Abstract
Background
Multiple cases of Candida auris infection have been reported with high mortality rates owing to its MDR nature. Rezafungin (previously CD101) is a novel echinocandin with enhanced stability and pharmacokinetics that achieves high plasma drug exposure and allows for once weekly dose administration.
Objectives
Evaluate the efficacy of rezafungin in the treatment of disseminated C. auris infection using a mouse model of disseminated candidiasis.
Methods
Mice were immunosuppressed 3 days prior to infection and 1 day post-infection. On the day of infection, mice were inoculated with 3 × 107C. auris blastospores via the tail vein. Mice were randomized into four groups (n = 20): rezafungin at 20 mg/kg, amphotericin B at 0.3 mg/kg, micafungin at 5 mg/kg and a vehicle control. Treatments were administered 2 h post-infection. Rezafungin was given additionally on days 3 and 6 for a total of three doses, while the remaining groups were treated every day for a total of seven doses. Five mice from each group were sacrificed on days 1, 4, 7 and 10 of the study. Kidneys were removed from each mouse to determine the number of cfu for each respective day.
Results
Rezafungin had significantly lower average log10 cfu/g of tissue compared with amphotericin B- and vehicle-treated mice on all days when kidneys were harvested. Additionally, rezafungin-treated mice had significantly lower average log10 cfu/g of tissue compared with micafungin-treated mice on day 10.
Conclusions
Our findings show that rezafungin possesses potent antifungal activity against C. auris in a disseminated model of candidiasis.
Introduction
The emergence of drug-resistant pathogens is an ever-growing concern. Originally reported in 2009,1Candida auris causes serious invasive infections with mortality rates approaching ∼60%.2 Reports of invasive infections caused by C. auris have emerged globally, including in Japan, South Korea, India, Kuwait, South Africa, Pakistan, the UK and, more recently, in Venezuela, Colombia and the USA.3–6 The majority of C. auris infections have been reported as secondary nosocomial infections.3,6,7 A high percentage of clinical strains of C. auris exhibit resistance to fluconazole and there are varying levels of resistance to the three major classes of currently available antifungals (azoles, polyenes and echinocandins), thus limiting treatment options.1,3–10
Given the MDR nature of C. auris, development of new antifungal drugs that can combat this resistance issue is critical.
The echinocandin class of drugs is used to treat serious invasive fungal infections and they are recommended for the first-line treatment of suspected or confirmed invasive Candida infections.11 Three echinocandins (caspofungin, micafungin and anidulafungin) are currently approved for the treatment of candidaemia, as well as other types of invasive candidiasis, by the US FDA. Although resistance to fluconazole and amphotericin B have been widely reported for different isolates of C. auris, only a limited number of strains of this emerging species have shown elevated MICs to currently available echinocandins. Rezafungin (previously CD101), a novel echinocandin with an extended t½ and high plasma drug exposure that may help counter resistance,12,13 is in development as a once weekly intravenous formulation for the treatment and prevention of systemic fungal infections.14 Although its pharmacokinetic profile is a major distinguishing characteristic, rezafungin was designed to first be chemically and metabolically stable to avoid hepatotoxicity while retaining the antifungal activity of an echinocandin.15 In our recent evaluation of antifungal susceptibility of a panel of C. auris isolates, in which 8 out of 16 isolates possessed susceptibility profiles that for other Candida spp. would be characterized as resistant to at least three of the antifungal agents tested, rezafungin demonstrated potent in vitro activity against both echinocandin-resistant and -susceptible C. auris isolates.16,17
Herein, we evaluated the efficacy of rezafungin in the treatment of C. auris infection, utilizing a mouse model of disseminated candidiasis. Specifically, the temporal effect of rezafungin on kidney tissue fungal burden was evaluated.
Materials and methods
Ethics
All procedures in this study were in compliance with the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, and the Office of Laboratory Animal Welfare.
Test compounds
The following antifungals were evaluated: rezafungin, micafungin and amphotericin B. Rezafungin and micafungin were provided by Cidara Therapeutics, Inc. Amphotericin B was purchased from Sigma–Aldrich (St Louis, MO, USA).
Organism and inoculum preparation
A clinical isolate of C. auris (MRL 35368) was obtained from the Center for Medical Mycology Culture Collection and used as the infecting fungus. The MIC values (determined using the CLSI broth microdilution method, as described in the M27-A3 document) of rezafungin, amphotericin B and micafungin were 0.063, 4 and 1 mg/L, respectively. C. auris inoculum was prepared by plating onto potato dextrose agar (PDA) (Becton Dickinson, Sparks, MD, USA) and incubating at 35°C for 2 days. Next, C. auris blastospores were harvested by centrifugation followed by three washes with PBS. A challenge inoculum of 3 × 107 was prepared using a haemocytometer.
Immunosuppression
Female, 6–8-week-old, CD-1 mice received cyclophosphamide (Sigma–Aldrich), 200 mg/kg administered by intraperitoneal injection 3 days prior to challenge and 150 mg/kg 1 day after challenge. On the day of infection, blood was collected (20 μL) from one mouse from each group for a white blood cell count to verify immunosuppression.
Infection and evaluation of treatment efficacy
To evaluate the temporal effect of rezafungin on tissue fungal burden, immunocompromised mice (n = 20 mice per group) were infected with 3 × 107C. auris blastospores in 100 μL of PBS via the lateral tail vein. Beginning 2 h post-infection, mice in the rezafungin group were given one dose (day 1) then subsequently treated on days 3 and 6 for a total of three doses. Additionally, mice in the remaining treatment groups (amphotericin B, micafungin and vehicle) were given one dose 2 h post-infection (day 1), then treated every day for a total of seven doses. Five mice from each group were sacrificed on days 1, 4, 7 and 10 of the study to determine the tissue fungal burden in the kidneys. Briefly, both kidneys from each animal were aseptically removed and weighed. Tissues were then homogenized in 1 mL of PBS and serially diluted. The dilutions were plated onto PDA and cultured at 35°C for 48 h to determine the number of cfu. Efficacy of rezafungin was evaluated as a reduction in log10 cfu compared with all other groups.
Statistical analysis
Differences in the mean cfu in the kidneys were compared with the control and different treatment groups using a one-way ANOVA with a post-hoc Tukey test. A P value of <0.05 was considered statistically significant.
Results
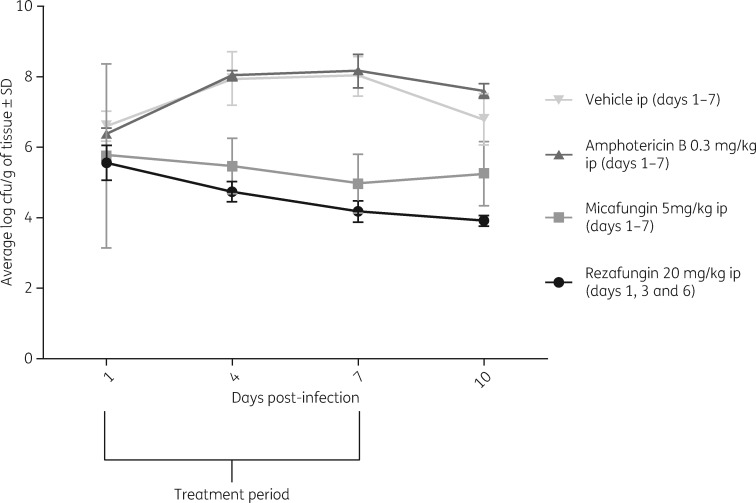
Figure 1 shows the average log10 cfu/g of tissue for mice by treatment group on the respective sacrifice day (days 1, 4, 7 and 10 post-infection). As shown in Figure 1, mice treated with rezafungin had significantly lower average log10 cfu compared with amphotericin B- and vehicle-treated mice on all days when kidneys were harvested (Table 1). Additionally, rezafungin-treated mice had significantly lower average log10 cfu compared with the micafungin-treated group on day 10 only.
Figure 1.
Average log cfu/g (±SD) in kidney tissue on days 1, 4, 7 and 10. ip, intraperitoneally.
Table 1.
Statistical summary of between-group comparisonsa of average log cfu/g of tissue
| Comparison |
Mean difference | 95% CI | Adjusted P value | |
|---|---|---|---|---|
| group A | group B | |||
| 1 day post-infection | ||||
| rezafungin 20 mg/kg ip | micafungin 5 mg/kg ip | −0.20 | −0.99 to 0.59 | 0.8801 |
| rezafungin 20 mg/kg ip | amphotericin B 0.3 mg/kg ip | −0.85 | −1.59 to −0.10 | 0.0231b |
| rezafungin 20 mg/kg ip | vehicle ip | −1.04 | −1.79 to −0.30 | 0.0052b |
| 4 days post-infection | ||||
| rezafungin 20 mg/kg ip | micafungin 5 mg/kg ip | −0.72 | −1.75 to 0.32 | 0.2339 |
| rezafungin 20 mg/kg ip | amphotericin B 0.3 mg/kg ip | −3.30 | −4.34 to −2.27 | <0.0001b |
| rezafungin 20 mg/kg ip | vehicle ip | −3.21 | −4.24 to −2.17 | <0.0001b |
| 7 days post-infection | ||||
| rezafungin 20 mg/kg ip | micafungin 5 mg/kg ip | −0.78 | −1.83 to 0.27 | 0.1836 |
| rezafungin 20 mg/kg ip | amphotericin B 0.3 mg/kg ip | −3.99 | −5.04 to −2.94 | <0.0001b |
| rezafungin 20 mg/kg ip | vehicle ip | −3.85 | −4.90 to −2.80 | <0.0001b |
| 10 days post-infection | ||||
| rezafungin 20 mg/kg ip | micafungin 5 mg/kg ip | −1.34 | −2.41 to −0.26 | 0.0128b |
| rezafungin 20 mg/kg ip | amphotericin B 0.3 mg/kg ip | −3.69 | −4.76 to −2.61 | <0.0001b |
| rezafungin 20 mg/kg ip | vehicle ip | −2.89 | −3.96 to −1.81 | <0.0001b |
ip, intraperitoneally.
Tukey’s multiple comparisons test.
Statistically significant results.
Discussion
In this study, we examined the temporal effect of rezafungin compared with amphotericin B and micafungin dosed daily for 7 days, while rezafungin was administered three times (days 1, 3 and 6). Although dosed less frequently than amphotericin B and micafungin, rezafungin was significantly more efficacious in reducing fungal burden. The demonstrated superior activity of rezafungin compared with the other agents, even with less frequent dosing, reflects the clinical potential of rezafungin over current daily dosing regimens of approved echinocandins and can be attributed to its pharmacokinetic/pharmacodynamic profile. As seen with other antimicrobials that exhibit concentration-dependent killing and a prolonged t½ (e.g. oritavancin), front-loaded dosing benefits rezafungin efficacy. Lakota et al.18 (2017) demonstrated that a single dose of rezafungin achieved efficacious drug exposures and greater efficacy than once daily and twice weekly regimens in a neutropenic mouse model of disseminated candidiasis. The time course of rezafungin concentrations (i.e. the shape of the AUC) was shown to be a determinant of its efficacy, which relates to the importance of achieving therapeutic drug exposure early in the course of therapy. The pharmacokinetics/pharmacodynamics of rezafungin may also play a role against resistance development, as suggested by the mutant prevention concentration (MPC) concept which calls for antifungal dosing above the MPC to inhibit growth of potentially resistant isolates.15
In our recent evaluation of the antifungal susceptibility of C. auris, 16 clinical isolates were tested against rezafungin and comparators, including currently available echinocandins, azole antifungals and amphotericin B. Eight of the 16 isolates possessed susceptibility profiles that for other Candida spp. would be characterized as resistant to at least three of the antifungal agents tested. These in vitro data showed that rezafungin possesses potent antifungal activity against a large panel of C. auris strains, including isolates that are resistant to other antifungal drugs, such as fluconazole and amphotericin B.17 Moreover, this study demonstrates that rezafungin possesses potent in vivo efficacy against C. auris. Further studies evaluating the efficacy of this novel antifungal against different strains of C. auris is recommended to rule out strain-specific findings.
Our findings are in agreement with recent studies that showed rezafungin possesses potent in vivo activity against Candida albicans and Aspergillus fumigatus in neutropenic mouse models of disseminated infection.15,19,20 These results collectively indicate the broad-spectrum in vivo activity of rezafungin against multiple fungal species and support further development of rezafungin for the treatment of C. auris infections.
Funding
This work was supported by Cidara Therapeutics, Inc.
Transparency declarations
M. A. G. has served as a consultant for Cidara Therapeutics, Inc. All other authors: none to declare.
References
- 1. CDC. Global Emergence of Invasive Infections Caused by the Multidrug-Resistant Yeast Candida auris. Atlanta, GA, USA: CDC, 2016. https://www.cdc.gov/fungal/diseases/candidiasis/candida-auris-alert.html. [Google Scholar]
- 2. Lee WG, Shin JH, Uh Y. et al. First three reported cases of nosocomial fungemia caused by Candida auris. J Clin Microbiol 2011; 49: 3139–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calvo B, Melo AS, Perozo-Mena A. et al. First report of Candida auris in America: clinical and microbiological aspects of 18 episodes of candidemia. J Infect 2016; 73: 369–74. [DOI] [PubMed] [Google Scholar]
- 4. Morales-López SE, Parra-Giraldo CM, Ceballos-Garzón A. et al. Invasive infections with multidrug-resistant yeast Candida auris, Colombia. Emerg Infect Dis 2016; 23: 162–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schelenz S, Hagen F, Rhodes JL. et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control 2016; 5: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vallabhaneni S, Kallen A, Tsay S. et al. Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug-resistant fungus—United States, May 2013–August 2016. Am J Transplant 2016; 17: 296–9. [DOI] [PubMed] [Google Scholar]
- 7. Chowdhary A, Anil Kumar V, Sharma C. et al. Multidrug-resistant endemic clonal strain of Candida auris in India. Eur J Clin Microbiol Infect Dis 2014; 33: 919–26. [DOI] [PubMed] [Google Scholar]
- 8. Khillan V, Rathore N, Kathuria S. et al. A rare case of breakthrough fungal pericarditis due to fluconazole-resistant Candida auris in a patient with chronic liver disease. JMM Case Rep 2014; doi:10.1099/jmmcr.0.T00018. [Google Scholar]
- 9. Kim MN, Shin JH, Sung H. et al. Candida haemulonii and closely related species at 5 university hospitals in Korea: identification, antifungal susceptibility, and clinical features. Clin Infect Dis 2009; 48: e57–61. [DOI] [PubMed] [Google Scholar]
- 10. Chakrabarti A, Sood P, Rudramurthy SM. et al. Incidence, characteristics and outcome of ICU-acquired candidemia in India. Intensive Care Med 2015; 41: 285–95. [DOI] [PubMed] [Google Scholar]
- 11. Pappas PG, Kauffman CA, Andes DR. et al. Executive summary: clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62: 409–17. [DOI] [PubMed] [Google Scholar]
- 12. Zhao Y, Perez WB, Jiménez-Ortigosa C. et al. CD101: a novel long-acting echinocandin. Cell Microbiol 2016; 18: 1308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lepak AJ, Zhao M, Vanscoy B. et al. In vivo pharmacokinetic/pharmacodynamic (PK/PD) target characterization of the novel, long acting echinocandin CD101 against C. albicans and C. glabrata in the neutropenic murine disseminated candidiasis model In: Abstracts of IDWeek 2017, San Diego, CA, USA. Abstract 1528. [Google Scholar]
- 14. Sandison T, Ong V, Lee J. et al. Safety and pharmacokinetics of CD101 IV, a novel echinocandin, in healthy adults. Antimicrob Agents Chemother 2017; 61: e01627–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krishnan BR, James KD, Polowy K. et al. CD101, a novel echinocandin with exceptional stability properties and enhanced aqueous solubility. J Antibiot (Tokyo) 2017; 70: 130–5. [DOI] [PubMed] [Google Scholar]
- 16. James KD, Laudeman CP, Malkar NB. et al. Structure-activity relationships of a series of echinocandins and the discovery of CD101, a highly stable and soluble echinocandin with distinctive pharmacokinetic properties. Antimicrob Agents Chemother 2017; 61: e01541–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Larkin E, Long L, Ghannoum MA.. Susceptibility of recent Candida auris isolates to the novel echinocandin CD101 and comparator antifungal agents In: Abstracts of the Twenty-seventh European Congress of Clinical Microbiology and Infectious Diseases, Vienna, Austria, 2017. Abstract 9037. ESCMID, Basel, Switzerland. [Google Scholar]
- 18. Lakota E, Bader J, Ong V. et al. Pharmacological basis of CD101 efficacy: exposure shape matters. Antimicrob Agents Chemother 2017; 71: e00758-17.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miesel L, Lin K-Y, Chien JC. et al. Efficacy of a novel echinocandin, CD101, in a mouse model of azole-resistant disseminated candidiasis In: Abstracts of ASM Microbe, Boston, MA, USA, 2016. [Google Scholar]
- 20. Miesel L, Huang H-H, You N-T. et al. Intravenous efficacy of a novel echinocandin, CD101, in neutropenic mouse models of disseminated candidiasis and aspergillosis In: Abstracts of the Fifty-fifth Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, CA, USA, 2015. American Society for Microbiology, Washington, DC, USA. [Google Scholar]