Abstract
Background
Identifying biomarkers to enrich prognostication and risk predictions in individuals at high risk of developing psychosis will enable stratified early intervention efforts. Brain-derived neurotrophic factor has been widely studied in schizophrenia and in first-episode psychosis with promising results. The aim of this study was to examine the levels of serum brain-derived neurotrophic factor between healthy controls and individuals with ultra-high risk of psychosis.
Methods
A sample of 106 healthy controls and 105 ultra-high risk of psychosis individuals from the Longitudinal Youth at Risk Study was included in this study. Ultra-high risk of psychosis status was determined using the Comprehensive Assessment of At-Risk Mental State at recruitment. Calgary Depression Scale for Schizophrenia was used to assess the severity of depression. All participants were followed up for 2 years, and ultra-high risk of psychosis remitters were defined by ultra-high risk of psychosis individuals who no longer fulfilled Comprehensive Assessment of At-Risk Mental State criteria at the end of the study period. Levels of brain-derived neurotrophic factor were measured in the serum by enzyme-linked immunosorbent assay method.
Results
The ultra-high risk of psychosis group had significantly higher baseline levels of serum brain-derived neurotrophic factor compared with the control group (3.7 vs 3.3 ng/mL, P=.018). However, baseline levels of serum brain-derived neurotrophic factor did not predict the development of psychosis (OR=0.64, CI=0.40–1.02) or remission (OR=0.83, CI=0.60–1.15) from ultra-high risk of psychosis status.
Conclusion
Findings from our study did not support a role for serum brain-derived neurotrophic factor in predicting outcomes in ultra-high risk of psychosis individuals. However, the finding of higher levels of serum brain-derived neurotrophic factor in ultra-high risk of psychosis individuals deserves further study.
Keywords: brain-derived neurotrophic factor, ultra-high risk of psychosis, peripheral markers
Significance Statement
Levels of serum brain-derived neurotrophic factor (BDNF) were significantly higher in participants identified to be at ultra-high risk of psychosis (UHR) than in healthy controls. This difference may be due to usage of antidepressants. In addition, baseline level of serum BDNF did not predict conversion to psychosis or remission status in the UHR group. Findings from our study did not support a role for serum BDNF in predicting outcomes in UHR individuals. However, the finding of higher levels of serum BDNF in UHR individuals deserves further study.
Introduction
The ultra-high risk state for psychosis (UHR) was conceived to be akin to a prodromal phase for psychosis but identified prospectively (Yung et al., 2005). Over the years, using well-researched and clinically operationalized criteria, conversion rates across studies have declined considerably (Yung et al., 2008; Ruhrmann et al., 2010; Simon and Umbricht, 2010). It is now clear that while a significant proportion develops psychosis, the majority do not. While studies have identified a list of predictors to aid clinicians in stratifying the UHR sample by different risk probabilities to guide clinical decision making in early intervention strategies, none of the predictors have included biological measurements (Fusar-Poli et al., 2013).
Brain-derived neurotrophic factor (BDNF) has been widely studied for association with development, regeneration, survival, and maintenance of neurons in the brain (Huang and Lee, 2006; Nurjono et al., 2012). Meta-analyses drew attention to the role of BDNF in mental disorders such as mood disorders, Alzheimer’s disease, and psychosis, suggesting BDNF as a candidate biomarker for diagnostic and prognostic purposes for disease outcomes and other comorbidities. (Brunoni et al., 2008; Green et al., 2011; Ji et al., 2015). Although BDNF was thought to influence the predisposition in schizophrenia development, there is a lack of longitudinal perspective in the evaluation of prognostic utility of BDNF in psychosis (Nurjono et al., 2012).
Levels of BDNF are influenced by obesity, smoking, aging, gender, and medications (Golden et al., 2010; B. H. Lee and Kim, 2010; Zhang et al., 2010; Bus et al., 2011; Toll and Mané, 2015), all of which have some association with mental disorders. BDNF’s role in metabolism and food regulation through modulation of hypothalamus has been implicated in the development of obesity (Lebrun et al., 2006; Araya et al., 2008; Lee et al., 2016). Smoking, which is prevalent in schizophrenia and ultra-high risk groups, has also been associated with an elevation in serum BDNF level due to the effects of chronic nicotine exposure on the hippocampus (Kenny et al., 2000; Lee et al., 2013, 2016). Depression was reported to be the most frequent diagnosis in UHR (Lim et al., 2015), and antidepressant treatments stimulate neurogenesis, synaptogenesis, and neuronal maturation and increase BDNF activity, thus increasing serum BDNF levels in depressed patients (Lee and Kim, 2010). These factors further confound the relationship between BDNF and mental disorders. However, in a meta-analysis of 17 studies by Green et al (2011), only 1 study included obesity and smoking as confounders in the comparison of serum BDNF levels between schizophrenia and healthy controls (Green et al., 2011).
The present study aims to evaluate the serum BDNF levels in a group of UHR individuals from the Longitudinal Youth at Risk Study (LYRIKS) (Lee et al., 2013; Lim et al., 2015) and to examine the association between conversion and remission status at 24-month follow-up with serum BDNF level at recruitment.
Methods
Study Settings and Subjects
LYRIKS was a prospective, observational study conducted in Singapore on youths aged 14 to 29 to assess risk factors for psychosis. Details of the study have been previously reported (Lee et al., 2013; Mitter et al., 2014; Lim et al., 2015). In brief, participants were recruited from psychiatric clinics, various community agencies including educational institutes and social services, or were self-referred. Participants were excluded if they had a past or current history of psychosis or mental retardation, were currently using illicit substances, were taking mood stabilizers, had previous antipsychotic exposure of >5 mg/d haloperidol for 3 weeks (or equivalent) or were on an antipsychotic at the point of recruitment, or had medical causes associated with their attenuated psychotic symptoms. Data collected at baseline and 24-month/final study visit was used for this study.
Ethics approval for this study was provided by the National Healthcare Group’s Domain Specific Review Board. After complete description of the study to the participants, written informed consent was obtained. Participants who provided a blood sample were included in the present study.
Assessments
The Comprehensive Assessment of At-Risk Mental State (CAARMS) is a semistructured interview used to evaluate if an individual meets the UHR criteria (Yung et al., 2005). The positive symptom subscale was used, which assesses 4 symptom domains: Unusual Thought Content (UTC), Non-bizarre Ideas (NBI), Perceptual Abnormalities (PA), and Disorganized Speech (DS). Each symptom was rated for the maximum intensity, frequency and duration, pattern, and related distress over the last 1 year.
UHR positive individuals fall into one or more of the following groups: vulnerability group (at risk of psychosis due to the combination of a trait risk factor and a significant deterioration in mental state and/or function), attenuated psychotic symptoms group (at risk of psychosis due to subthreshold psychotic syndrome), and brief limited intermittent psychotic symptoms group (at risk of psychosis due to a recent history of frank psychotic symptoms that resolved spontaneously within a week). The CAARMS interview was repeated at every time point. CAARMS total score refers to the sum of multiples of intensity and frequency of CAARMS subscales—UTC, NBI, PA, and DS (Morrison et al., 2012). Remission status was determined through a change in CAARMS status from positive at baseline to not meeting UHR criteria at 24 months or the final study visit, whichever is the latest.
The Structured Clinical Interview for DSM IV Axis I Disorders was used to assess for the presence of any psychiatric disorders. This interview was performed at recruitment and again at the end of the follow-up period or when a participant developed psychosis.
UHR positive participants were further assessed, at recruitment and 6-monthly intervals, on the Calgary Depression Scale for Schizophrenia (CDSS).
Measurement of Serum BDNF Levels
Venous blood was collected into a serum separating tube from study participants and allowed to coagulate at room temperature for approximately 30 minutes. Serum was collected after centrifugation for 10 minutes at 4°C using a clinical centrifuge (Hettich). BDNF was then measured using commercially available ELISA (Millipore) with the range of sensitivity from 7.8 to 500 pg/mL and intra-assay variations of <20%. Briefly, the serum samples were diluted 1:2 in sample diluent provided by the kit and ran in duplicates. The samples were subsequently incubated in 1:1000 biotinylated mouse anti–human BDNF monoclonal antibody overnight at 4°C before they were incubated in 1:1000 biotinylated mouse anti-BDNF monoclonal antibody for 2 hours and 1:1000 streptavidin–horseradish peroxidase conjugate solution for 1 hour at room temperature. TMB/E substrate was then added to each sample for 15 minutes, and the reaction was stopped with the addition of stop solution provided as part of the kit. Immediately after the reactions were stopped, the plate was read at 450 nm absorbance. Samples with readings outside the range of sensitivity were excluded from subsequent analyses.
Data Analysis
Data was analyzed on SPSS Statistics version 23 (IBM Co.). Descriptive statistics were tabulated for control and UHR positive groups. Statistical significance was set at P<.05. Categorical variables were examined using chi-squared test. Continuous variables were analyzed via Mann-Whitney U test and data were reported as mean and SD. Kruskal-Wallis H was used to compare serum BDNF levels among control, UHR on antidepressants, and UHR not on antidepressants at baseline. A multivariate linear regression model was employed to examine the associations between CAARMS scores, CDSS scores, and serum BDNF levels of UHR positive individuals. Logistic regression analysis was used to study the relationship between remission, conversion status, and serum BDNF level at baseline. Age, gender, body mass index (BMI), smoking status, and use of antidepressants at baseline were included in the regression models as they have been shown to influence serum BDNF levels (Green et al., 2011; Björkholm and Monteggia, 2016; Lee et al., 2016).
Results
Participant Demographics
A total of 106 healthy controls and 105 individuals with UHR were included in the present study. A detailed description of the demographics can be found in Table 1. There were no differences in ethnicity, gender, age, BMI, and smoking status between the 2 groups. Approximately 44% of UHR individuals were on antidepressants at the time of recruitment.
Table 1.
Study Participants’ Demographics at Baseline
| Control (n=106) |
UHR (n=105) |
||
|---|---|---|---|
| n (%) | n (%) | P Valuea | |
| Ethnicity | .754 | ||
| Chinese | 72 (68.6) | 75 (71.4) | |
| Malay | 20 (19.0) | 20 (19.0) | |
| Indian | 12 (11.4) | 8 (7.6) | |
| Others | 1 (1.0) | 2 (1.9) | |
| Gender | .251 | ||
| Male | 63 (60.0) | 71 (67.6) | |
| Female | 42 (40.0) | 34 (32.4) | |
| Smoking status | .052 | ||
| No | 86 (81.9) | 74 (70.5) | |
| Yes | 19 (18.1) | 31 (29.5) | |
| Psychiatric comorbidities | |||
| Bipolar disorder | 2 (1.9) | ||
| Depressive disorders | 1 (0.9) | 39 (37) | |
| Anxiety disorders | 1 (0.9) | 41 (39) | |
| Substance use disorders | 2 (1.9) | ||
| Adjustment disorders | 6 (5.7) | ||
| Antidepressants usageb | |||
| No | 105 (100.0) | 59 (56.2) | |
| Yes | 0 (0.0) | 46 (43.8) | |
| Mean (SD) | Mean (SD) | ||
| Age | 22.0 (3.8) | 21.8 (3.6) | .13 |
| BMI | 22.3 (3.6) | 22.5 (4.9) | .546 |
| CAARMS totalc | 1.0 (2.6) | 24.1 (15.1) | <.005 |
| CDSS | — | 5.8 (5.0) | |
| Serum BDNF (ng/mL) | 3.3 (1.5) | 3.7 (1.4) | 0.018 |
Abbreviations: BMI, body mass index; CAARMS,Comprehensive Assessment of At-Risk Mental State; CDSS,Calgary Depression Scale for Schizophrenia; BDNF,brain derived neurotrophic factor.
aChi-squared test for categorical variables, Mann-Whitney U test for continuous variables.
bAntidepressants used in current study were amitriptyline, clomipramine, venlafaxine, dothiepin, mirtazapine, fluvoxamine, fluoxetine, sertraline, paroxetine, and escitalopram.
cCAARMS=(IUTC*FUTC) + (INBI*FNBI) + (IPA*FPA) + (IDS*FDS).
There were no differences in ethnicity, gender, smoking status, age, and BMI among healthy controls, remitters, nonremitters, and converters at baseline. While healthy controls (0.9±2.8) had a significantly lowest baseline CAARMS total score (P<.05), there was no difference among the remitters (24.8±17.0), nonremitters (22.9±13.8), and converters (25.6±12.5) CAARMS total score. There was also no difference in baseline CDSS scores among remitters (5.5±4.9), nonremitters (5.8±5.3), and converters (6.3±5.0) (P=.879). There was a significant number of remitters (37%), nonremitters (33%), and converters (45%) who were on antidepressants since baseline (P<.005).
Serum BDNF Level at Baseline and 24-Month Follow-Up
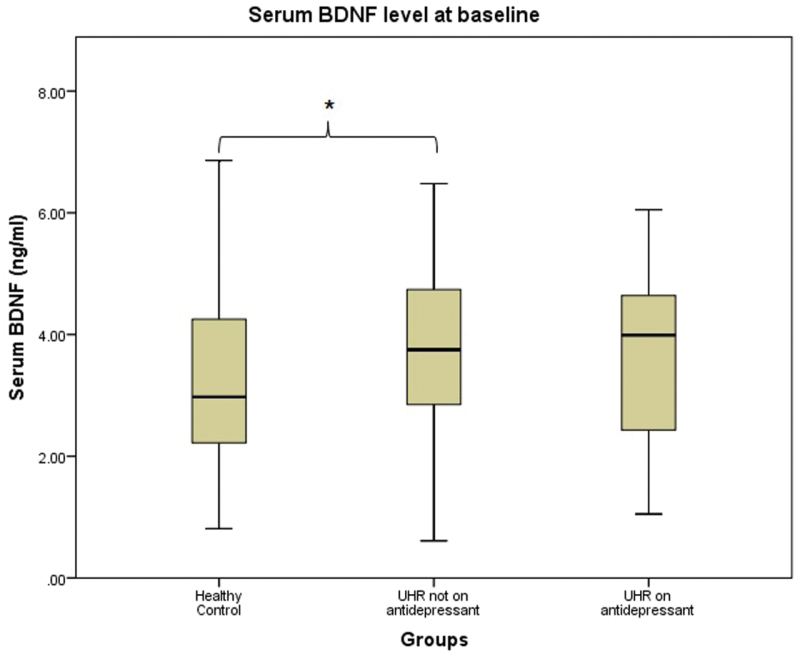
The UHR group was observed to have a significantly higher serum BDNF level than healthy controls at baseline (U=4519.5, P=.018). To investigate the effects of antidepressants on serum BDNF in the current dataset, UHR group was further divided into 2 groups—on antidepressants (n=46) and not on antidepressants (n=59). Mann-Whitney U test was performed to compare serum BDNF levels at baseline between groups. UHR not on antidepressants had a significantly higher baseline serum BDNF level than healthy controls (3.7±1.4 vs 3.3±1.5 ng/mL, U=2473, P=.026). There was no significant difference in baseline serum BDNF levels between healthy controls and UHR on antidepressants, at 3.3±1.5 ng/mL and 3.6±1.5 ng/mL, respectively (U=2046.5, P=.116). A similar observation was made for the difference between UHR on antidepressants and UHR not on antidepressants (U=1322, P=.821) (Figure 1).
Figure 1.
Baseline serum brain-derived neurotropic factor (BDNF) levels between groups.
Follow-up data were available for 71 controls and 71 UHR individuals. Of the 71 UHR individuals with follow-up data, 35 were remitters, 26 were nonremitters, and 10 were converters. We did not observe a significant difference in serum BDNF levels between both time points (see Table 2).
Table 2.
Serum BDNF Levels at Baseline and 24-Month Follow-Up
| Serum BDNF (ng/mL) | |||
|---|---|---|---|
| Baseline | 24-month follow-up | P valuea | |
| Healthy control | n=76 3.3 (1.5) | n=71 3.0 (1.0) | .254 |
| Remitter | n=41 3.7 (1.3) | n=35 3.4 (0.7) | .147 |
| Nonremitter | n=27 3.6 (1.5) | n=26 3.7 (1.5) | .949 |
| Converter | n=11 2.9 (1.8) | n=10 3.4 (1.3) | .241 |
aWilcoxon signed ranks test.
Associations between Baseline Serum BDNF Level and Psychopathology Indices, Conversion, and Remission Status
There was no significant association between baseline levels of serum BDNF with baseline CAARMS and CDSS scores (see Table 3). Baseline levels of serum BDNF were also not associated with conversion (OR=0.64, CI=0.40–1.02) or remission (OR=0.83, CI=0.60–1.15) in the UHR group (see Tables 4 and 5).
Table 3.
Associations between Serum BDNF and Psychopathology at Baseline
| Unadjusted | Adjusted for Gender, Age, BMI, Smoking, and Use of Antidepressants | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | P value | β | 95% CI | P value | |
| CAARMS | 0.10 | -1.03–3.10 | .323 | 0.10 | -1.00–3.11 | .308 |
| CDSS | 0.12 | -0.27–1.08 | .239 | 0.12 | -0.28–1.08 | .248 |
Table 4.
Prediction of Conversion Status at 24-Month Follow-Up Using Serum BDNF at Baseline
| Unadjusted | Adjusted for Gender, Age, BMI, Smoking, and Use of Antidepressants | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P value | Odds Ratio | 95% CI | P value | |
| Serum BDNF | 0.63 | 0.40–1.00 | .051 | 0.64 | 0.40–1.02 | .060 |
Table 5.
Prediction of Remission Status at 24-Month Follow-Up Using Serum BDNF at Baseline
| Unadjusted | Adjusted for Gender, Age, BMI, Smoking, and Use of Antidepressants | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P value | Odds Ratio | 95% CI | P value | |
| Serum BDNF | 0.84 | 0.62–1.14 | .266 | 0.83 | 0.60–1.15 | .183 |
Discussion
Levels of serum BDNF have been commonly reported to be lower in patients with mental disorders, suggesting the utility of BDNF as a biomarker in mood disorders, schizophrenia, and Alzheimer’s disorder (Brunoni et al., 2008; Green et al., 2011; Ji et al., 2015). However, there is no longitudinal study of the application of BDNF as a biomarker. To our knowledge, this study is the first to report the levels of serum BDNF in individuals with UHR. The present study found a higher baseline serum BDNF level in the UHR group. However, baseline levels of BDNF were not associated with severity of symptoms—both psychotic and depressive symptoms. There was also no association between baseline serum BDNF level and conversion or remission status at 24-month follow-up visit.
Contrary to a recent systemic review on drug naïve, first-episode psychosis patients, the present study observed a higher baseline serum BDNF level in UHR individuals (Toll and Mané, 2015). As suggested by previous studies, this difference in serum BDNF level may be due to usage of antidepressants, as almost one-half of UHR individuals were on antidepressants (Fusar-Poli et al., 2014; Lim et al., 2015). However, we did not observe the effect of antidepressant on serum BDNF levels in our dataset. This could be due to varying duration and dosage of psychotropic medications of the UHR group. There has been a continuous effort to identify predictors of psychosis over the past decade. The North American Prodrome Longitudinal Study reported 5 clinical predictors in their participants with high risk of psychosis: genetic risk with functional decline, high unusual thought content scores, high suspicion or paranoia scores, low social functioning, and history of substance abuse (Seidman et al., 2010). Similar to the North American Prodrome Longitudinal Study, the Personal Assessment and Crisis Evaluation Study identified high unusual thought content scores, low functioning, and genetic risk with functional decline to be predictors of psychosis in their cohort (Thompson et al., 2011). Participants with high risk of psychosis in the Basel Early Detection of Psychosis Clinic study, high suspiciousness, high anhedonia, or asociality scores contributed to high transition risk (Riecher-Rössler et al., 2009). Clinical markers, family history of psychosis, neurocognitive, electrophysiological measures, environmental factors, lifestyles, and conducting multicenter studies were suggested to aid in improving the prediction of psychosis (Fusar-Poli et al., 2013). While the abovementioned studies identified predictors through psychopathology indices, the present study is the first attempt to explore the utility of serum BDNF levels in the prediction of conversion and remission in UHR individuals.
The present study has some limitations. Psychotropic medications have been studied to influence serum BDNF levels (Björkholm and Monteggia, 2016). In this observational study, we were unable to adjust or control for participants’ prior use of psychotropic medications. Serum BDNF may not provide a direct index of BDNF level in the central nervous system and may not represent the degree of synaptic plasticity or neuronal maintenance. The small sample size for converters might limit the statistical power to detect a difference. Genotypic information on val66met BDNF polymorphism was unavailable and might have affected expression of serum BDNF levels (Toll and Mané, 2015).
The present study is the first to explore serum BDNF levels in an UHR group and if it adds additional prognostic accuracy in predicting clinical outcomes. While we found, rather unexpectedly, higher levels of serum BDNF in individuals with UHR, this difference might have been caused by antidepressant use. Our study did not support the use of serum BDNF levels in UHR individuals to predict development of psychosis or remission from the UHR state. Due to the diversity of BDNF’s functions, its concentrations may be regulated by lifestyle changes and clinical intervention; future studies of blood BDNF should include the potential confounding effects of clinical interventions and environment factors.
Statement of Interest
None.
Acknowledgments
This study was supported by the National Research Foundation Singapore under the National Medical Research Council Translational and Clinical Research Flagship Programme (grant no. NMRC/TCR/003/2008) and Singapore Ministry of Health’s National Medical Research Council under the Centre Grant Programme (grant no. NMRC/CG/004/2013).
References
- Araya AV, Orellana X, Espinoza J(2008)Evaluation of the effect of caloric restriction on serum BDNF in overweight and obese subjects: preliminary evidences. Endocrine 33:300–304. [DOI] [PubMed] [Google Scholar]
- Björkholm C, Monteggia LM(2016)BDNF—a key transducer of antidepressant effects. Neuropharmacology 102:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni AR, Lopes M, Fregni F(2008)A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol 11:1169–1180. [DOI] [PubMed] [Google Scholar]
- Bus BA, Molendijk ML, Penninx BJ, Buitelaar JK, Kenis G, Prickaerts J, Elzinga BM, Voshaar RC(2011)Determinants of serum brain-derived neurotrophic factor. Psychoneuroendocrinology 36:228–239. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Nelson B, Valmaggia L, Yung AR, McGuire PK(2014)Comorbid depressive and anxiety disorders in 509 individuals with an at-risk mental state: impact on psychopathology and transition to psychosis. Schizophr Bull 40:120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, et al. (2013)The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry 70:107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden E, Emiliano A, Maudsley S, Windham BG, Carlson OD, Egan JM, Driscoll I, Ferrucci L, Martin B, Mattson MP(2010)Circulating brain-derived neurotrophic factor and indices of metabolic and cardiovascular health: data from the Baltimore longitudinal study of aging. Plos One 5:e10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MJ, Matheson SL, Shepherd A, Weickert CS, Carr VJ(2011)Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol Psychiatry 16:960–972. [DOI] [PubMed] [Google Scholar]
- Huang TL, Lee CT(2006)Associations between serum brain-derived neurotrophic factor levels and clinical phenotypes in schizophrenia patients. J Psychiatr Res 40:664–668. [DOI] [PubMed] [Google Scholar]
- Ji H, Dai D, Wang Y, Jiang D, Zhou X, Lin P, Ji X, Li J, Zhang Y, Yin H, Chen R, Zhang L, Xu M, Duan S, Wang Q(2015)Association of bdnf and bche with Alzheimer’s disease: meta-analysis based on 56 genetic case-control studies of 12,563 cases and 12,622 controls. Exp Ther Med 9:1831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, File SE, Rattray M(2000)Acute nicotine decreases, and chronic nicotine increases the expression of brain-derived neurotrophic factor MRNA in rat hippocampus. Brain Res Mol Brain Res 85:234–238. [DOI] [PubMed] [Google Scholar]
- Lebrun B, Bariohay B, Moyse E, Jean A(2006)Brain-derived neurotrophic factor (BDNF) and food intake regulation: a minireview. Auton Neurosci 126–127:30–38. [DOI] [PubMed] [Google Scholar]
- Lee BH, Kim YK(2010)The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investig 7:231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Nurjono M, Lee TS(2016)Levels of serum brain-derived neurotrophic factor in schizophrenia. J Nerv Ment Dis 204:636–639. [DOI] [PubMed] [Google Scholar]
- Lee J, Rekhi G, Mitter N, Bong YL, Kraus MS, Lam M, Rapisarda A, Lee TS, Subramaniam M, Chong SA, Keefe RS(2013)The longitudinal youth at risk study (LYRIKS)–an Asian UHR perspective. Schizophr Res 151:279–283. [DOI] [PubMed] [Google Scholar]
- Lim J, Rekhi G, Rapisarda A, Lam M, Kraus M, Keefe RS, Lee J(2015)Impact of psychiatric comorbidity in individuals at ultra high risk of psychosis—findings from the longitudinal youth at risk study (LYRIKS). Schizophr Res 164:8–14. [DOI] [PubMed] [Google Scholar]
- Mitter N, Nah GQ, Bong YL, Lee J, Chong SA(2014)Longitudinal youth-at-risk study (LYRIKS): outreach strategies based on a community-engaged framework. Early Interv Psychiatry 8:298–303. [DOI] [PubMed] [Google Scholar]
- Morrison AP, French P, Stewart SL, Birchwood M, Fowler D, Gumley AI, Jones PB, Bentall RP, Lewis SW, Murray GK, Patterson P, Brunet K, Conroy J, Parker S, Reilly T, Byrne R, Davies LM, Dunn G(2012)Early detection and intervention evaluation for people at risk of psychosis: multisite randomised controlled trial. Bmj 344:e2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurjono M, Lee J, Chong SA(2012)A review of brain-derived neurotrophic factor as a candidate biomarker in schizophrenia. Clin Psychopharmacol Neurosci 10:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecher-Rössler A, Pflueger MO, Aston J, Borgwardt SJ, Brewer WJ, Gschwandtner U, Stieglitz RD(2009)Efficacy of using cognitive status in predicting psychosis: a 7-year follow-up. Biol Psychiatry 66:1023–1030. [DOI] [PubMed] [Google Scholar]
- Ruhrmann S, Schultze-Lutter F, Salokangas RK, Heinimaa M, Linszen D, Dingemans P, Birchwood M, Patterson P, Juckel G, Heinz A, Morrison A, Lewis S, von Reventlow HG, Klosterkötter J(2010)Prediction of psychosis in adolescents and young adults at high risk: results from the prospective european prediction of psychosis study. Arch Gen Psychiatry 67:241–251. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD, McGlashan TH, Perkins DO, Tsuang MT, Walker EF, Woods SW, Bearden CE, Christensen BK, Hawkins K, Heaton R, Keefe RS, Heinssen R, Cornblatt BA, North American Prodrome Longitudinal Study (NAPLS) Group (2010)Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Arch Gen Psychiatry 67:578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AE, Umbricht D(2010)High remission rates from an initial ultra-high risk state for psychosis. Schizophr Res 116:168–172. [DOI] [PubMed] [Google Scholar]
- Thompson A, Nelson B, Yung A(2011)Predictive validity of clinical variables in the “at risk” for psychosis population: international comparison with results from the North American prodrome longitudinal study. Schizophr Res 126:51–57. [DOI] [PubMed] [Google Scholar]
- Toll A, Mané A(2015)Brain-derived neurotrophic factor levels in first episode of psychosis: a systematic review. World J Psychiatry 5:154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung AR, Nelson B, Stanford C, Simmons MB, Cosgrave EM, Killackey E, Phillips LJ, Bechdolf A, Buckby J, McGorry PD(2008)Validation of “prodromal” criteria to detect individuals at ultra high risk of psychosis: 2 year follow-up. Schizophr Res 105:10–17. [DOI] [PubMed] [Google Scholar]
- Yung AR, Yuen HP, McGorry PD, Phillips LJ, Kelly D, Dell’Olio M, Francey SM, Cosgrave EM, Killackey E, Stanford C, Godfrey K, Buckby J(2005)Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Aust N Z J Psychiatry 39:964–971. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Xiu MH, Chen DC, Yang FD, Wu GY, Lu L, Kosten TA, Kosten TR(2010)Nicotine dependence and serum BDNF levels in male patients with schizophrenia. Psychopharmacology (Berl) 212:301–307. [DOI] [PubMed] [Google Scholar]