Abstract
We report identification of Madariaga virus (MADV) in plasma and urine samples from a child with acute undifferentiated febrile illness in Venezuela. Our data document the occurrence of milder MADV infections (ie, without encephalitis), with a symptom complex that resembles that seen with other arboviral infections, including dengue and zika.
Keywords: acute febrile illness, epizootic, Madariaga virus, Venezuela
(See the Editorial Commentary by Powers on pages 622–3.)
Recent ecologic and genetic studies of eastern equine encephalitis virus (EEEV; Togaviridae: Alphavirus) have demonstrated clear separation between North and South American EEEV strains: North American EEEV cluster in a single genetic lineage (lineage I, in the system proposed by Arrigo et al [1]), with South American EEEV strains (now known as Madariaga virus [MADV]) clustering in EEEV lineages II, III, and IV. Although there is reasonable understanding of clinical and epidemiologic features of North American EEE, much less is known about MADV infections. MADV can cause outbreaks in horses and appears to infect a variety of mammals, including rats and bats, with a study in Panama finding the highest rates of seropositivity in short-tailed cane rats (8.3%) [2, 3]. However, less than a dozen human cases of MADV infection have been documented, and almost all have been encephalitis cases in children seen as part of an outbreak in Panama in 2010 [3, 4]. In population-based serologic surveys in Panama and the Peruvian Amazon, between 2% and 5% of the general population had evidence of prior infection [2, 3, 5], suggesting that mild or asymptomatic human infection is relatively common. We report here a case of MADV infection in Venezuela in a child with acute undifferentiated febrile illness, diagnosed after initial screening tests for Zika virus (ZIKV) were negative.
CASE REPORT
In June 2016, during an outbreak of Zika, a 12-year-old girl presented to Hospital Internacional de Barquisimeto (HI), Cabudare, Venezuela, with a history of fever of abrupt onset, asthenia, malaise, headache, and nausea. She had been previously healthy with no significant past medical history. The fever was high grade (39°–40° C), lasting 2 days, and was followed by severe fatigue; the patient denied arthralgias or mialgias. Concomitantly, she developed an intensely pruritic maculopapular rash that persisted for 5 days after the onset of fever. Physical examination revealed a conscious, febrile patient with rash, mild pallor, and congestion of palpebral conjunctiva. She had no petechiae, icterus, or lymphadenopathy, and an examination that was otherwise unremarkable. Complete blood count and chemistry were unremarkable except for mild lymphocytic leukocytosis. She was treated with oral acetaminophen 500 mg qid and adequate fluid intake, fully recovering after 5 days.
The patient lived in an urban area of the city of Barquisimeto (population of approximately 1 million; capital of the state of Lara). She had no exposure to sick contacts, pets, or animals, and no contact with horses or farms. Ten days before onset of illness she had traveled to the beach at Tucacas, a coastal town in the northern state of Falcón. During this time period there were reports of an outbreak of equine encephalitis near Tucacas, with veterinarians in the region reporting horses with fever, lethargy, extreme weakness, unstable gait, muscle twitches, and fatalities. There were also sporadic reports of equine illness in Lara and neighboring states, including Yaracuy, Portuguesa, and Barinas. Illness in the horses was only evaluated clinically, with no diagnostic testing done.
DIAGNOSTIC STUDIES
At HI, the child’s plasma tested negative for DENV, CHIKV, EBV, CMV, and parvovirus. As part of a collaborative effort to identify other possible viral etiologies among children presenting with febrile illness during the ZIKV epidemic, plasma and urine samples were further evaluated by the Lednicky Laboratory at the University of Florida, where they tested negative by real-time reverse transcription polymerase chain reaction with primers specific for CHIKV, DENV types 1–4, and ZIKV [6, 7]. Samples were then tested with universal primer systems for the detection and identification of both alpha- and flaviviruses [8, 9]. A very weak alphavirus amplicon was generated from both plasma and urine viral RNA (vRNA) samples, though the putative alphavirus amplicon did not correspond in size to the alphaviruses identified by de Morais Bronzoni et al [8].
To gain insights whether an alphavirus had been detected, an aliquot of plasma was treated with cyanase nuclease using a Nucleic Acid Removal Kit (RiboSolutions, Inc., Cedar Creek, Texas), and vRNA extracted from the treated material [6, 7]. Synthesis of complementary DNA was achieved as previously described [6] using non-ribosomal hexamers to favor the reverse transcription of viral genomes over ribosomal RNA [9]. PCR was performed with random hexamers and One Taq DNA polymerase (New England Biolabs). One prominent approx. 330 bp amplicon purified from a 2% agarose gel stained with ethidium bromide was cloned into a TA cloning kit (Invitrogen TA cloning kit. with pCR™2.1 Vector and One Shot™ TOP10 Chemically Competent Escherichia coli) and sequenced, revealing the insert was from MADV (data not shown). Neither MADV nor North American EEEV were present in the laboratory at the time of these studies.
SEQUENCING
Sanger sequencing was performed on vRNA from plasma to obtain the MADV consensus sequence using methods similar to a previously published genome walking approach using overlapping primers (Supplemental Table S1) [6] that produced specific amplicons in the 800 bp range. Because primers described in published literature were suboptimal, the primer list depicts primers that were purpose designed for this work (data not shown). The 5′ end of the MADV genome was determined using primers given in the Supplemental Table, and the 3′ end required use of a reverse primer T25A (5′—TTTTTTTTTTTTTTTTTTTTTTTTTA—3′).
PHYLOGENETIC ANALYSIS
In order to classify our MADV isolate within the EEEV complex, nucleotide sequences (3729 bp) encoding for the structural polyprotein open reading frame (capsid, E3, E2, 6K, E1) for NA EEEV and MADV isolates were aligned using the MAFFT. After confirming absence of nucleotide substitution saturation and presence of phyogentic signal using DAMBE6 and IQ-TREE, respectively (data not shown), maximum likelihood (ML) phylogeny was obtained based on the best-fit model (GTR+G4) chosen according to Bayesian information criterion using IQ-TREE [10]. Statistical robustness for internal branching order was assessed by UFBoot—Ultrafast Bootstrap (BB) Approximation (2000 replicates), and defining strong statistical support along the branches as BB > 90% [11].
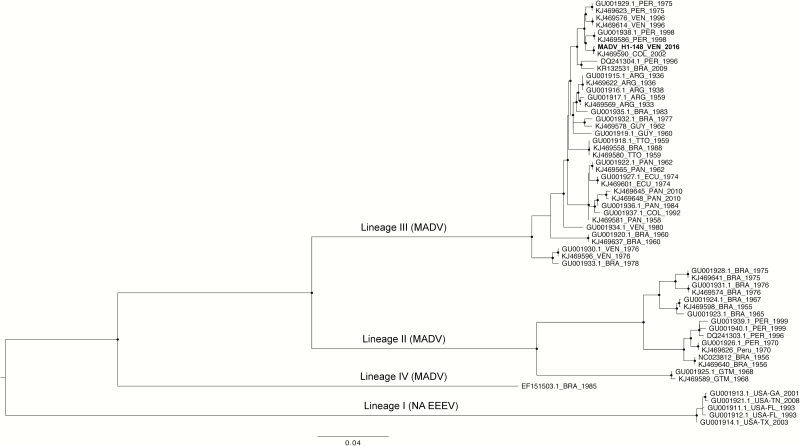
Consistent with previous findings [1], MADV isolates cluster within 4 lineages (Figure 1). Based on the ML phylogeny, our new isolate obtained from Venezuela clusters within the Lineage III that comprises isolates from Central and South America, and in particular strains isolated in Colombia, Venezuela, and Peru.
Figure 1.
Maximum likelihood tree of EEV complex based on structural polyprotein open reading frame. Maximum likelihood phylogeny was inferred using the software IQ-TREE based on of the structural polyprotein open reading frame of the NA EEEV (lineage I) and MADV (former SA EEEV). The new MADV isolate from Venezuela, shown in bold, clusters within the Lineage III. Branch lengths reflect genetic distances, and strong statistical support based on ultrafast-bootstrap (BB > 90%) is indicated at each node with circles. Abbreviations: EEEV, eastern equine encephalitis virus; MADV, Madariaga virus.
COMMENT
MADV (previously known as South American EEEV), appears to be distinct genetically from both North American EEEV and Venezuelan Equine Encephalitis Virus, with a distinct ecology, epidemiology, and clinical presentation [1–3, 5]. Little is known about MADV transmission, although it is assumed that Culex mosquitoes are the primary vectors, with, possibly, reservoirs in rat or bat populations in more rural areas of Latin America [2, 3]. Documented human cases of MADV infection are rare in the literature, and when identified, have generally been in children who presented with encephalitis, often with severe neurologic sequelae [3]. At the same time, cross-sectional serologic studies (conducted with solid methodology, including use of plaque-reduction neutralization tests for confirmation), have reported seropositivity rates of between 2% and 5% in Panama and Peru [2, 3, 5]. Based on these observations, it has been hypothesized that illness in humans can be asymptomatic or relatively mild, resulting in few clinical diagnoses despite an underlying level of endemicity within the population.
This report adds yet another piece to the puzzle of understanding this emerging pathogen. In this instance, we were looking for ZIKV, and had a child with symptoms that were broadly consistent with ZIKV infection. When studies were negative for ZIKV, DENV, and CHIKV, all of which are known to be endemic in Venezuela, we looked for other possible viruses, and found MADV, demonstrating that MADV can cause relatively mild, self-limited febrile illness in children. These findings again underscore the potential for misdiagnosis when using clinical criteria to identify specific arbovirus infections, in keeping with data which we have reported from Haiti on other arbovirus species such as Mayaro [6, 12]. It also reinforces the need for caution before automatically assuming that all cases presenting in the midst of an epidemic (in this instance, a ZIKV epidemic) are due to the epidemic microorganism. From a diagnostic standpoint, our data provide evidence that the virus is present in urine, as has been seen with ZIKV, which may help guide future development of diagnostic tests.
From an epidemiologic standpoint, there are anecdotal data that the case occurred at a time when there was an epizootic of presumed viral equine encephalitis in the region. However, the case patient lived in the city and did not have any history of direct exposure to horses (or farms). If we postulate an incubation period of up to 10 days, it is possible that she was exposed during her trip to the beach at Tucacas, an area where equine encephalitis cases were reported. Alternatively, what we are seeing may reflect urban transmission by Culex within Barquisimeto (the 4th largest city in Venezuela)—ie, there may be both urban and sylvatic cycles, with sylvatic transmission dependent on small mammal reservoirs, and the urban transmission reflecting direct transmission among humans. Further studies are needed to explore these hypotheses and better define the epidemiology of this pathogen.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Funding. Work was supported in part by the National Institutes of Health grant R01 AI126357-01S1, awarded to J. G. M. and by the National Science Foundation, grant 1515734.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Arrigo NC, Adams AP, Weaver SC. Evolutionary patterns of eastern equine encephalitis virus in North versus South America suggest ecological differences and taxonomic revision. J Virol 2010; 84:1014–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vittor AY, Armien B, Gonzalez P et al. Epidemiology of emergent madariaga encephalitis in a region with endemic Venezuelan equine encephalitis: initial host studies and human cross-sectional study in Darien, Panama. PLoS Negl Trop Dis 2016; 10:e0004554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carrera JP, Forrester N, Wang E et al. Eastern equine encephalitis in Latin America. N Engl J Med 2013; 369:732–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luciani K, Abadía I, Martínez-Torres AO et al. Madariaga virus infection associated with a case of acute disseminated encephalomyelitis. Am J Trop Med Hyg 2015; 92:1130–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aguilar PV, Robich RM, Turell MJ et al. Endemic eastern equine encephalitis in the Amazon region of Peru. Am J Trop Med Hyg 2007; 76:293–8. [PubMed] [Google Scholar]
- 6. Lednicky J, De Rochars VM, Elbadry M et al. Mayaro virus in child with acute febrile illness, Haiti, 2015. Emerg Infect Dis 2016; 22:2000–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lednicky J, Beau De Rochars VM, El Badry M et al. Zika virus outbreak in Haiti in 2014: molecular and clinical data. PLoS Negl Trop Dis 2016; 10:e0004687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Morais Bronzoni RV, Baleotti FG, Ribeiro Nogueira RM, Nunes M, Moraes Figueiredo LT. Duplex reverse transcription-PCR followed by nested PCR assays for detection and identification of Brazilian alphaviruses and flaviviruses. J Clin Microbiol 2005; 43:696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maher-Sturgess SL, Forrester NL, Wayper PJ et al. Universal primers that amplify RNA from all three flavivirus subgroups. Virol J 2008; 5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trifinopoulos J, Nguyen LT, von Haeseler A, Minh BQ. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res 2016; 44:W232–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Minh BQ, Nguyen MA, von Haeseler A. Ultrafast approximation for phylogenetic bootstrap. Mol Biol Evol 2013; 30:1188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mavian C, Rife BD, Dollar JJ et al. Emergence of recombinant Mayaro virus strains from the Amazon basin. Sci Rep 2017; 7:8718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.