Abstract
Background
Neutrophil to lymphocyte ratio (NLR) was introduced to predict poor prognosis in various diseases, but not all variants of ANCA-associated vasculitis (AAV). In this study, we aimed to investigate whether NLR at diagnosis can estimate vasculitis activity at diagnosis and poor prognosis during follow-up in patients with AAV.
Methods
We retrospectively reviewed the medical records of 160 patients with AAV. We collected clinical and laboratory data at diagnosis and obtained remission and death as poor prognosis. We stratified AAV patients into three groups according to tertile and defined the lower limit of each highest tertile as the optimal cut-off (5.9 for NLR and 15.0 of Birmingham vasculitis activity score [BVAS] for severe AAV).
Results
The mean age at diagnosis was 55.2 years and 48 patients were men. In the univariable linear regression analysis, BVAS was negatively correlated with lymphocyte count and positively correlated with erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and NLR. In the multivariable linear regression analyses of ESR and CRP with either lymphocyte count or NLR, lymphocyte count (β = − 0.160) and NLR (β = 0.169) were associated with BVAS. Patients having NLR ≥ 5.9 exhibited severe AAV more frequently than those having NLR < 5.9 at diagnosis (relative 2.189, P = 0.023). Patients having NLR ≥ 5.9 exhibited a higher frequency of AAV relapse, but not death, than those having NLR < 5.9 (P = 0.016).
Conclusions
NLR at diagnosis can estimate vasculitis activity at diagnosis and predict relapse during follow-up in patients with AAV.
Keywords: Antineutrophil cytoplasmic antibody-associated vasculitis, Neutrophil to lymphocyte ratio, Vasculitis activity, Prognosis
Background
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a group of three systemic vasculitides involving small vessels from capillaries to intraparenchymal arterioles and venules: microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA) and eosinophilic granulomatosis with polyangiitis (EGPA) [1]. MPA and GPA exhibit similar clinical manifestations of pulmonary, renal and ear-nose-throat manifestations, despite differences in genetic backgrounds, aetiologies, ANCA type, and histologic findings [1–3], whereas, EGPA shows both necrotising vasculitis and allergic components such as asthma and eosinophilia [1, 2, 4].
Neutrophil to lymphocyte ratio (NLR) has been recently introduced and widely used to predict poor prognosis in several cancers and inflammatory diseases [5, 6]. Neutrophil count may be often directly proportional to the inflammatory burdens, furthermore, activated neutrophils are very closely associated with the pathogenesis of AAV. By contrast, lymphocyte count may decrease in autoimmune inflammatory diseases [7]. Particularly, lymphopenia with low numbers of CD4+ T cells can be observed in GPA due to an extensive recruitment of peripheral T cells to the affected tissues [8]. Therefore, it can be reasonably speculated that NLR may reflect the inflammatory burdens in patients with systemic vasculitis. So far, NLR has been reported to be associated with disease activity and prognosis in Takayasu arteritis, Behcet disease, Kawasaki vasculitis and Henoch Schonlein purpura [9–11]. However, there were only a few reports the clinical role of NLR in patients with AAV [12, 13]. Furthermore, there was no study to demonstrate the association of NLR with both vasculitis activity and poor prognosis including relapse and death in a considerable number of patients with MPA, GPA and EGPA to date. Hence, in this study, we aimed to investigate whether NLR at diagnosis can estimate vasculitis activity at diagnosis and poor prognosis during follow-up in 160 patients with AAV, who were not administered immunosuppressive drugs before AAV diagnosis.
Methods
Patients
We retrospectively reviewed the medical records of 160 patients with AAV according the inclusion criteria as follows: i) patients who were first classified as AAV from October 2000 to September 2017 at the Department of Internal Medicine, Yonsei University College of Medicine, Severance Hospital, where this study was conducted as a monocentric investigation; ii) patients who fulfilled the American College of Rheumatology 1990 criteria for the classification for AAV and then reclassified by the 2007 European Medicines Agency algorithm modified by the 2012 revised Chapel Hill Consensus Conferences Nomenclature of Vasculitis [1–4]; iii) patients who had well-documented medical records with which to calculate items of Birmingham vasculitis activity score (BVAS) and five factor score (FFS (2009)) at diagnosis [14–16]; iv) patients who had results on perinuclear (P)-ANCA and cytoplasmic (C)-ANCA or myeloperoxidase (MPO)-ANCA and proteinase 3 (PR3)-ANCA at diagnosis [17]; v) patients who had no concomitant or previous medical conditions to disturb AAV classification, which was confirmed by the 10th revised International Classification of Diseases [18]; and vi) patients who received no immunosuppressive drugs prior to diagnosis of AAV, which was searched by the Korean Drug Utilization Review system. This study was approved by the Institutional Review Board of Severance Hospital (4–2017-0673), who waived the need for patient written informed consent, as this was a retrospective study.
Clinical data
We obtained age and gender as demographic data at the time of the first diagnosis of AAV and searched the initial ANCAs. When an AAV patient exhibited an item described in BVAS, we considered him or her to have an organ-specific involvement of AAV, regardless of tissue biopsy findings as below: general manifestation including muscle pain, joint symptoms, fever and weight loss ≥2 kg; cutaneous manifestation including skin rashes and ulcerations; mucous membrane / eyes manifestation including oral or genital ulceration, inflammation in sclera or conjunctiva, impairment in visual function, uveitis and retinitis; ear nose throat manifestation including inflammation in nasal passage or paranasal sinus and hearing loss; chest manifestation including inflammation in both lung parenchyma and pleura; cardiovascular manifestation including coronary arterial occlusion, heart failure and pericarditis; abdominal manifestation including gastrointestinal bleeding and mesenteric arterial occlusion; renal manifestation including proteinuria > 1+ on urine stick, haematuria ≥10 RBCs/HPF and renal dysfunction; nervous system manifestation including central and peripheral neuropathies. We also calculated the total score of BVAS and FFS (2009) at diagnosis.
Laboratory data
We collected laboratory results at diagnosis, which represent the inflammatory burdens in the real clinical settings including complete blood counts [white blood cell (WBC), neutrophil, lymphocyte, and platelet counts]; erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) before the administration of immunosuppressive drugs to AAV patients. The follow-up duration was defined as the duration from diagnosis to the last visit in patients without relapse, whereas it was defined as the time from diagnosis to the first relapse in patients with relapse. Therefore, the follow-up duration in this study meant the relapse free period of AAV.
Prognosis
Remission was determined as no active disease requiring the maintenance therapy, relapse was defined as active disease after remission [19]. We also counted all cause death in AAV patients.
Equations of NLR and optimal cut-off
NLR was calculated as a ratio of neutrophil count over lymphocyte count at diagnosis [NLR = neutrophil count (/uL) / lymphocyte count (/uL)] [9, 10]. We stratified AAV patients into three groups according to tertile and define the lower limit of each highest tertile as the optimal cut-off [6]. The optimal cut-offs were set at 15.0 for severe AAV based on BVAS and 5.9 for NLR. In particular, in this study, we discretionally define severe AAV when BVAS is 15.0 or greater.
Statistical analyses
We expressed continuous variables as a mean ± standard deviation, and categorical variables as number (%). We assessed the standardised correlation coefficient by the multivariable linear regression analysis using variables with significance in the univariable analysis. We compared categorical variables between the two groups, and analysed the relative risk (RR) using the chi square and Fisher’s exact tests. Also we compared cumulative relapse free and patient survivals between the two groups using the Kaplan-Meier survival analysis. We conducted all statistical analyses using SPSS software (version 23 for windows; IBM Corp., Armonk, NY, USA). P-values < 0.05 were considered statistically significant.
Results
Baseline characteristics of 160 patients with AAV
The baseline characteristics are described in Table 1. Eight-five of 160 patients (53.1%) were classified as MPA, 41 patients (25.6%) as GPA and 34 patients (21.3%) as EGPA. The mean age at diagnosis was 55.2 years and 48 patients (30.0%) were men. Ninety-nine patients (61.9%) and 27 patients (16.9%) had MPO-ANCA (or P-ANCA) and PR3-ANCA (or C-ANCA), respectively. Seven patients (4.4%) had MPO-ANCA (or P-ANCA) as well as PR3-ANCA (or C-ANCA), and 41 patients (25.6%) had no ANCA. The most common clinical manifestation of AAV at diagnosis was renal manifestation (59.4%), followed by chest (52.5%) and general (44.4%) manifestations. The mean BVAS at diagnosis was 11.9 and the mean FFS (2009) at diagnosis was 1.3. In terms of laboratory results related to the inflammatory burdens at diagnosis, the mean WBC, neutrophil, lymphocyte and platelet counts were 10,175.6/mm3, 7227.5/mm3, 1564.0/mm3 and 327,500.0/mm3, respectively. The mean NLR was 6.6. The mean follow-up duration was 55.6 months. During the follow-up of more than 12 weeks, 43 patients (26.9%) exhibited relapse after remission and 14 patients (8.8%) died.
Table 1.
Baseline characteristics of 160 patients with AAV
| Variables | Values |
|---|---|
| Variants of AAV | |
| MPA | 85 (53.1) |
| GPA | 41 (25.6) |
| EGPA | 34 (21.3) |
| Demographic data at diagnosis | |
| Age (year old) | 55.2 ± 15.1 |
| Male gender (N, (%)) | 48 (30.0) |
| ANCA at diagnosis (N, (%)) | |
| MPO-ANCA (or P-ANCA) | 99 (61.9) |
| PR3-ANCA (or C-ANCA) | 27 (16.9) |
| MPO-ANCA (or P-ANCA) and PR3-ANCA (or C-ANCA) | 7 (4.4) |
| ANCA negative | 41 (25.6) |
| Clinical manifestations at diagnosis (N, (%)) | |
| General | 71 (44.4) |
| Cutaneous | 37 (23.1) |
| Mucous membranes/eyes | 12 (7.5) |
| Ear Nose Throat (ENT) | 56 (35.0) |
| Chest | 84 (52.5) |
| Cardiovascular | 45 (28.1) |
| Abdominal | 10 (6.3) |
| Renal | 95 (59.4) |
| Nervous system | 52 (32.5) |
| Vasculitis activity and prognostic factors at diagnosis | |
| BVAS or BVAS for GPA | 11.9 ± 7.6 |
| FFS (2009) | 1.3 ± 1.0 |
| Laboratory results at diagnosis | |
| WBC (/mm3) | 10,175.6 ± 4758.2 |
| Neutrophil (/mm3) | 7227.5 ± 4047.2 |
| Lymphocyte (/mm3) | 1564.0 ± 721.2 |
| Platelet (×1,000/mm3) | 327.5 ± 141.9 |
| ESR (mm/hr) | 60.1 ± 37.4 |
| CRP (mg/L) | 43.0 ± 56.5 |
| NLR at diagnosis | 6.6 ± 8.3 |
| Prognosis | |
| Follow-up duration (months) | 55.6 ± 51.5 |
| Relapse (N, (%)) | 43 (26.9) |
| Death (N, (%)) | 14 (8.8) |
Values are expressed as mean and standard deviation or N (%)
AAV antineutrophil associated vasculitis, MPA microscopic polyangiitis, GPA granulomatosis with polyangiitis, EGPA eosinophilic granulomatosis with polyangiitis, MPO myeloperoxidase, ANCA antineutrophil cytoplasmic antibody, P-ANCA perinuclear ANCA, PR3 proteinase 3, C-ANCA cytoplasmic ANCA, BVAS Birmingham vasculitis activity score, FFS five factor score, WBC white blood cell, ESR erythrocyte sedimentation rate, CRP C-reactive protein, NLR neutrophil to lymphocyte ratio
Univariable and multivariable linear regression analyses
In the univariable linear regression analysis, BVAS was negatively correlated with lymphocyte count (r = − 0.198, P = 0.012) and positively correlated with ESR (r = 0.218, P = 0.006) and CRP (r = 0.169, P = 0.033). BVAS was also significantly correlated with NLR (r = 0.204, P = 0.009) (Table 2). We performed the multivariable linear regression analyses of ESR and CRP with either lymphocyte count or NLR. In terms of multivariable linear regression analysis of lymphocyte, ESR and CRP, only lymphocyte count was significantly associated with BVAS (β = − 0.160, 95% confidence interval [CI] -0.003, 0.000, P = 0.045). In terms of multivariable linear regression analysis of NLR, ESR and CRP (R = 0.279), only NLR was significantly associated with BVAS (β = 0.169, 95% CI 0.010, 0.299, P = 0.036) (Table 2).
Table 2.
Univariable and multivariable linear regression analyses of BVAS and variables related to the inflammatory burdens in 160 patients with AAV
| Univariable analysis | Multivariable analysis (ESR, CRP and Lymphocyte) |
Multivariable analysis (ESR, CRP and NLR) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Regression Coefficient (Crude B) |
Correlation Coefficient (R = β) |
P value | Standardized β* | 95% confidence interval | P value | Standardized β* | 95% confidence interval | P value | |
| Demographic data at diagnosis | |||||||||
| Age (years old) | 0.027 | 0.053 | 0.504 | ||||||
| Laboratory data at diagnosis | |||||||||
| WBC (/mm3) | 0.000 | 0.095 | 0.232 | ||||||
| Neutrophil (/mm3) | 0.000 | 0.114 | 0.150 | ||||||
| Lymphocyte (/mm3)* | − 0.002 | −0.198 | 0.012 | −0.160 | − 0.003, 0.000 | 0.045 | N/A | N/A | N/A |
| Platelet (×1,000/mm3) | 0.003 | 0.060 | 0.449 | ||||||
| ESR (mm/hr) | 0.044 | 0.218 | 0.006 | 0.167 | −0.003, 0.071 | 0.074 | 0.177 | −0.001, 0.073 | 0.058 |
| CRP (mg/L) | 0.023 | 0.169 | 0.033 | 0.044 | −0.019, 0.031 | 0.644 | 0.029 | −0.021, 0.029 | 0.757 |
| NLR | 0.187 | 0.204 | 0.009 | N/A | N/A | N/A | 0.169 | 0.010, 0.299 | 0.036 |
*We performed multivariable linear regression analyses of ESR and CRP with either lymphocyte count or NLR
BVAS Birmingham vasculitis activity score, AAV antineutrophil associated vasculitis, ESR erythrocyte sedimentation rate, CRP C-reactive protein, WBC white blood cell, NLR neutrophil to lymphocyte ratio
RR of severe AAV based on BVAS
When we classified AAV patients into two groups based on the cut-off of NLR, patients having NLR ≥ 5.9 exhibited the higher frequency of severe AAV than those having NLR < 5.9 (47.2% vs. 29.0%, P = 0.023). Particularly, patients having NLR ≥ 5.9 had a significantly higher risk of severe AAV than those not having (RR 2.189, 95% CI 1.107, 4.330) (Fig. 1).
Fig. 1.
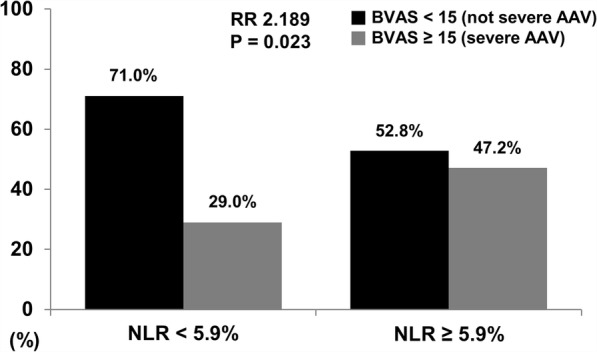
Relative risk of severe AAV based on BVAS. Patients having NLR ≥ 5.9 exhibited the higher frequency of severe AAV than those having NLR < 5.9 (47.2% vs. 29.0%, RR 2.189, P = 0.023). AAV; ANCA-associated vasculitis: ANCA: antineutrophil cytoplasmic antibody; BVAS: Birmingham vasculitis activity score; NLR: neutrophil to lymphocyte ratio
A predictor of relapse and death during follow-up
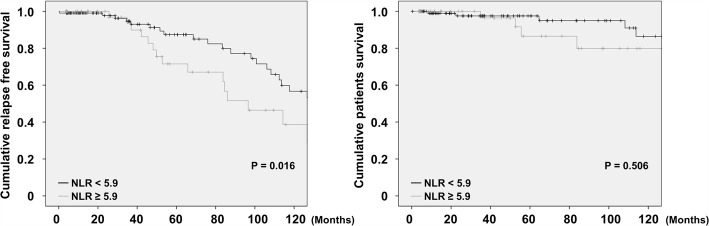
We evaluated whether the highest tertile of NLR (5.9 or greater) at diagnosis can predict relapse of AAV and death during the follow-up using Kaplan-Meier survival analysis. Cumulative relapse free and patient survival rates were depicted in Fig. 2. Patients having NLR ≥ 5.9 exhibited the higher frequency of relapse of AAV than those having NLR < 5.9 (P = 0.016). Thus, NLR has a potential of a predictor of relapse of AAV during the follow-up. However, there was no significant difference in cumulative patient survival rate between patients having NLR ≥ 5.9 and those NLR < 5.9 at diagnosis.
Fig. 2.
A predictor of relapse of AAV. Patients having NLR ≥ 5.9 exhibited the higher frequency of relapse of AAV than those having NLR < 5.9 (P = 0.016). AAV; ANCA-associated vasculitis: ANCA: antineutrophil cytoplasmic antibody; NLR: neutrophil to lymphocyte ratio
Discussion
In this study, we investigated whether NLR at diagnosis can estimate vasculitis activity at diagnosis and poor prognosis during follow-up in 160 patients with AAV in a single centre. First, in terms of vasculitis activity of AAV, we conclude that lymphocyte count and NLR are significantly correlated with BVAS, comparable to ESR and CRP. Meanwhile, among four variables, lymphocyte count and NLR are significantly associated with BVAS. The statistical significance of the association between BVAS and NLR was slightly higher than that between BVAS and lymphocyte count (β = 0.169 vs. β = − 0.160). In addition, NLR ≥ 5.9 (RR 2.189) can estimate severe AAV based on BVAS. Second, in terms of prognosis of AAV, we conclude that NLR ≥ 5.9 at diagnosis is a predictor of relapse of AAV, but not death, during follow-up. Therefore, we believe that NLR at diagnosis is a useful marker to estimate vasculitis activity at diagnosis and poor prognosis during follow-up in AAV patients.
In addition to NLR, lymphocyte count also exhibited a significant correlation and association with BVAS along with ESR and CRP. Unlike NLR, lymphocyte count is automatically counted and reported, suggesting that lymphocyte count is much more convenient than NLR. Nonetheless, NLR has been widely proposed to estimate the inflammatory burdens and predict prognosis than lymphocyte count in various diseaes. NLR includes two different lineages of immune cells, neutrophils and lymphocytes. Neutrophils are mainly in charge of nonspecific and early systemic inflammation. Neutrophil count may be elevated by infections or temporarily by glucocorticoid use, whereas it may be reduced by neutrophil-consuming medical conditions or immunosuppressive drugs. Meanwhile, lymphocyte participates in relatively late immune reactions. Lymphocyte count may also be affected by general health and stress or various autoimmune diseases. Therefore, NLR, in which two lineages of immune cells possessing different characters are integrated, is considered a more reliable and complementary marker than counts of single immune cells such as lymphocytes [4, 20].
In this study, we first demonstrated that NLR ≥ 5.9 at diagnosis can predict relapse of AAV during follow-up. Calculating NLR at diagnosis of AAV implies that they mainly reflect the vasculitis activity of AAV before the administration of immunosuppressive drugs. In our previous studies, we demonstrated that BVAS at diagnosis representing the initial inflammatory burdens could predict poor prognosis such as relapse or refractory disease in patients with AAV [21, 22]. Therefore, the clinical role of NLR at diagnosis to predict relapse of AAV can be explained by the positive link between NLR and BVAS. With these results, we suggest that physicians may calculate NLR at diagnosis to predict poor prognosis of AAV during follow-up. Furthermore, we also suggest that the more frequent visits, laboratory tests and evaluation of treatment efficacy may be necessary in AAV patients having NLR ≥ 5.9.
The number of neutrophils can be increased by the use of glucocorticoids, whereas it can be decreased by the use of immunosuppressive drugs by bone marrow suppression. Therefore, we included only patients who had neither glucocorticoid nor immunosuppressive drugs in this study. In addition, we excluded 12 patients with ischaemic heart disease or peripheral vascular diseases, as NLR might be influenced by atherosclerosis or peripheral vascular diseases [23, 24].
Our study has two advantages. First, we demonstrated the clinical roles of NLR at diagnosis to not only estimate vasculitis activity at diagnosis, but also predict relapse during follow-up in patients with all variants of AAV. Second, we could control the clinical and laboratory confounding factors including inter-observer or inter-centric variation due to a single-centric study. However, this study also has several limitations. First, we could not perform the subgroup analysis of clinical features of patients with NLR ≥ 5.9, but without severe AAV or relapse due to the retrospective study-design. Second, the number of AAV patients in this study was not large enough to represent the ethnic feature of Korean patients with AAV. If future studies with the larger number of patients can calculate BVAS and NLR prospectively, they might reveal the reliable and valuable results of NLR to not only estimate the current BVAS, but also predict poor prognosis during follow-up.
Conclusions
NLR at diagnosis can estimate vasculitis activity at diagnosis and predict relapse during the follow-up in patients with AAV. Thus, we suggest that physicians should pay more attention to patients with NRL at diagnosis ≥5.9, encourage them to visit more often and prolong the period of maintenance therapy even in those achieving remission.
Acknowledgments
Consent to publication
Not applicable.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1D1A1B03029050) and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (HI14C1324).
Availability of data and materials
The data used and analysed in this study are available from the corresponding author on reasonable request.
Author’s contributions
All authors contributed to the study concept, design, acquisition and interpretation of data. SSA and SWL performed the statistical analysis. SSA, YBP and SWL drafted and revised manuscript. All authors have read and approved the manuscript for publication.
Abbreviations
- AAV
ANCA-associated vasculitis
- ANCA
Antineutrophil cytoplasmic antibody
- BVAS
Birmingham vasculitis activity score
- C
Cytoplasmic
- CI
Confidence interval
- CRP
C-reactive protein
- EGPA
Eosinophilic granulomatosis with polyangiitis
- ESR
Erythrocyte sedimentation rate
- FFS
Five factor score
- GPA
Granulomatosis with polyangiitis
- MPA
Microscopic polyangiitis
- MPO
Myeloperoxidase
- NLR
Neutrophil to lymphocyte ratio
- P
Perinuclear
- PR3
Proteinase 3
- RR
Relative risk
- WBC
White blood cell
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Severance Hospital (4–2017-0673), who waived the need for patient written informed consent, as this was a retrospective study.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sung Soo Ahn, Email: SANETH@yuhs.ac.
Seung Min Jung, Email: JSMIN00@yuhs.ac.
Jason Jungsik Song, Email: JSKSONG@yuhs.ac.
Yong-Beom Park, Email: YONGBPARK@yuhs.ac.
Sang-Won Lee, Phone: 82-2-2228-1987, Email: sangwonlee@yuhs.ac, Email: SANGWONLEE@yuhs.ac.
References
- 1.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised international Chapel Hill consensus conference nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 2.Watts R, Lane S, Hanslik T, Hauser T, Hellmich B, Koldingsnes W, et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis. 2007;66:222–227. doi: 10.1136/ard.2006.054593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leavitt RY, Fauci AS, Bloch DA, Michel BA, Hunder GG, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Wegener's granulomatosis. Arthritis Rheum. 1990;33:1101–1107. doi: 10.1002/art.1780330807. [DOI] [PubMed] [Google Scholar]
- 4.Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis) Arthritis Rheum. 1990;33:1094–1100. doi: 10.1002/art.1780330806. [DOI] [PubMed] [Google Scholar]
- 5.Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. [DOI] [PubMed]
- 6.Benites-Zapata VA, Hernandez AV, Nagarajan V, Cauthen CA, Starling RC, Tang WH. Usefulness of neutrophil-to-lymphocyte ratio in risk stratification of patients with advanced heart failure. Am J Cardiol. 2015;115:57–61. doi: 10.1016/j.amjcard.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulze-Koops H. Lymphopenia and autoimmune diseases. Arthritis Res Ther. 2004;6:178–180. doi: 10.1186/ar1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berden AE, Kallenberg CG, Savage CO, Yard BA, Abdulahad WH, de Heer E, et al. Cellular immunity in Wegener's granulomatosis: characterizing T lymphocytes. Arthritis Rheum. 2009;60:1578–1587. doi: 10.1002/art.24576. [DOI] [PubMed] [Google Scholar]
- 9.Pan L, Du J, Li T, Liao H. Platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio associated with disease activity in patients with Takayasu's arteritis: a case-control study. BMJ Open. 2017;7:e014451. doi: 10.1136/bmjopen-2016-014451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuksel M, Yildiz A, Oylumlu M, Turkcu FM, Bilik MZ, Ekinci A, et al. Novel markers of endothelial dysfunction and inflammation in Behçet's disease patients with ocular involvement: epicardial fat thickness, carotid intima media thickness, serum ADMA level, and neutrophil-to-lymphocyte ratio. Clin Rheumatol. 2016;35:701–708. doi: 10.1007/s10067-015-2907-0. [DOI] [PubMed] [Google Scholar]
- 11.Park CH, Han DS, Jeong JY, Eun CS, Yoo KS, Jeon YC, et al. The optimal cut-off value of neutrophil-to-lymphocyte ratio for predicting prognosis in adult patients with Henoch-Schönlein Purpura. PLoS One. 2016;11:e0153238. doi: 10.1371/journal.pone.0153238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Küçük H, Göker B, Varan Ö, Dumludag B, Haznedaroğlu Ş, Öztürk MA, et al. Predictive value of neutrophil/lymphocyte ratio in renal prognosis of patients with granulomatosis with polyangiitis. Ren Fail. 2017;39:273–276. doi: 10.1080/0886022X.2016.1259633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abaza NM, El-Latif EMA, Gheita TA. Clinical significance of neutrophil/lymphocyte ratio in patients with granulomatosis with Polyangiitis. Reumatol Clin. 2017; 10.1016/j.reuma.2017.11.003. [Epub ahead of print] [DOI] [PubMed]
- 14.Mukhtyar C, Lee R, Brown D, Carruthers D, Dasgupta B, Dubey S, et al. Modification and validation of the Birmingham Vasculitis activity score (version 3) Ann Rheum Dis. 2009;68:1827–1832. doi: 10.1136/ard.2008.101279. [DOI] [PubMed] [Google Scholar]
- 15.Stone JH, Hoffman GS, Merkel PA, Min YI, Uhlfelder ML, Hellmann DB, et al. A disease-specific activity index for Wegener's granulomatosis: modification of the Birmingham Vasculitis activity score. International network for the study of the systemic Vasculitides (INSSYS) Arthritis Rheum. 2001;44:912–920. doi: 10.1002/1529-0131(200104)44:4<912::AID-ANR148>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Guillevin L, Pagnoux C, Seror R, Mahr A, Mouthon L, Le Toumelin P; French Vasculitis Study Group (FVSG). The five-factor score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis study group (FVSG) cohort. Medicine (Baltimore) 2011;90:19–27. [DOI] [PubMed]
- 17.Csernok E, Moosig F. Current and emerging techniques for ANCA detection in vasculitis. Nat Rev Rheumatol. 2014;10:494–501. doi: 10.1038/nrrheum.2014.78. [DOI] [PubMed] [Google Scholar]
- 18.Noel N, André C, Bengoufa D, Dehoulle C, Mahler M, Limal N, et al. Performance evaluation of three assays for the detection of PR3-ANCA in granulomatosis with polyangiitis in daily practice. Autoimmun Rev. 2013;12:1118–1122. doi: 10.1016/j.autrev.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Mukhtyar C, Hellmich B, Jayne D, Flossmann O, Luqmani R. Remission in antineutrophil cytoplasmic antibody-associated systemic vasculitis. Clin Exp Rheumatol 2006;24:S-93-8. [PubMed]
- 20.Park JJ, Jang HJ, Oh IY, Yoon CH, Suh JW, Cho YS, et al. Prognostic value of neutrophil to lymphocyte ratio in patients presenting with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2013;111:636–642. doi: 10.1016/j.amjcard.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Yoo J, Kim HJ, Jung SM, Song JJ, Park YB, Lee SW. Birmingham vasculitis activity score of more than 9.5 at diagnosis is an independent predictor of refractory disease in granulomatosis with polyangiitis. Int J Rheum Dis. 2017;20:1593–1605. doi: 10.1111/1756-185X.13144. [DOI] [PubMed] [Google Scholar]
- 22.Oh YJ, Ahn SS, Park ES, Jung SM, Song JJ, Park YB, et al. Chest and renal involvements, Birmingham vascular activity score more than 13.5 and five factor score (1996) more than 1 at diagnosis are significant predictors of relapse of microscopic polyangiitis. Clin Exp Rheumatol. 2017;35:47–54. [PubMed] [Google Scholar]
- 23.Bhat TM, Afari ME, Garcia LA. Neutrophil lymphocyte ratio in peripheral vascular disease: a review. Expert Rev Cardiovasc Ther. 2016;14:871–875. doi: 10.1586/14779072.2016.1165091. [DOI] [PubMed] [Google Scholar]
- 24.Balta S, Celik T, Mikhailidis DP, Ozturk C, Demirkol S, Aparci M, et al. The relation between atherosclerosis and the neutrophil-lymphocyte ratio. Clin Appl Thromb Hemost. 2016;22:405–411. doi: 10.1177/1076029615569568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used and analysed in this study are available from the corresponding author on reasonable request.