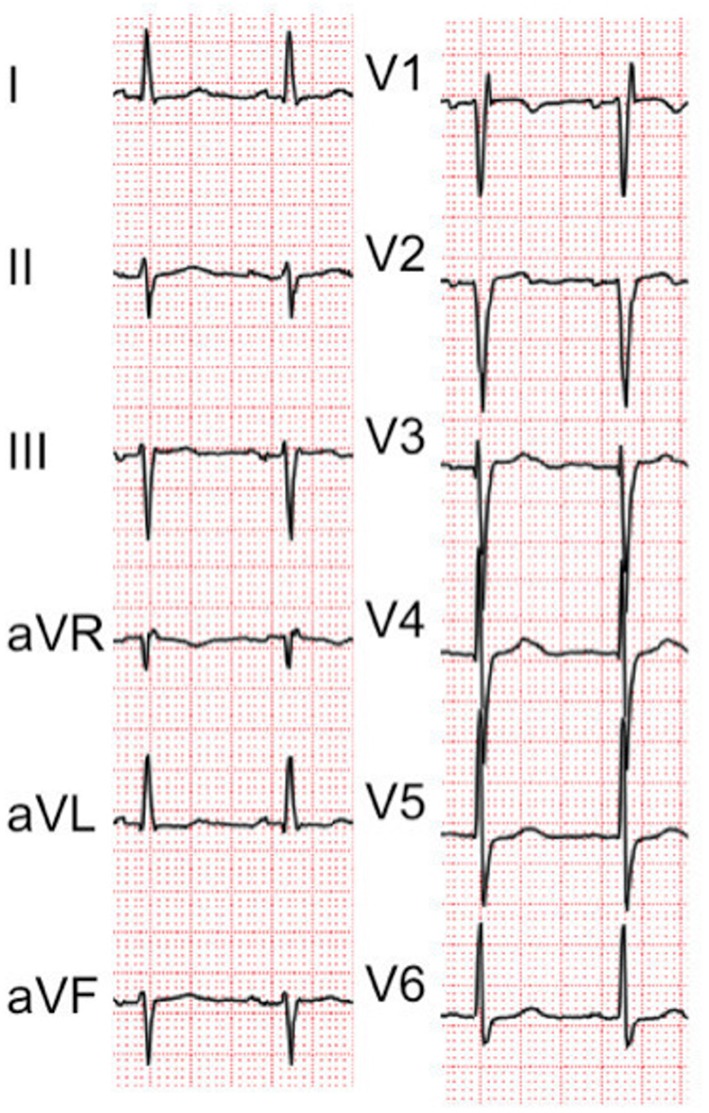
Left axis deviation and complete right bundle branch block.
Table 1.
Comparison of arterial blood gas analysis in supine and upright positions
| Arterial blood gas analysis | Supine position | Upright position |
| Arterial oxygen tension | 61 mm Hg | 53.9 mm Hg |
| Arterial carbon dioxide tension | 29.5 mm Hg | 32.6 mm Hg |
Figure 3.
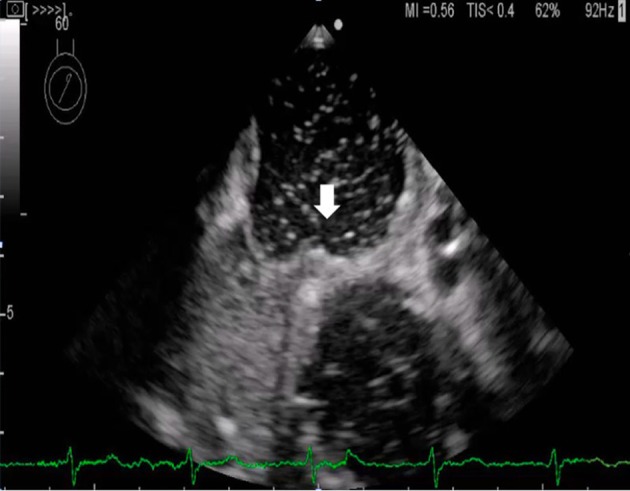
Right-to-left shunt flow through the patent foramen ovale (white arrow).
Figure 4.
The amount of bubbles appearing in the left atrium and ventricle was remarkably higher in the upright position than in the supine position.
Treatment
In this case, percutaneous closure of the PFO using a 25 mm Amplatzer Cribriform Occluder was performed instead of surgical closure (figure 5), which we felt would be too risky because of her age and history of cardiac surgery.
Figure 5.
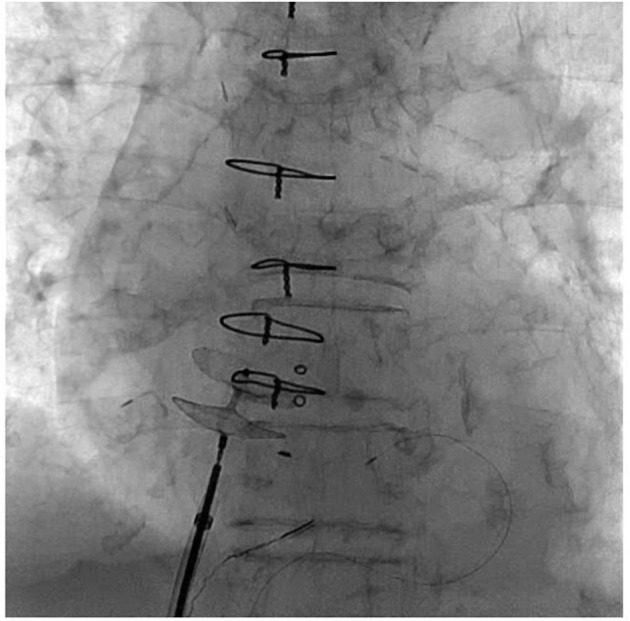
Percutaneous closure of the patent foramen ovale using a 25 mm Amplatzer Cribriform.
Outcome and follow-up
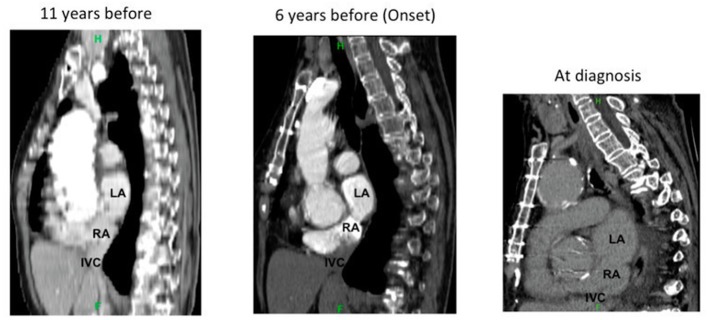
After treatment, the patient’s arterial oxygen saturation gradually recovered to 98% on room air while she was in the sitting position and her symptoms disappeared. When we retrospectively reviewed this case, we determined that the deviation of the spine with kyphosis progression had apparently proceeded as POS worsened over time (figure 6). We therefore hypothesised that kyphosis progression had played a major role in the POS progression.
Figure 6.
Deviation of the spine with kyphosis progression apparently proceeding over time and changing the positional relationship of the left atrium (LA), right atrium (RA), atrial septum and inferior vena cava (IVC).
Discussion
We described a case of progressive POS that occurred in an elderly patient after cardiac surgery. The diagnosis was triggered by the finding of hypoxaemia (ie, SpO2 around 70%) even during supplemental oxygen administration when in the upright position, such as when taking a shower, that rapidly improved when the patient returned to the supine position. Cardiac surgery was performed approximately 5 years before the onset of POS. The existence of a PFO was not recognised before the surgery. The chronic hypoxaemia was considered to be caused by age-related changes, such as decreased chest cavity compliance after open chest surgery, respiratory muscle weakness and respiratory function decline, which increased the amount of time needed to make a final diagnosis.
Burchell1 first described POS more than half a century ago. POS is an uncommon but serious clinical syndrome characterised by dyspnoea and hypoxaemia that occur during changes from a supine position to a sitting or upright position. Some anatomical features and functional components must coexist as causes of POS. Anatomical features include PFO, an atrial septal defect and an atrial septal aneurysm. Functional factors, which cause changes in the direction of right-to-left shunt flow by excavation of the heart or deformation of the atrial septum in the upright position, include pulmonary emphysema, pulmonary arteriovenous malformation, pulmonary embolism, pulmonary resection, contractile pericarditis, scoliosis, cirrhosis of the liver and elongation of the ascending aorta.2 Rodrigues et al stated that physicians should define this syndrome with the cardiac anatomic feature of right-to-left shunt as platypnoea–orthodeoxia disease, with was defined by the existence of interatrial communication, a right-to-left shunt, normal pressure in the right atrium and, of course, platypnoea and orthodeoxia.2 Many POS cases involve elderly people over the age of 70 years. There is a possibility that it becomes easier for venous blood from the inferior vena cava to flow toward the fossa ovale as a patient ages due to a deviation of the mediastinum, a decrease in the compliance of the heart or a counter-clockwise rotation or twist of the heart.3 4 PFO accounts for a majority of POS anatomical elements. When pulmonary hypertension occurs in the presence of PFO, it causes a right-to-left shunt; however, in the case of POS, it produces a right-to-left shunt despite the absence of pulmonary hypertension. Treatment of POS requires closure of the atrial shunt. Recently, more reports have been made of atrial septal defects and closure of the PFO by percutaneous closure methods. Effects of PFO and atrial septal defect closure in cases of severe chronic obstructive pulmonary disease exhibiting marked pulmonary hypertension or other comorbid conditions that can cause POS, such as hepatic lung syndrome, may be limited, so it is important to determine the main cause of right-to-left shunt by contrast echography or right heart catheterisation examination.5
In this case, the breathing difficulty gradually progressed, making a definite diagnosis difficult. It seems that the POS onset in this case was affected by an atrial septum deformity, elongation of the ascending aorta, the influence of cardiac surgery and kyphosis progression. A case of POS onset due to a thoracic fracture after a traffic accident has been reported.6 As shown in figure 6, the deviation ofthe spine due to the progression of kyphosis apparently proceeded over time. We considered the progression of the kyphosis to be a major contributor to the breathing difficulty and onset of POS, hypothesising that it became easier for the blood flow originating from the inferior vena cava to enter directly into the atrial septum due to a change in the heart position.
PFO has also been reported to be present in 25%–30% of otherwise healthy people.7 8 In the current ageing society, when dealing with hypoxaemia of unknown causes, physicians should be aware of the possibility of POS and examine the patient for unexpected changes in SpO2 while they are in the supine and upright positions.
Learning points.
Platypnoea–orthodeoxia syndrome (POS) is an uncommon clinical condition that is characterised by dyspnoea and hypoxaemia occurring during a change from a supine to an upright position.
We should be aware of the possibility of POS when dealing with hypoxaemia of unknown causes.
Kyphosis can be major contributor to the breathing difficulty and onset of POS.
Footnotes
Contributors: TI wrote the initial draft of the manuscript. TM assisted in taking care of the patient, and in the preparation of the manuscript. RM and TN critically reviewed the manuscript. All authors approved the final version of the manuscript, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Burchell HB. Reflex orthostatic dyspnea associated with pulmonary hypotension. Am J Physiol 1949;159:563–4. [Google Scholar]
- 2.Rodrigues P, Palma P, Sousa-Pereira L. Platypnea-orthodeoxia syndrome in review: defining a new disease? Cardiology 2012;123:15–23. 10.1159/000339872 [DOI] [PubMed] [Google Scholar]
- 3.Cheng TO. Platypnea-orthodeoxia syndrome: etiology, differential diagnosis, and management. Catheter Cardiovasc Interv 1999;47:64–6. [DOI] [PubMed] [Google Scholar]
- 4.Meier B, Lock JE. Contemporary management of patent foramen ovale. Circulation 2003;107:5–9. 10.1161/01.CIR.0000046073.34261.C1 [DOI] [PubMed] [Google Scholar]
- 5.Mojadidi MK, Gevorgyan R, Noureddin N, et al. The effect of patent foramen ovale closure in patients with platypnea-orthodeoxia syndrome. Catheter Cardiovasc Interv 2015;86:701–7. 10.1002/ccd.25953 [DOI] [PubMed] [Google Scholar]
- 6.Fukuoka R, Kawasaki T, Koga H, et al. Platypnea-orthodeoxia syndrome associated with a thoracic vertebral fracture following a car accident. Intern Med 2014;53:35–8. 10.2169/internalmedicine.53.0797 [DOI] [PubMed] [Google Scholar]
- 7.Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc 1984;59:17–20. 10.1016/S0025-6196(12)60336-X [DOI] [PubMed] [Google Scholar]
- 8.Meissner I, Whisnant JP, Khandheria BK, et al. Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study. Stroke Prevention: Assessment of Risk in a Community. Mayo Clin Proc 1999;74:862–9. [DOI] [PubMed] [Google Scholar]