Abstract
The cerebellum plays major role in motor coordination and learning. It contains half of the neurons in the brain. Thus, deciphering the mechanisms by which cerebellar neurons are generated is essential to understand the cerebellar functions and the pathologies associated with it. In a recent study, Wojcinski et al. (2017) by using in vivo Cre/loxP technologies reveal that Nestin-expressing progenitors repopulated the external granular cell layer after injury. Depletion of postnatal external granular cell layer is not sufficient to induce motor behavior defects in adults, as the cerebellum recovers these neurons. Strikingly, Nestin-expressing progenitors differentiate into granule cell precursors and mature granule neurons after ablation of perinatal external granular layer, either by irradiation or by genetic ablation. This work identified a novel role of Nestin-expressing progenitors in the cerebellar microenvironment during development, and revealed that extracellular signals can convert specified progenitors into multipotent stem cells. Here, we discuss the findings from this study, and evaluate recent advances in our understanding of the cerebellar neurogenesis.
Keywords: Nestin, cerebellum, neurogenesis, progenitors
INTRODUCTION
The cerebellum, anatomically, has a singular geometric arrangement. It comprises an extensive folded format consisting of two cerebellar hemispheres, separated from the cerebrum by the dura mater layer. The cerebellum is united by a central component called the vermis situated in the posterior cranial fossa, lying inferior to the occipital lobes and dorsal to the brainstem (Schmahmann, 1996). Embryonically, it derives from the rhombomere 1, and it is located at the anterior end of the hindbrain (Buckner, 2013). Interestingly, in mammals, the cerebellum encloses most of the mature neurons in the adult brain (Wingate and Hatten, 1999). The cerebellum plays key roles in motor coordination, and it is implicated in cognition, learning, emotion, and behavior as well as in several neurological disorders associated with the impairment of both motor and non-motor functions, such as verbal memory impairments, cerebellar ataxia, dystonia, autism, and visuospatial dysfunctions (Reeber et al., 2013). Thus, elucidating the mechanisms involved in cerebellar formation is critical to understand its roles and the pathologies with which it is associated.
The neural cells that form the cerebellum originate in a sequential way during the embryonic and postnatal development from two different primary germinal areas, the ventricular zone and the rhombic lip (Millen and Gleeson, 2008). During mid-gestation, a group of cells from the ventricular zone migrate to the rhombic lip, where they become committed to the neural lineage (Alder et al., 1999). These cells form the external granule layer of the cerebellar primordia, becoming precursors of the cerebellar granule neurons (Machold and Fishell, 2005). After this, these cells migrate to the internal granule layer, differentiating into mature granule neurons (Rakic, 1971). Interestingly, the external granular layer can be reconstituted after its ablation by irradiation (Altman et al., 1969). Nevertheless, the cellular mechanisms responsible for the replenishment of the external granular layer remain unknown. Now, in an article in Nature Neuroscience, Wojcinski and colleagues reveal that cerebellar Nestin-expressing progenitors, which during normal development only form interneurons and astroglia, extend their differentiation capacity, and originate mature granule neurons after injury (Wojcinski et al., 2017). The authors investigated the role of Nestin-expressing progenitors in the recovery of perinatal external granular layer after depletion by using in vivo lineage-tracing Cre/loxP technologies combined with live imaging. Wojcinski and colleagues, by tracking the fate of Nestin-expressing cells, show that cerebellar Nestin-expressing progenitors do not give rise to granule cell precursors or mature granule neurons in normal conditions in vivo. Interestingly, the authors found that Nestin-expressing progenitors self-renew and have multipotent potential in vitro, suggesting that in special conditions Nestin-expressing progenitors may expand their differentiation ability. Indeed, after ablation of perinatal external granular layer, either by irradiation or by genetic ablation, Nestin-expressing progenitors differentiate into granule cell precursors and mature granule neurons (Wojcinski et al., 2017). Importantly, by tracking the migration of individual cells in Nestin-CFP mice, the authors observed Nestin-expressing progenitors actively re-populating the external granule layer after injury. Moreover, Wojcinski and colleagues demonstrated, by genetic deletion of Smoothened in Nestin-expressing cells, that sonic hedgehog signaling was essential for external granule layer repopulation post-irradiation.
Here, we discuss the findings from this work, and evaluate recent advances in our understanding of the cerebellar neurogenesis (Figure 1).
Figure 1. Cerebellar Nestin-expressing progenitors replenish the external granular cell layer after injury.
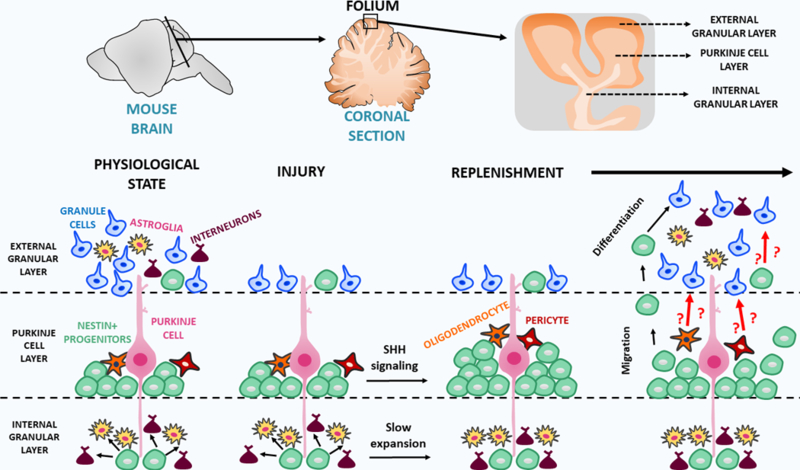
The neural cells that form the cerebellum originate in a sequential way during the embryonic and postnatal development. The study of Wojcinski and colleagues now reveals that cerebellar Nestin-expressing progenitors in the Purkinje cell layer, which during normal development only form interneurons and astroglia, extend their differentiation capacity, and originate mature granule neurons after injury (Wojcinski et al., 2017).
PERSPECTIVES / FUTURE DIRECTIONS
The main findings from this study are based on the data obtained from Nestin-FlpoER mice (Wojcinski et al., 2017). These transgenic mice were designed using a construct with the second intron of the nestin gene, which is known to drive expression in different cell types in various tissues (Birbrair et al., 2011). Therefore, the exact identity of Nestin-expressing progenitors that repopulate the external granule cell layer post-injury remains to be elucidated. Even though Wojcinski and colleagues show that Nestin-expressing progenitors residing in the Purkinje cell layer participate in the repopulation of the external granule cell layer post-injury, they also mention that rare Nestin-expressing progenitors located at the external granular cell layer itself may contribute with this repopulation (Wojcinski et al., 2017). Quiescent Nestin-expressing progenitors residing in the deep part of the external granular cell layer have been recently characterized to be the cells of origin for medulloblastoma (Li et al., 2013). Several other cells in the central nervous system with capacity to differentiate into the neural lineage are targeted in those mice as well, including quiescent neural stem cells (Encinas et al., 2011), amplifying neural stem cells (Encinas et al., 2011), oligodendrocyte progenitors (Rafalski et al., 2013), and pericytes (Birbrair et al., 2014). As some of these cells are not present in the cerebellum, are central nervous system cells from outside the cerebellum able to move to the cerebellar external granule cell layer, and assist in its repopulation after irradiation? If yes, what is the percentage of the newly formed granule cell precursors and mature granule neurons derived from these cells?
Interestingly, the function of cerebellar Nestin-expressing progenitors could be confounded with pericytes and oligodendrocyte progenitors, as both may express Nestin (Birbrair et al., 2013a; Encinas et al., 2011; Walker et al., 2010). Pericytes are fundamental in central nervous system physiology both in health and disease (Azevedo et al., 2017; Birbrair et al., 2015). They stabilize blood vessels (Costa et al., 2018), regulate the blood flow (Almeida et al., 2017; Pallone et al., 2003), and collaborate with other cells to regulate the maintenance of functional integrity of the blood-brain barrier (Santos et al., 2017). Importantly, pericytes have the capacity to behave as progenitors (Birbrair et al., 2017), forming neural cell types (Birbrair et al., 2013b). Pericytes reside around the cerebellar blood vessels (Peppiatt et al., 2006). Whether they have capacity to form granule cells or other neuronal types during development or in specific pathological conditions remains completely unknown, and should be explored in future studies. Crossing pericyte-specific inducible CreER drivers with inducible reporter lines such as TdTomato (Asada et al., 2017; Khan et al., 2016), will allow to specifically track the fate of pericytes in the cerebellum at different stages. In addition to studying genetic mouse models, transcriptomic and single pericytes analyses represent fundamental tools that will help us understand the roles of pericytes within the cerebellum.
Oligodendrocyte progenitors are highly proliferative (Clarke et al., 2012), can self-renew, and give rise to several cell types from the neural lineage (Kondo and Raff, 2004; Noble et al., 2003), including functional neurons (Belachew et al., 2003). Whether oligodendrocyte progenitors are able to originate cerebellar neurons during development or in disease settings also remains unexplored. Crossing oligodendrocyte prongeitor-specific inducible CreER drivers, such as Plp1CreER (Guo et al., 2010), with inducible reporter lines such as TdTomato (Madisen et al., 2010), will allow for the fate mapping of cerebellar oligodendrocyte progenitors at different stages. So far, whether cerebellar pericytes and oligodendrocyte progenitors contribute to the repopulation of external granule cell layer in physiologic or pathologic conditions, and if so to what extent remains unknown. Importantly, Wojcinski and colleagues show that Nestin-expressing progenitors from the internal granular layer, in contrast to what happens in the Purkinje cell layer, decrease their proliferation (Wojcinski et al., 2017). Future studies should also explore the behavior post-injury of oligodendrocyte progenitors which are abundant in this region.
Wojcinski and colleagues reveal an unexpected plasticity of cerebellar Nestin-expressing progenitors after postnatal injury in vivo (Wojcinski et al., 2017). These findings suggest that initial specification can be reversed in the postnatal life under special conditions. Whether this can also occur in adult cerebellum after injury remains unknown. Wojcinski and colleagues observed an increase in the number of Nestin-expressing cells in the adult Purkinje cell layer after ablation of granule cell precursors. Nevertheless, these cells did not proliferate or produce granule cells (Wojcinski et al., 2017). Future studies need to explore in more detail adult neurogenesis in the cerebellum upon injury. Adult neurogenesis in the brain was described in the subventricular zone of the lateral ventricle and in the subgranular zone of the dentate gyrus in the hippocampus (Ming and Song, 2005). Interestingly, in the adult cerebellum, a cell population has been described with capacity to give rise to neuronal progeny in vitro and after transplantation (Klein et al., 2005; Lee et al., 2005). However, artificial environment that characterize cell culture systems may induce progenitor ability in these cells that not necessarily is shared by the corresponding endogenous cerebellar cells under physiological conditions in vivo (Snippert and Clevers, 2011). Further investigation will be needed to understand whether neurogenesis may occur in the adult cerebellum and under which conditions.
Precise control over progenitor differentiation is essential for organogenesis and tissue homeostasis in different organs (Lousado et al., 2017). Progenitors reside in specialized microenvironments, also called niches, which maintain them in an undifferentiated and self-renewing state (Andreotti et al., 2017). Understanding the signaling mechanisms by which the niche controls the progenitors fate is crucial for the success of clinical applications (Birbrair, 2017). Dissecting the complex pathways leading to progenitor activation may be very challenging. Wojcinski and colleagues show that Smoothened expressed in Nestin-expressing progenitors is essential for the repopulation of the perinatal external granular layer after post-irradiation (Wojcinski et al., 2017). Smoothened is a 7-transmembrane protein regulated by the 12-transmembrane protein Patched1 and by secreted Sonic hedgehog glycoproteins (Varjosalo and Taipale, 2008). Although it was suggested that Purkinje cells are an important source of Sonic hedgehog (Lewis et al., 2004), it remains to be explored whether they are the main/only functionally important source. Therefore, future studies should explore which cells in the cerebellar microenvironment provide the functionally important signals for the Sonic hedgehog signaling. This could be done by analyzing specific Sonic hedgehog gene deletion from various cell types in the cerebellar microenvironment. Wojcinski and colleagues proposed an interesting hypothesis that Purkinje cells may regulate Sonic hedgehog signaling via sensing the depletion in the external granular cell layer due to the altered excitatory input produced by the lack of newly produced granule cells (Wojcinski et al., 2017). This hypothesis can be tested taking advantage of optogenetic tools (Bao et al., 2017), by which we could alter excitatory input without altering the cell number.
The generation of granule cell precursors and mature granule neurons in the external granule cell layer can be subjected to diverse signals, including epigenetic phenomena or the natural environmental influences thought to contribute to several neurodevelopmental disorders, such as medulloblastoma formation. Whatever the case, much work remains to be done to increase our knowledge on the neurodevelopmental regulation of cerebellar neurogenesis. Defining and understanding the mechanisms that restrict niche signaling exclusively to progenitors is crucial to determine how these cells undergo self-renewal while their progeny differentiate (Birbrair, 2017). Although significant progress has been made towards understanding the differentiation capacity of distinct progenitors in the cerebellum, the components of the cerebellar progenitors’ microenvironments remain unknown due to the probable complexity of niche composition and its dynamics. Further insights into the cellular and molecular mechanisms that regulate cerebellar neurogenesis at distinct stages of development will have important implications for our understanding of cerebellar homeostasis and disease.
In conclusion, the study by Wojcinski and colleagues report a novel role of Nestin-expressing progenitors in the cerebellar microenvironment during development, and reveal that extracellular signals can convert specified progenitors into multipotent stem cells. However, our understanding of cerebellar biology still remains limited, and the complexity and interactions of different cellular components and molecules of the microenvironment in the cerebellum in health and disease should be elucidated in future works.
ACKNOWLEDGMENTS
Alexander Birbrair is supported by a grant from Instituto Serrapilheira/Serra-1708–15285, a grant from Pró-reitoria de Pesquisa/Universidade Federal de Minas Gerais (PRPq/UFMG) (Edital 05/2016); a grant from FAPEMIG [Rede Mineira de Engenharia de Tecidos e Terapia Celular (REMETTEC, RED-00570–16)], and a grant from FAPEMIG [Rede De Pesquisa Em Doenças Infecciosas Humanas E Animais Do Estado De Minas Gerais (RED-00313–16)]; Akiva Mintz is supported by the National Institute of Health (1R01CA179072–01A1) and by the American Cancer Society Mentored Research Scholar grant (124443-MRSG-13–121-01-CDD).
Footnotes
DISCLOSURES:
The authors indicate no potential conflicts of interest.
REFERENCES
- Alder J, Lee KJ, Jessell TM, Hatten ME (1999) Generation of cerebellar granule neurons in vivo by transplantation of BMP-treated neural progenitor cells. Nat Neurosci 2, 535–540. [DOI] [PubMed] [Google Scholar]
- Almeida VM, Paiva AE, Sena IFG, Mintz A, Magno LAV, Birbrair A (2017) Pericytes Make Spinal Cord Breathless after Injury. Neuroscientist [DOI] [PMC free article] [PubMed]
- Altman J, Anderson WJ, Wright KA (1969) Early effects of x-irradiation of the cerebellum in infant rats: decimation and reconstitution of the external granular layer. Exp Neurol 24, 196–216. [DOI] [PubMed] [Google Scholar]
- Andreotti JP, Lousado L, Magno LAV, Birbrair A (2017) Hypothalamic Neurons Take Center Stage in the Neural Stem Cell Niche. Cell Stem Cell 21, 293–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada N, Kunisaki Y, Pierce H, Wang Z, Fernandez NF, Birbrair A, Ma’ayan A, Frenette PS (2017) Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol 19, 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo PO, Sena IFG, Andreotti JP, Carvalho-Tavares J, Alves-Filho JC, Cunha TM, Cunha FQ, Mintz A, Birbrair A (2017) Pericytes modulate myelination in the central nervous system. J Cell Physiol [DOI] [PMC free article] [PubMed]
- Bao H, Asrican B, Li W, Gu B, Wen Z, Lim SA, Haniff I, Ramakrishnan C, Deisseroth K, Philpot B, Song J (2017) Long-Range GABAergic Inputs Regulate Neural Stem Cell Quiescence and Control Adult Hippocampal Neurogenesis. Cell Stem Cell 21, 604–617 e605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V (2003) Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol 161, 169–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A (2017) Stem Cell Microenvironments and Beyond. Adv Exp Med Biol 1041, 1–3. [DOI] [PubMed] [Google Scholar]
- Birbrair A, Borges IDT, Gilson Sena IF, Almeida GG, da Silva Meirelles L, Goncalves R, Mintz A, Delbono O (2017) How Plastic Are Pericytes? Stem Cells Dev 26, 1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Wang ZM, Messi ML, Enikolopov GN, Delbono O (2011) Nestin-GFP transgene reveals neural precursor cells in adult skeletal muscle. PLoS One 6, e16816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O (2013a) Skeletal muscle neural progenitor cells exhibit properties of NG2-glia. Exp Cell Res 319, 45–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O (2013b) Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Res 10, 67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O (2015) Pericytes at the intersection between tissue regeneration and pathology. Clin Sci (Lond) 128, 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Olson JD, Mintz A, Delbono O (2014) Type-2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol Cell Physiol 307, C25‘38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL (2013) The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 80, 807–815. [DOI] [PubMed] [Google Scholar]
- Clarke LE, Young KM, Hamilton NB, Li H, Richardson WD, Attwell D (2012) Properties and fate of oligodendrocyte progenitor cells in the corpus callosum, motor cortex, and piriform cortex of the mouse. J Neurosci 32, 8173–8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa MA, Paiva AE, Andreotti JP, Cardoso MV, Cardoso CD, Mintz A, Birbrair A (2018) Pericytes constrict blood vessels after myocardial ischemia. J Mol Cell Cardiol [DOI] [PMC free article] [PubMed]
- Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G (2011) Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 8, 566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Maeda Y, Ma J, Xu J, Horiuchi M, Miers L, Vaccarino F, Pleasure D (2010) Pyramidal neurons are generated from oligodendroglial progenitor cells in adult piriform cortex. J Neurosci 30, 12036–12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan JA, Mendelson A, Kunisaki Y, Birbrair A, Kou Y, Arnal-Estape A, Pinho S, Ciero P, Nakahara F, Ma’ayan A, Bergman A, Merad M, Frenette PS (2016) Fetal liver hematopoietic stem cell niches associate with portal vessels. Science 351, 176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Butt SJ, Machold RP, Johnson JE, Fishell G (2005) Cerebellum- and forebrain-derived stem cells possess intrinsic regional character. Development 132, 4497–4508. [DOI] [PubMed] [Google Scholar]
- Kondo T, Raff M (2004) Chromatin remodeling and histone modification in the conversion of oligodendrocyte precursors to neural stem cells. Genes Dev 18, 2963–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Kessler JD, Read TA, Kaiser C, Corbeil D, Huttner WB, Johnson JE, Wechsler-Reya RJ (2005) Isolation of neural stem cells from the postnatal cerebellum. Nat Neurosci 8, 723–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PM, Gritli-Linde A, Smeyne R, Kottmann A, McMahon AP (2004) Sonic hedgehog signaling is required for expansion of granule neuron precursors and patterning of the mouse cerebellum. Dev Biol 270, 393–410. [DOI] [PubMed] [Google Scholar]
- Li P, Du F, Yuelling LW, Lin T, Muradimova RE, Tricarico R, Wang J, Enikolopov G, Bellacosa A, Wechsler-Reya RJ, Yang ZJ (2013) A population of Nestin-expressing progenitors in the cerebellum exhibits increased tumorigenicity. Nat Neurosci 16, 1737–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lousado L, Prazeres P, Andreotti JP, Paiva AE, Azevedo PO, Santos GSP, Filev R, Mintz A, Birbrair A (2017) Schwann cell precursors as a source for adrenal gland chromaffin cells. Cell Death Dis 8, e3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machold R, Fishell G (2005) Math1 is expressed in temporally discrete pools of cerebellar rhombic-lip neural progenitors. Neuron 48, 17–24. [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H (2010) A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13, 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millen KJ, Gleeson JG (2008) Cerebellar development and disease. Curr Opin Neurobiol 18, 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H (2005) Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 28, 223–250. [DOI] [PubMed] [Google Scholar]
- Noble M, Arhin A, Gass D, Mayer-Proschel M (2003) The cortical ancestry of oligodendrocytes: common principles and novel features. Dev Neurosci 25, 217–233. [DOI] [PubMed] [Google Scholar]
- Pallone TL, Zhang Z, Rhinehart K (2003) Physiology of the renal medullary microcirculation. Am J Physiol Renal Physiol 284, F253–266. [DOI] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D (2006) Bidirectional control of CNS capillary diameter by pericytes. Nature 443, 700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafalski VA, Ho PP, Brett JO, Ucar D, Dugas JC, Pollina EA, Chow LM, Ibrahim A, Baker SJ, Barres BA, Steinman L, Brunet A (2013) Expansion of oligodendrocyte progenitor cells following SIRT1 inactivation in the adult brain. Nat Cell Biol 15, 614–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P (1971) Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electronmicroscopic study in Macacus Rhesus. J Comp Neurol 141, 283–312. [DOI] [PubMed] [Google Scholar]
- Reeber SL, Otis TS, Sillitoe RV (2013) New roles for the cerebellum in health and disease. Front Syst Neurosci 7, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos GSP, Prazeres P, Mintz A, Birbrair A (2017) Role of pericytes in the retina. Eye (Lond: ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD (1996) From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp 4, 174–198. [DOI] [PubMed] [Google Scholar]
- Snippert HJ, Clevers H (2011) Tracking adult stem cells. EMBO Rep 12, 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varjosalo M, Taipale J (2008) Hedgehog: functions and mechanisms. Genes Dev 22, 2454–2472. [DOI] [PubMed] [Google Scholar]
- Walker AS, Goings GE, Kim Y, Miller RJ, Chenn A, Szele FG (2010) Nestin reporter transgene labels multiple central nervous system precursor cells. Neural Plast 2010, 894374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingate RJ, Hatten ME (1999) The role of the rhombic lip in avian cerebellum development. Development 126, 4395–4404. [DOI] [PubMed] [Google Scholar]
- Wojcinski A, Lawton AK, Bayin NS, Lao Z, Stephen DN, Joyner AL (2017) Cerebellar granule cell replenishment postinjury by adaptive reprogramming of Nestin(+) progenitors. Nat Neurosci 20, 1361–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]


