Abstract
Background
The glutamatergic modulator ketamine has rapid antidepressant effects in individuals with major depressive disorder and bipolar depression. Thus, modulating glutamatergic transmission may be critical to effectively treating depression, though the mechanisms by which this occurs are not fully understood.
Methods
This double-blind, crossover, placebo-controlled study analyzed data from 18 drug-free major depressive disorder subjects and 18 heathy controls who received a single i.v. infusion of ketamine hydrochloride (0.5 mg/kg) as well as an i.v. saline placebo. Magnetoencephalographic recordings were collected prior to the first infusion and 6 to 9 hours after both ketamine and placebo infusions. During scanning, participants passively received tactile stimulation to the right index finger. Antidepressant response was assessed across timepoints using the Montgomery-Asberg Depression Rating Scale. Dynamic causal modeling was used to measure changes in α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)- and N-methyl-D-aspartate (NMDA)-mediated connectivity estimates in major depressive disorder subjects and controls using a simple model of somatosensory evoked responses.
Results
Both major depressive disorder and healthy subjects showed ketamine-mediated NMDA-blockade sensitization, with major depressive disorder subjects showing enhanced NMDA connectivity estimates in backward connections and controls showing enhanced NMDA connectivity estimates in forward connections in our model. Within our major depressive disorder subject group, ketamine efficacy, as measured by improved mood ratings, correlated with reduced NMDA and AMPA connectivity estimates in discrete extrinsic connections within the somatosensory cortical network.
Conclusions
These findings suggest that AMPA- and NMDA-mediated glutamatergic signaling play a key role in antidepressant response to ketamine and, further, that dynamic causal modeling is a powerful tool for modeling AMPA- and NMDA-mediated connectivity in vivo.
Clinicaltrials.gov
NCT#00088699.
Keywords: ketamine, major depressive disorder, magnetoencephalography, dynamic causal modeling
Significance Statement
This research demonstrates that ketamine administration leads to short-term changes in modeled elecytrophysiological estimates of NMDA-mediated connectivity in a simple model of somatosensory evoked responses in a group of patients with major depressive disorder (MDD) and healthy controls. Further, this research demonstrates that specific changes in AMPA- and NMDA-mediated extrinsic connectivity estimates correlate with change in depression scores within MDD patients following ketamine administration. These findings are the first to demonstrate changes in modeled electrophysiological glutamatergic signaling estimates in MDD patients following ketamine administration.
Introduction
Glutamatergic signaling abnormalities are thought to be an underlying factor in mood disorders (Yüksel and Öngür, 2010), including major depressive disorder (MDD) (Choudary et al., 2005; Bernard et al., 2011) and bipolar depression (Eastwood and Harrison, 2010). Interest in targeting this system for treatment has grown exponentially (Ohgi et al., 2015), with particular focus on the glutamatergic modulator ketamine (Zarate et al., 2006; Diazgranados et al., 2010) as a clinical treatment option. Several studies have now demonstrated that a single subanesthetic dose of ketamine can rapidly relieve depressive symptoms in individuals with MDD (Zarate et al., 2006; Murrough et al., 2013) and bipolar depression (Diazgranados et al., 2010; Zarate et al., 2012), including treatment-resistant subjects. Understanding the mechanisms underlying ketamine’s rapid antidepressant effects could help identify novel biomarkers for antidepressant response as well as expedite the development of fast-acting and more effective therapeutics to treat depressive symptoms.
Ketamine is a noncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist, although recent studies suggest that NMDA antagonism may not be the mechanism underlying its antidepressant effects. For instance, recent work has shown that the ketamine metabolite (2R,6R)-hydroxynorketamine, which is not an NMDA antagonist, exerts antidepressant effects in animal models, potentially by enhancing α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) throughput (Zanos et al., 2016). Subanesthetic-dose ketamine administration leads to immediate presynaptic disinhibition of glutamatergic neurons, producing a glutamate surge (Moghaddam et al., 1997). This surge is thought to result from the blockade of NMDA receptors targeting γ-aminobutyric acid-ergic interneurons, leading to local inhibition of interneuron tonic firing and subsequent disinhibition of glutamate transmission (Homayoun and Moghaddam, 2007). Due to a blockade of NMDA receptors on postsynaptic excitatory neurons, excess synaptic glutamate is primarily taken up by AMPA receptors, thereby activating neuroplasticity-related signaling pathways (including mammalian target of rapamycin complex 1 (Li et al., 2010; Li et al., 2011) and brain-derived neurotrophic factor (Liu et al., 2012), both of which result in increased synaptogenesis and synaptic potentiation). AMPA’s role is supported by studies demonstrating that administration of an AMPA receptor antagonist can neutralize ketamine’s antidepressant effects (Maeng et al., 2008; Koike et al., 2011) in animal models of depression.
In addition, several electrophysiological studies have demonstrated that ketamine directly induces spontaneous γ synchrony (30–80 Hz) within cortical networks (Hong et al., 2010; Lazarewicz et al., 2010; Cornwell et al., 2012; Shaw et al., 2015). This increased γ synchrony is thought to result from ketamine-induced pyramidal cell disinhibition (Homayoun and Moghaddam, 2007), thus resulting in increased pyramidal cell excitation. While ketamine-induced disinhibition and the subsequent glutamate surge are transient phenomena, one recent study found that, roughly 6.5 hours post-ketamine infusion, MDD subjects who responded to ketamine had increased stimulus-evoked γ-band responses compared with nonresponders (Cornwell et al., 2012). These findings were thought to result from AMPA-mediated glutamatergic neurotransmission following synaptic potentiation, which could provide one explanation for how ketamine influences mood. Indeed, a few studies using modeling in tandem with electrophysiology to measure changes in effective connectivity found changes in both AMPA and NMDA signaling post-ketamine administration (Moran et al., 2015; Muthukumaraswamy et al., 2015). Importantly, one of these studies used ketamine to model schizophrenia symptoms in rats (Moran et al., 2015), administering much higher ketamine doses than typically used to treat depressive symptoms. The second study administered subanesthetic doses of ketamine to healthy control subjects (Muthukumaraswamy et al., 2015), leaving open the question of how ketamine influences mood in MDD subjects.
This double-blind, crossover, placebo-controlled study used magnetoencephalography (MEG) in tandem with dynamic causal modeling (DCM) to measure AMPA and NMDA signaling in vivo during a passive somatosensory stimulation task in both MDD subjects and healthy controls post-ketamine administration. The post-ketamine condition was compared with both a baseline scan collected prior to ketamine infusion and a control scan during which an i.v. infusion of a saline placebo was administered. DCM was used to estimate effective connectivity within a simple network activated by the task following ketamine administration. DCM fits a biophysically plausible model of neural dynamics to measure electrophysiological signals. These models provide intrinsic (within-region) and extrinsic (between-region) estimates of synaptic response in specific neuronal ensembles (Moran et al., 2011), providing a powerful means to measure brain dynamics in vivo. We were particularly interested in measuring AMPA- and NMDA-mediated extrinsic connectivity to compare baseline estimates (here, both baseline and placebo) with estimates obtained within the window of antidepressant response to ketamine.
Methods
Participants
All participants were studied at the National Institute of Mental Health (NIMH) in Bethesda, Maryland between September 2011 and August 2016. The present study used data drawn from a larger clinical trial (NCT#00088699) that assessed ketamine’s antidepressant effects (n=60). The present study included only those subjects who completed all study scans; the sample comprised 18 healthy controls (11 F/7 M, mean age=33.9±10.3 years) and 18 subjects with a DSM-IV-TR diagnosis of MDD (American Psychiatric Association, 1994) without psychotic features (10 F/8 M, mean age=36.9±10.7 years). MDD subjects were 18 to 65 years old, were experiencing a major depressive episode lasting at least 4 weeks, and had a Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979) score of ≥20 at screening. Treatment resistance was also confirmed by prior failure to respond to at least one adequate antidepressant trial, as assessed using the Antidepressant Treatment History Form (Sackeim, 2001). Diagnosis was determined by Structured Clinical Interviews for Axis I DSM-IV-TR Disorders–Patient Edition (First et al., 2002). Healthy controls were 18 to 65 years old, had no Axis I disorder as determined by the Structured Clinical Interviews for Axis I DSM-IV-TR Disorders-Non-Patient Edition, and had no family history of Axis I disorders in first-degree relatives. All MDD subjects were hospitalized for the duration of the study and drug-free from psychotropic medications for at least 2 weeks prior to MEG testing. Healthy controls completed study procedures as inpatients but were otherwise outpatients.
All participants were in good health as evaluated by a medical history and physical examination, toxicology screens and urinalysis, blood laboratory results, clinical MRI, and electrocardiogram. The Combined Neuroscience Institutional Review Board at the NIH approved the study. All participants provided informed written consent, and MDD subjects were matched with an NIMH advocate from the Human Subjects Protection Unit to monitor consent and participation.
Clinical Measurements
The primary outcome measure, the MADRS (Montgomery and Asberg, 1979), was administered 60 minutes prior to infusion (both ketamine and placebo) and at multiple time points (40, 80, 120, and 230 minutes postinfusion as well as at Days 1, 2, 3, 10, and 11). For efficiency, we classified the preinfusion rating as a baseline measurement, and we classified the 230-minute postinfusion rating as either the post-ketamine or post-placebo rating, depending on condition. This timepoint was chosen because it was closest to the time of the MEG recording. Finally, we classified the Day 11 post-ketamine timepoint as the rating for sustained post-ketamine response. Immediate change in MADRS score was calculated by subtracting baseline and post-placebo ratings from post-ketamine ratings. Sustained change in MADRS scores was calculated by subtracting baseline ratings from sustained post-ketamine ratings.
MEG Acquisition and Preprocessing
MEG recordings were collected 2 to 4 days prior to the first infusion and 6 to 9 hours after both ketamine and placebo experimenter-blinded infusions. The order of ketamine and placebo infusions was randomized across participants. During each session, participants received tactile stimulation of the right index finger (500 stimuli, 25-millisecond duration, 2-Hz average rate) during a 250-second experimental run. Tactile stimulation was controlled by a pneumatic stimulating device emitting brief bursts of air (30 psi) displacing a plastic membrane resting against the skin of the distal phalange (see, e.g., Cornwell et al., 2012). Throughout the duration of the task, participants were asked to focus on a stationary dot projected on a screen in the subject’s field of view.
Neuromagnetic data were collected using a 275-channel CTF system with SQUID-based axial gradiometers (VSM MedTech Ltd.) housed in a magnetically-shielded room (Vacuumschmelze). Data were collected at 1200 Hz with a bandwidth of 0 to 300 Hz. Synthetic third-order balancing was used for active noise cancellation. Offline, MEG data were first visually inspected, and trials were removed where visible artifacts (e.g., head movements, jaw clenches, eye blinks, and muscle movements) were present. In addition, individual channels showing excessive sensor noise were marked as bad and removed from the analysis. Data were then bandpass filtered from 1 to 58 Hz and epoched from -100 to 300 milliseconds peristimulus time. The analysis routines available in the academic freeware SPM12 (Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm/) were used for data processing. This work used the computational resources of the NIH HPC Biowulf cluster (http://hpc.nih.gov).
Source Localization and Source Activity Extraction
The multiple sparse priors routine implemented in SPM12 was used to identify gamma frequency (30–58 Hz) sources of activity from each participant’s sensor-level data over a peristimulus event time window from -100 to 300 milliseconds. Gamma frequency was targeted, as recent findings using a similar paradigm demonstrated robust, ketamine-mediated cortical responses in that band (Cornwell et al., 2012). In addition, a host of studies have demonstrated increased gamma synchrony/power following ketamine administration (Hong et al., 2010; Lazarewicz et al., 2010; Muthukumaraswamy et al., 2015; Shaw et al., 2015). Evoked responses to airpuff stimulation were localized to 512 potential mesh points using a variational Bayesian approach following co-registration of sensor positions to a canonical template brain. Participant-level activation maps were constructed following inversion of each data session (i.e., baseline, placebo, ketamine) separately for all subjects. No prior constraints on source location were used. Following the inversion, statistical maps of group activity were computed, and a mixed-effects ANOVA was used to define source-localized cortical regions showing a main effect of the airpuff stimulus in the ketamine condition, thresholded at P<.05 family-wise error correction.
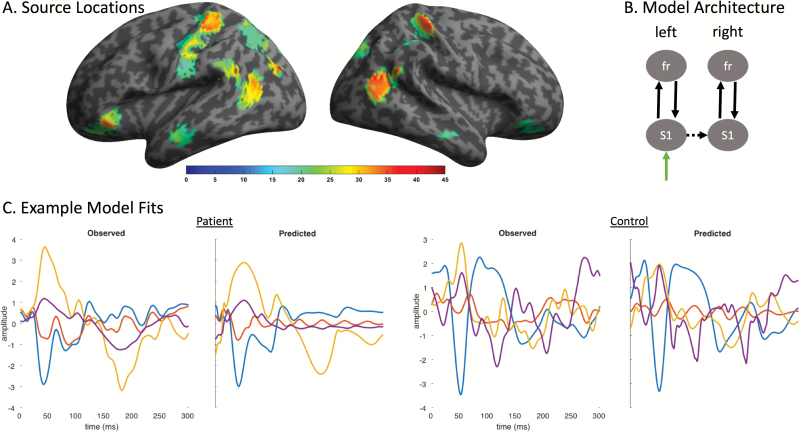
Group-level statistical activation maps demonstrated stimulus-evoked gamma-band activity in a network of brain regions including bilateral somatosensory cortices, posterior parietal and middle to superior temporal sulci, and anterior/inferior temporal and frontal lobes (Figure 1A). Because our aim was to characterize AMPA- and NMDA-mediated extrinsic connectivity in a simple model of ketamine’s effects, we focused on 2 regions to bilaterally model forward and backward connections in our network: bilateral S1 and inferior frontal cortex (see Figure 1A and below for source locations). Source activity at each region of interest was extracted using SPM’s source extraction algorithm using a 5-mm radius, extracting individual airpuff trials from the initial preprocessed data. Subsequent analyses used these ‘virtual electrode’ signals in the 1- to 58-Hz band.
Figure 1.
Source locations, model architecture, and example model fits. (A) Evoked gamma frequency (30–58 Hz) source-localized estimates of the main effect of ketamine for all participants, thresholded at P<.05 corrected. (B) A simple model architecture was used that included subcortical inputs to left primary somatosensory cortex and lateral connections to right primary somatosensory cortex. Forward and backward recurrent extrinsic connections carried signals from S1 to frontal cortex, bilaterally. (C) Example model fits showing measured wide-band (1–42 Hz) virtual electrode signals (observed) from left S1 (blue), right S1 (red), left frontal (orange), and right frontal (purple) sources compared with the estimated signal from the fitted model (predicted).
Dynamic Causal Modeling
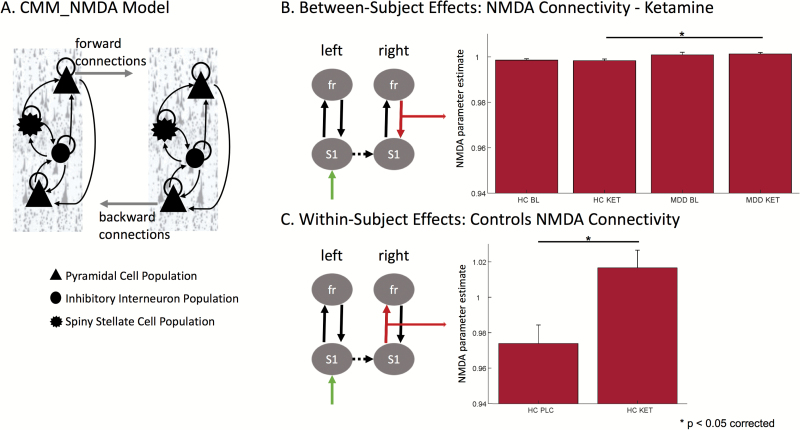
DCM uses a biophysical model of neural responses based on neural mass models to predict recorded electrophysiological data (David et al., 2006). The present study specifically used a conductance-based neural mass model for DCM for electrophysiology, the standard CMM_NMDA model as implemented in SPM12 (http://www.fil.ion.ucl.ac.uk/spm/), to model responses between S1 and frontal cortex. Within the model, synaptic responses are modeled within (i.e., intrinsic) and between (i.e., extrinsic) regions. Intrinsic excitatory connections are mediated by both AMPA and NMDA receptor types, while intrinsic inhibitory connections use γ-aminobutyricA acid receptors. Each receptor type is furnished with its own time constants and dynamics. Extrinsic connection parameters for both fast (AMPA-mediated) and slow (NMDA-mediated) glutamatergic signaling are mediated by 2 connection types: “feedforward” connections and “feedback” connections. Within the model, superficial pyramidal cells encode and carry feedforward signaling to stellate cells, while deep pyramidal cells carry feedback signaling to both superficial pyramidal cells and inhibitory interneurons (Figure 2A). More detailed information on this model architecture can be found in the spm_fx_cmm_nmda.m file, freely available in SPM12. Thalamic (stimulus-bound) input was modeled with a Gaussian bump function that drove activity in left S1 (-40, -32, 60) in our model. Left S1 was laterally connected with right S1 (42, -30, 62). Signals were then passed via forward connections from bilateral S1 to bilateral inferior frontal cortex (left: -46, 28, -14; right: 44, 28, -14). Backward connections from frontal cortex to S1 ensured recurrent extrinsic connections (Figure 1B).
Figure 2.
Dynamic causal modeling (DCM) model and NMDA-mediated effects in vivo. (A) The CMM_NMDA model included four distinct cell layers: superficial pyramidal cells, spiny stellates, inhibitory interneurons, and deep pyramidal cells. Superficial pyramidal cells carry forward extrinsic signals to excitatory spiny stellate cells. Deep pyramidal cells carry backward extrinsic signals to both superficial pyramidal cells and inhibitory interneurons. B. A comparison of between-subject differences in α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)- and N-methyl-D-aspartate (NMDA)-mediated connectivity estimates found significantly increased NMDA-mediated connectivity following ketamine administration in the backward connection from right frontal cortex to right S1 in subjects with major depressive disorder (MDD) compared to controls. C. A comparison of within-subject differences in AMPA- and NMDA-mediated connectivity estimates found significantly increased NMDA-mediated connectivity following ketamine administration compared to placebo administration in the forward connection from right S1 to right frontal cortex in our controls.
For the DCM analyses, MEG activity for the extracted time series was fitted over 1 to 300 milliseconds peristimulus time in a wide-frequency band from 1 to 42 Hz using an LFP model to capture event-related potentials of evoked activity. For computational efficiency, DCM optimizes a posterior density over free parameters (parameterized by its mean and covariance) via a standard variational Bayesian inversion procedure (Friston et al., 2007). Model inversion results in optimized parameters of different receptor-mediated synaptic responses given the model architecture that best predicts a given dataset (here, the virtual electrode signals in the 1- to 42-Hz band from bilateral S1 and bilateral frontal cortex). We were specifically interested in the optimized parameters for AMPA- and NMDA-mediated extrinsic connectivity between our regions of interest. In the present analysis, initial DCMs were computed for each participant and condition and model fits were assessed. The posterior estimates were then used to initialize a second set of DCMs for each participant and condition, and model fits were again assessed. The negative free energy bound on the log-model evidence was then used to adjudicate between the first and second model for each subject and condition, selecting the model with greater log-model evidence for subsequent analyses. Parameter estimates were harvested from optimized DCMs for the winning model for each subject and condition separately to compare ketamine-mediated effects on extrinsic AMPA- and NMDA-mediated connectivity estimates.
Statistical Analyses
To first examine whether there were biases in model fits between conditions, model fits for the winning DCM were computed by correlating the estimated data from the fitted model to the extracted virtual electrode data. The model fits were compared within and between groups using paired and 2-sample t tests. To then determine whether there were differences between the MDD and control groups based on condition, the extracted parameter estimates for AMPA and NMDA connectivity were entered separately into mixed-effects ANOVAs. We specifically tested for group (controls vs MDD subjects) by condition (baseline, placebo, and ketamine) effects. Posthoc t tests were used to compare between- and within-group differences separately, using Bonferonni correction to correct for multiple comparisons over connections. We subsequently tested whether the variance between MDD and control estimates differed for any statistically significant between-group effects identified in our posthoc comparisons using a 2-sample F-test for equal variances. Finally, to establish whether AMPA- and NMDA-mediated extrinsic connectivity parameter estimates from MDD subjects were related to change in depressive symptom scores, we computed pairwise linear correlation coefficients between the NMDA and AMPA parameter estimates from the ketamine scan and change in MADRS scores from baseline to ketamine, placebo to ketamine, and baseline to Day 11 post-ketamine.
Results
A multiple sparse priors routine was used to infer the generators of the MEG signal. Significant group-level evoked gamma-band activation was identified in response to the airpuff stimulus specifically in the ketamine session (Figure 1A) for both MDD subjects and controls. The network of regions activated following ketamine administration included robust bilateral responses in S1 and surrounding somatosensory cortex, more posterior regions in parietal and temporal cortices, inferior regions in the anterior temporal lobes, and inferior frontal cortex. We focused on characterizing parameter estimates of AMPA and NMDA signaling using DCM for electrophysiology within a simple model that included bilateral regions in S1 and inferior frontal cortex (Figure 1B). Our decision to focus on frontal cortex was motivated by previous findings demonstrating changes in frontal-to-parietal connectivity following ketamine administration in healthy subjects (Muthukumaraswamy et al., 2015).
An iterative procedure was used to fit the data, using estimated model complexity to choose the winning DCM for each subject and condition. Model fits for the winning DCM were computed by correlating the estimated data from the fitted model to the extracted virtual electrode data. Model fits were compared using paired t tests within MDD and control groups separately for all conditions (i.e., baseline, placebo, ketamine), and no significant differences in estimated fits were observed within groups (MDD subjects: baseline mean=0.648±0.042 SE, placebo mean=0.593±0.053 SE, ketamine mean=0.669±0.047 SE; controls: baseline mean=0.699±0.052 SE, placebo mean=0.743±0.051 SE, ketamine mean=0.638±0.054 SE). Model fits were also compared between groups (i.e., control vs MDD) for each condition using 2-sample t tests; similarly, no significant differences in model fits were found. Example model fits for a single control and MDD subject are shown in Figure 1C.
Following model comparisons, the extrinsic connectivity parameter estimates for both fast (i.e., AMPA) and slow (i.e., NMDA) glutamatergic signaling were extracted to determine if there were differences in the fitted model estimates between groups. Our analysis of extrinsic AMPA signaling showed no significant effects (FGroupxCondition(1,2)=1, P=.3676). Our analysis of extrinsic NMDA signaling showed a significant group by condition effect (FGroupxCondition(1,2)=3.8, P=.0229). To examine this further, we first asked how depression impacted NMDA signaling estimates for each condition separately (i.e., control baseline vs MDD subject baseline, control placebo vs MDD subject placebo, control ketamine vs MDD subject ketamine) using t tests to compare each pair. Posthoc tests between diagnostic groups identified a single parameter estimate showing a significant between-group difference; specifically, the NMDA parameter estimate for the feedback signal from right frontal cortex to right S1 (t(34)=-2.85, P=.0354 corrected) during the ketamine condition showed increased NMDA-mediated backward connectivity for MDD subjects compared with controls (Figure 2B). Subsequent comparisons of the variance between MDD subjects and controls on this connection over recording sessions showed unequal variance between groups for the baseline scan (F=4.1, P=.0058), but not for the ketamine (F=0.83, P=.7064) or placebo (F=1.07, P=.8896) scans. No NMDA-mediated differences were observed between groups for either the baseline or placebo conditions. Posthoc t tests between sessions within each group separately identified a single parameter estimate that showed a significant ketamine-mediated effect; specifically, the NMDA parameter estimate for the forward signal from right S1 to right frontal cortex showed an increase in NMDA-mediated forward connectivity for ketamine compared with placebo (but not baseline) for controls only (t(17)=-3.00, P=.0337 corrected; Figure 2C). No significant effects for MDD subjects were observed, and no other NMDA-mediated connectivity estimates showed ketamine-induced effects. No AMPA-mediated connectivity estimates showed between-session effects for either the MDD subjects or controls.
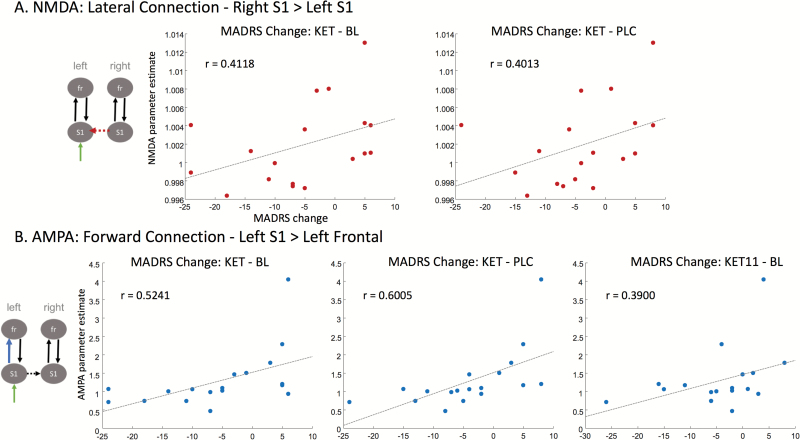
Finally, as an additional exploratory analysis, we sought to determine whether the AMPA- and NMDA-mediated extrinsic connectivity parameter estimates correlated with change in MADRS scores within the MDD subject group. Here, a more liberal criterion of P<.05 uncorrected was used to determine significance. For the NMDA estimates, a significant correlation was observed between the lateral connection from right S1 to left S1 following ketamine administration and change in MADRS scores from both baseline to ketamine (r=0.4118, P<.05) and placebo to ketamine (r=0.4013, P<.05) (Figure 3A). These results indicate that reduced NMDA connectivity estimates between right and left S1 were correlated with improved mood scores. For the AMPA estimates, a significant correlation was observed between the forward connection from left S1 to left frontal cortex following ketamine administration and change in MADRS scores from both baseline to ketamine (r=0.5241, P<.05) and placebo to ketamine (r=0.6005, P<.01) (Figure 3B). This parameter also approached significance with the sustained change in MADRS scores from baseline to Day 11 post-ketamine infusion (r=0.3900, P=.05) (Figure 3B). These results indicate that reduced AMPA connectivity estimates between left S1 and left frontal cortex were correlated with improved mood scores.
Figure 3.
N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) estimates following ketamine administration and improved mood. (A) NMDA estimates following ketamine administration were compared with change in Montgomery-Asberg Depression Rating Scale (MADRS) scores at several timepoints: ketamine minus baseline, ketamine minus placebo, and 11 days post-ketamine minus baseline. A significant correlation was observed between the NMDA parameter estimate from the lateral connection between right S1 and left S1 and change in MADRS scores from baseline to ketamine and placebo to ketamine. (B) Post-ketamine administration, AMPA estimates were compared with change in MADRS score at the same timepoints. A significant correlation was observed between the AMPA parameter estimate from the forward connection between left S1 and left frontal cortex and change in MADRS scores from baseline to ketamine and placebo to ketamine. This same parameter estimate approached significance with change in MADRS score from baseline to 11 days post-ketamine infusion (P=.05).
Discussion
This study used MEG recordings in tandem with DCM and an airpuff somatosensory stimulation paradigm to investigate AMPA- and NMDA-mediated connectivity changes following ketamine administration in subjects with treatment-resistant MDD and healthy controls. We found 2 distinct NMDA-mediated effects of ketamine in vivo. The first involved greater NMDA-mediated connectivity in the backward connection from right frontal cortex to right somatosensory cortex for MDD subjects relative to controls following ketamine administration. The second involved an increase in NMDA-mediated connectivity in the forward connection from right somatosensory cortex to right frontal cortex for controls following ketamine administration compared to placebo (but not baseline).
Interestingly, in the first set of results, significant increases in the estimated NMDA-mediated connectivity in these regions were observed, though there appeared to be distinct effects in MDD subjects compared with controls. In particular, we found NMDA-mediated, top-down, modulatory connectivity differences when comparing MDD subjects with controls, while we found that ketamine administration increased the NMDA-mediated bottom-up, stimulus-driven connections in controls. Similar findings of enhanced glutamatergic connectivity were reported in a model of schizophrenia effects (Moran et al., 2008) and have been attributed to upregulation and sensitization effects (McLennan, 1980; van den Pol et al., 1996). That is, ketamine-induced NMDA antagonism might lead to short-term sensitization of postsynaptic mechanisms, affecting forward and backward NMDA connectivity separately for both MDD subjects and healthy controls. This sensitization was evident in controls who demonstrated a significant increase in NMDA-mediated connectivity, while ketamine served to stabilize NMDA-mediated connectivity estimates for MDD subjects (reflected by a stabilization of the variance estimates for MDD subjects from baseline to ketamine and placebo scans).
Secondarily, we examined whether changes in AMPA- or NMDA-mediated extrinsic connectivity estimates correlated with change in MADRS rating scale scores within our MDD group following ketamine administration. Again, we found 2 distinct effects. The first was a correlation between the strength of the lateral connection from right S1 to left S1 and depression rating scale scores, where decreased NMDA-mediated connectivity was related to improved mood. The second was a correlation between the strength of the frontal connection from left S1 and left frontal cortex and depression rating scale scores, where decreased AMPA-mediated connectivity was related to improved mood. When comparing change in depression rating scale scores across time, longer lasting effects were observed for AMPA-mediated connectivity relative to NMDA connectivity, with the correlation between AMPA estimates post-ketamine and improved mood approaching significance at even 11 days postinfusion. Decreases in NMDA- and AMPA-mediated connectivity have been reported elsewhere following ketamine administration (Muthukumaraswamy et al., 2015). Notably, these previous findings showed that ketamine modulated the backward connections from frontal to parietal regions during the resting state for both AMPA and NMDA. Our findings build on this work by showing that ketamine-mediated modulation of early lateral connections for NMDA and forward connections for AMPA correlated with improvements in mood in individuals with treatment-resistant MDD. Thus, reductions in both AMPA- and NMDA-mediated connectivity following ketamine administration lead to positive behavioral outcomes in MDD subjects.
Taken together, these findings suggest that post-ketamine administration, NMDA-mediated glutamatergic sensitivity was evident in MEG recordings collected 6 to 9 hours postinfusion, as demonstrated by increased NMDA-mediated extrinsic connectivity estimates in healthy controls and stabilization of NMDA-mediated extrinsic connectivity estimates (i.e, no differences in variance) in MDD subjects. However, ketamine’s antidepressant efficacy, as assessed via improved mood ratings in MDD subjects, correlated with reduced NMDA and AMPA extrinsic connectivity estimates in discrete connections within the somatosensory cortical network. Interestingly, while NMDA estimates correlated with improved mood only when comparing depression rating scale scores following ketamine administration with baseline scans (in this case, baseline and placebo), the effects of AMPA appeared to be of longer duration (specifically, 11 days post-ketamine), suggesting that AMPA receptor effects might be critical for longer-term antidepressant response to ketamine. These results are in line with previous findings that demonstrate a key role for AMPA throughput in regulating mood and behavior (Maeng et al., 2008; Zanos et al., 2016).
It should be noted that one key limitation of our study is that we could not model the acute effects of ketamine administration on AMPA and NMDA connectivity estimates. While this has certainly been done using healthy subjects (Muthukumaraswamy et al., 2015), the question of ketamine’s acute effects on MDD subjects will need to be examined further.
In conclusion, our findings demonstrate that ketamine administration leads to key differences in NMDA- and AMPA-mediated connectivity estimates measured using magnetoencephalography in tandem with DCM. They add to a growing body of evidence that glutamatergic signaling differences are key to ketamine’s antidepressant efficacy, with AMPA receptor differences supporting longer-term antidepressant response in MDD subjects. In addition, our findings underscore the usefulness of DCM as a tool to model AMPA- and NMDA-mediated connectivity in vivo.
Statement of Interest
Dr Zarate is listed as a coinventor on a patent for the use of ketamine and its metabolites in major depression and suicidal ideation. Dr Zarate is listed as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain. Dr Zarate is listed as co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and posttraumatic stress disorders; he has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. All other authors have no conflict of interest to disclose, financial or otherwise.
Acknowledgments
The authors thank the 7SE research unit and staff for their support. We also thank Rosalyn Moran for insightful discussion and helpful comments. Ioline Henter (NIMH) provided invaluable editorial assistance. The authors are entirely responsible for the scientific content of the paper.
Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIA MH002857), by a NARSAD Independent Investigator Award to Dr Zarate, and by a Brain and Behavior Mood Disorders Research Award to Dr Zarate.
References
- American Psychiatric Association (1994)Diagnostic and Statistical Manual of Mental Disorders, 4th ed Washington, DC: American Psyciatric Association. [Google Scholar]
- Bernard R, Kerman IA, Thompson RC, Jones EG, Bunney WE, Barchas JD, Schatzberg AF, Myers RM, Akil H, Watson SJ(2011)Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry 16:634–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, Myers RM, Bunney WE Jr, Akil H, Watson SJ, Jones EG(2005)Altered cortical glutamatergic and gabaergic signal transmission with glial involvement in depression. Proc Natl Acad Sci U S A 102:15653–15658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell BR, Salvadore G, Furey M, Marquardt CA, Brutsche NE, Grillon C, Zarate CA Jr(2012)Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biol Psychiatry 72:555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David O, Kiebel SJ, Harrison LM, Mattout J, Kilner JM, Friston KJ(2006)Dynamic causal modeling of evoked responses in EEG and MEG. Neuroimage 30:1255–1272. [DOI] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA Jr(2010)A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 67:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ(2010)Markers of glutamate synaptic transmission and plasticity are increased in the anterior cingulate cortex in bipolar disorder. Biol Psychiatry 67:1010–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB(2002)Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. In: SCID-I/P. New York: State Psychiatric Institute, Biometrics Research. [Google Scholar]
- Friston K, Mattout J, Trujillo-Barreto N, Ashburner J, Penny W(2007)Variational free energy and the laplace approximation. Neuroimage 34:220–234. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B(2007)NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27:11496–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, Buchanan RW, O’Donnell P, Thaker GK, Weiler MA, Lahti AC(2010)Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology 35:632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, Iijima M, Chaki S(2011)Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res 224:107–111. [DOI] [PubMed] [Google Scholar]
- Lazarewicz MT, Ehrlichman RS, Maxwell CR, Gandal MJ, Finkel LH, Siegel SJ(2010)Ketamine modulates theta and gamma oscillations. J Cogn Neurosci 22:1452–1464. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS(2010)Mtor-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS(2011)Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69:754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK(2012)Brain-derived neurotrophic factor val66met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry 71:996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK(2008)Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 63:349–352. [DOI] [PubMed] [Google Scholar]
- McLennan H.(1980)The effect of decortication on the excitatory amino acid sensitivity of striatal neurones. Neurosci Lett 18:313–316. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D(1997)Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17:2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M(1979)A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389. [DOI] [PubMed] [Google Scholar]
- Moran RJ, Stephan KE, Kiebel SJ, Rombach N, O’Connor WT, Murphy KJ, Reilly RB, Friston KJ(2008)Bayesian estimation of synaptic physiology from the spectral responses of neural masses. Neuroimage 42:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran RJ, Symmonds M, Stephan KE, Friston KJ, Dolan RJ(2011)An in vivo assay of synaptic function mediating human cognition. Curr Biol 21:1320–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran RJ, Jones MW, Blockeel AJ, Adams RA, Stephan KE, Friston KJ(2015)Losing control under ketamine: suppressed cortico-hippocampal drive following acute ketamine in rats. Neuropsychopharmacology 40:268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ(2013)Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 170:1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Shaw AD, Jackson LE, Hall J, Moran R, Saxena N(2015)Evidence that subanesthetic doses of ketamine cause sustained disruptions of NMDA and AMPA-mediated frontoparietal connectivity in humans. J Neurosci 35:11694–11706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgi Y, Futamura T, Hashimoto K(2015)Glutamate signaling in synaptogenesis and Nmda receptors as potential therapeutic targets for psychiatric disorders. Curr Mol Med 15:206–221. [DOI] [PubMed] [Google Scholar]
- Sackeim HA.(2001)The definition and meaning of treatment-resistant depression. J Clin Psychiatry 62:10–17. [PubMed] [Google Scholar]
- Shaw AD, Saxena N, E Jackson L, Hall JE, Singh KD, Muthukumaraswamy SD(2015)Ketamine amplifies induced gamma frequency oscillations in the human cerebral cortex. Eur Neuropsychopharmacol 25:1136–1146. [DOI] [PubMed] [Google Scholar]
- Van Den Pol AN, Obrietan K, Belousov A(1996)Glutamate hyperexcitability and seizure-like activity throughout the brain and spinal cord upon relief from chronic glutamate receptor blockade in culture. Neuroscience 74:653–674. [DOI] [PubMed] [Google Scholar]
- Yüksel C, Öngür D(2010)Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry 68:785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr, Gould TD(2016)NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK(2006)A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA(2012)Replication of Ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 71:939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]