Abstract
Background
The volatile anesthetic isoflurane may exert a rapid and long-lasting antidepressant effect in patients with medication-resistant depression. The mechanism underlying the putative therapeutic actions of the anesthetic have been attributed to its ability to elicit cortical burst suppression, a distinct EEG pattern with features resembling the characteristic changes that occur following electroconvulsive therapy. It is currently unknown whether the antidepressant actions of isoflurane are shared by anesthetics that do not elicit cortical burst suppression.
Methods
In vivo electrophysiological techniques were used to determine the effects of isoflurane and halothane, 2 structurally unrelated volatile anesthetics, on cortical EEG. The effects of anesthesia with either halothane or isoflurane were also compared on stress-induced learned helplessness behavior in rats and mice.
Results
Isoflurane, but not halothane, anesthesia elicited a dose-dependent cortical burst suppression EEG in rats and mice. Two hours of isoflurane, but not halothane, anesthesia reduced the incidence of learned helplessness in rats evaluated 2 weeks following exposure. In mice exhibiting a learned helplessness phenotype, a 1-hour exposure to isoflurane, but not halothane, reversed escape failures 24 hours following burst suppression anesthesia.
Conclusions
These results are consistent with a role for cortical burst suppression in mediating the antidepressant effects of isoflurane. They provide rationale for additional mechanistic studies in relevant animal models as well as a properly controlled clinical evaluation of the therapeutic benefits associated with isoflurane anesthesia in major depressive disorder.
Keywords: learned helplessness, treatment-resistant depression, halothane, electroconvulsive therapy, fast-acting antidepressant
Significance Statement
Preliminary clinical trials have suggested that isoflurane anesthesia is as effective as electroconvulsive therapy in medication-resistant depression. Both treatments produce a temporary suppression in electroencephalographic activity that could be functionally linked to their therapeutic effects. Recently, it was shown that isoflurane and another volatile anesthetic, halothane, activate TrkB receptors, implying that isoflurane and the rapidly acting antidepressant ketamine share a common mechanism of action. Here we show that isoflurane, in contrast to halothane, induces burst suppression in cortical electroencephalographic activity. Isoflurane, but not halothane, also reverses helpless behavior in mice and prevents its development in rats. These results provide rationale for additional mechanistic studies in animal models and a definitive evaluation of the potential therapeutic benefits of isoflurane anesthesia in major depressive disorder. In addition to providing an alternative treatment strategy for patients, these studies may converge on mechanisms that provide an explicit functional link between burst suppression anesthesia and electroconvulsive therapy.
Introduction
Major depressive disorder is a disabling and life-threatening disease with a lifetime prevalence of 17% in the US population (Kessler et al., 2003). While 50% to 70% of patients diagnosed with this disorder respond to conventional antidepressant medications, only one-half to one-third achieve full remission (Olchanski et al., 2013). Upwards of 30% of patients with medication-resistant depression, defined as the failure to achieve full remission with an adequate dose and duration of treatment (Fava, 2003), also fail to achieve remission after 4 sequential trials with different antidepressant medications. This type of chronic medication-resistant depression is associated with persistent vocational disability, substantially higher risk of suicide, and significantly higher health care utilization costs (Russell et al., 2004; Dunner et al., 2006; Olchanski et al., 2013).
Electroconvulsive therapy (ECT) is an effective treatment for medication-resistant depression (Kellner et al., 2012). ECT induces a generalized tonic-clonic seizure characterized by paroxysmal EEG discharges followed by the abrupt onset of a flat (i.e., isoelectric) EEG referred to as postictal suppression. Efforts to associate specific EEG changes with treatment response have implicated the duration of postictal suppression as a determinant of clinical outcome following ECT (Krystal et al., 1993; Suppes et al., 1996; Perera et al., 2004; Azuma et al., 2007, 2010; Kranaster et al., 2013). Support for the role of cortical isoelectricity in the mechanism of action of ECT was obtained from early clinical studies comparing its efficacy with repeated isoflurane (ISO) treatments. Like ECT, deep anesthesia with ISO elicits distinctive EEG changes characterized by brief, high-amplitude, low-frequency bursts followed by prolonged intervals of electrical suppression. Several preliminary clinical trials found that repeated ISO anesthesia to EEG burst suppression is as effective as ECT in reducing symptoms in patients with major depressive disorder (Langer et al., 1985, 1995; Carl et al., 1988; Engelhardt et al., 1993; Weeks et al., 2013) without producing the deleterious side effects typically associated with ECT (Carl et al., 1988; Weeks et al., 2013). However, 2 groups reporting limited benefit of burst suppression anesthesia in patients (Greenberg et al., 1987; García-Toro et al., 2001, 2004) along with the lack of preclinical data supporting a role for ISO anesthesia in animal models of MDD stalled interest in this potential treatment.
Recently, Antila and colleagues (2017) reported that a single exposure to ISO anesthesia prevented the development of learned helplessness in rats, an established model of depression-related maladaptive behavior (Maier and Seligman, 2016). The antidepressant-like effects of ISO were accompanied by activation of the brain-derived neurotropic factor receptor, TrkB, an effect implicated in the mechanism of action of the rapid-acting antidepressant, ketamine (Li et al., 2010; Autry et al., 2011; Liu et al., 2012). Notably, halothane (HALO), a structurally unrelated volatile anesthetic, was found to induce comparable changes in TrkB signaling, suggesting that it may share ISO’s antidepressant actions (Antila et al., 2017). To test this hypothesis, we compared the effects of ISO and HALO anesthesia on learned helplessness in rats and mice. The doses used were comparable with those previously shown to activate TrkB but differed in their ability to produce EEG burst suppression. The results indicate that ISO, but not HALO anesthesia, prevents the development and reverses learned helplessness. These findings provide additional support for the efficacy of ISO as a rapidly-acting antidepressant and suggest the involvement of burst suppression as the relevant mechanism underlying these effects.
Methods
Animals
Adult male Sprague-Dawley rats (n=46) and adult male CD1 mice (n=101) obtained from Charles River were maintained on a 12-hour-light/-dark cycle and provided ad libitum access to food and water. All experiments and procedures were conducted in strict accordance with recommendations in The Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (2011) and the University of Maryland School of Medicine Institutional Animal Care and Use Committee.
EEG Recording
Anesthesia was induced in rats (n=6) and mice (n=12) with HALO or ISO (3% in 100% O2, 1 Liter/min) in a ventilated induction chamber, and the animals were mounted in a stereotaxic instrument equipped with a nose cone from which additional anesthetic was delivered using precision vaporizers (HALO, Ohmeda Fluotech 4; ISO, VetEquip). Body temperature was monitored continuously and maintained at 36°C using a feedback-controlled heating pad. The scalp was incised and 2 small burr holes drilled in the skull overlying the frontal cortex. A pair of stainless-steel (SNEX, Rhodes Medical Equipment) or Ag/AgCl pellet electrodes (Warner Instruments) were positioned on the surface of the cortex and submerged in mineral oil. EEG was recorded between the left and right hemispheres using a differential amplifier (DAM-80, World Precision Instruments), filtered (1–100 Hz bandpass), and digitized at 1 KHz using a 16-bit laboratory interface (Digidata 1321A; Molecular Devices). During the recording, ISO or HALO concentration was increased in a stepwise fashion. After obtaining the response to the highest dose of the first anesthetic administered, animals were switched to an equivalent concentration of the alternate anesthetic and recordings continued for an additional 10 to 15 minutes. Cortical EEG was acquired continuously during the experiment and recorded for offline analysis using Spike 2 (CED). The burst suppression ratio (BSR) was computed using a thresholding algorithm that determined the time of occurrence and the duration of each burst in the EEG record. The interval between consecutive burst starts was taken as the event duration and the BSR computed as follows:
Learned Helplessness in Rats
Rats were individually housed and assigned to 1 of 3 treatment groups including ISO (n=12), HALO (n=12), or control (CON, n=22). Animals in the ISO and HALO groups were induced with isoflurane (3%) or halothane (3%), respectively, in 100% O2 (1 l/min) using a ventilated induction chamber. Once unconscious, rats were placed in a stereotaxic instrument using atraumatic earbars, and a nosecone was used to deliver either ISO (2%) or HALO (1.5%) continuously for 2 hours. A feedback-controlled heating pad was used to maintain body temperature at 36°C. Following anesthesia exposure, rats recovered on a warm heating pad until ambulatory at which point they were returned to their home cages. CON rats were brought to the surgery room and returned to the animal quarters 2 hours later without having received either anesthetic.
Two weeks following the initial treatment, all rats underwent a modified 2-day learned helplessness procedure based on previously published studies in the rat (Vollmayr and Henn, 2001; Shirayama et al., 2002). On Day 1, individual animals were placed on one side of a 2-chamber shuttle-box (21×21×16 cm; Med Associates) configured to deliver scrambled electric shocks to metal floor bars. The chamber was equipped with 4 pairs of parallel horizontal infrared photobeams positioned 12 cm apart and 3 cm above the grid floor. Following a brief acclimation period, the rats received a total of 120 shocks (0.8 mA) of variable duration (5–15 seconds) applied at random intervals (5–15 seconds) during a 40-minute session. At the end of the session, all rats were returned to their home cages and the shuttle box wiped clean with 70% ethanol.
The following day, rats were returned to the same chamber used on Day 1 where they received an additional 30 inescapable foot-shocks using the stimulus parameters described above. Following the last shock, the house lights came on initiating a new set of 30 trials that began with a 5-second tone signaling the opening of a guillotine door (9 cm wide by 11 cm high) separating the 2 compartments. At the termination of the tone, a scrambled electrical shock (0.8 mA) was applied to the floor grid for a maximum of 15 seconds. Crossing to the opposite, non-electrified side of the chamber and breaking the far photobeam at any point during the shock triggered closure of the guillotine door and termination of the trial which was scored as an escape. Crossing to the nonelectrified side of the box in response to the predictive tone and thus prior to shock delivery rarely occurred but was scored as an avoidance response. Failure to escape the shock within the 15-second interval terminated the trial, which was scored as an escape failure.
Learned Helplessness in Mice
On arrival, mice were housed in groups of 5 animals per cage. Consistent with previously published reports (Zanos et al., 2016), the learned helplessness procedure consisted of 4 phases: inescapable shock training, screening, treatment, and test. To avoid disrupting their social environment, mice were housed with their original cage-mates throughout all phases of the experiment. For the inescapable shock training phase (Day 1 to induction), mice were placed in one side of a 2-chambered shuttle box (34×37×18 cm; Coulbourn Instruments). A door separating the chambers remained closed. Following a 5-minute adaptation period, 120 inescapable foot-shocks (0.45 mA, 15-second duration, randomized 45-second average, range 40–50 second inter-shock interval) were delivered through a grid-floor. Following the session, mice were returned to their home cage. The following day (Day 2 to screening), mice were placed in one of the 2 compartments of the apparatus for a 5-min period and immediately after a 3-second foot-shock (0.45 mA) was delivered, and the door between the 2 chambers was raised simultaneously. Crossing over into the second chamber terminated the shock. If the animal did not cross over, the shock terminated after 3 seconds. A total of 30 screening trials of escapable shocks was presented to each mouse with an average of 30 seconds (range 25–35 seconds) delay between each trial.
On Day 3 (treatment) mice that developed helplessness behavior (>20 total escape failures and >5 escape failures during the last 10 screening shocks) received the assigned treatment (controls: no exposure, 2.0% HALO for 1 hour, or 2.5% ISO for 1 hour) 24 hours following screening. Control mice remained in their home cages while mice in the ISO and HALO groups were induced individually with their respective anesthetics and subsequently transferred to a divided and ventilated holding chamber (4 mice/chamber) maintained at 37°C where they continued to receive their respective anesthetic for a total of 1 hour. Mice were allowed to recover from the effects of the anesthesia in individual cages before they were returned to their home cage. During the LH test phase (Day 4), the animals were placed on one side of the shuttle box and after a 5-minute adaptation period, a 0.45-mA shock was delivered concomitantly with the door opening for the first 5 trials. For the next 40 trials, the shock was delivered for 2 seconds prior to opening the door. Crossing over to the second chamber terminated the shock. If the animal did not cross over to the other chamber, the shock was terminated after 24 seconds. A total of 45 trials of escapable shocks was presented to each mouse with 30-second inter-trial intervals. The number of escape failures was recorded for each mouse by automated computer software (Graphic State v3.1; Coulbourn Instruments).
Statistical Analysis
All data are expressed as the mean±SEM. Sample sizes were estimated from previous studies using similar techniques and animals were randomly assigned to their respective treatment groups. All statistical analyses were conducted in SigmaPlot ν.12.5 (Systat Software). Omnibus testing was conducted using a 1- or 2-way ANOVA unless the underlying assumptions of normality and/or equal variance were violated, in which case the Kruskal–Wallis 1-way ANOVA on ranks was substituted.
Results
Differential Effects of Halothane and Isoflurane on Cortical EEG
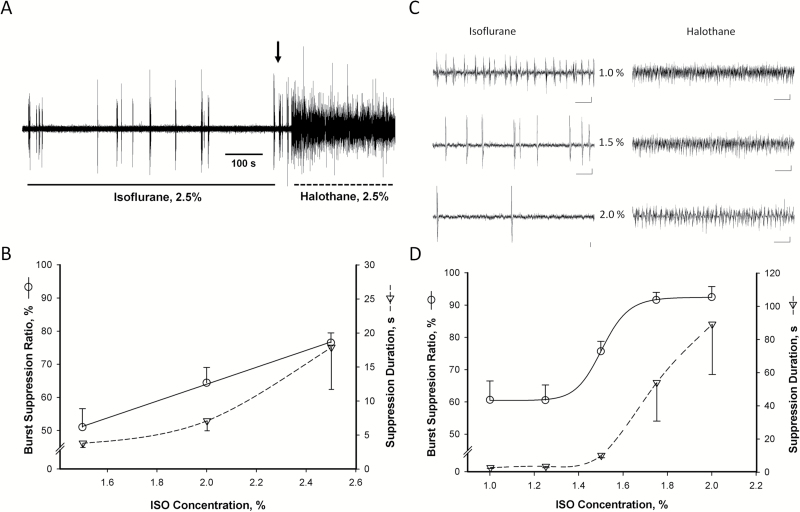
EEG recordings obtained from Sprague-Dawley rats anesthetized with ISO exhibited pronounced burst suppression consisting of an initial burst of high-amplitude activity followed by a prolonged interval of electrical quiescence (Figure 1A). At the lowest concentration tested (1.5%), the duration of the burst and the subsequent interval of isoelectric activity were equivalent, yielding a BSR of ~50%. Increasing the concentration of ISO resulted in a dose-dependent increase in BSR (Figure 1B, F(2,12)=7.3, P<.01) that ranged from 69% to 82% at the highest concentration tested (2.5%). Although a small reduction in burst duration typically occurred at higher concentrations, the dose-dependent increase in BSR under ISO was largely attributable to an increase in the duration of the postburst suppression in EEG activity (Figure 1B, H(2)=7.7, P<.05), which at the highest concentration tested ranged from 7 to 41 seconds. By contrast, HALO did not elicit EEG burst suppression at any of the concentrations tested (up to 3%; the highest dose that did not induce respiratory depression) even after a prolonged exposure to ISO (Figure 1A).
Figure 1.
Isoflurane (ISO) but not halothane (HALO) elicits cortical burst suppression in rodents. (A) Representative tracing illustrating burst suppression EEG pattern in a rat anesthetized with ISO (2.5%). Switching from ISO to HALO (arrow) results in a short latency cessation in burst suppression and the emergence of a high-voltage continuous slow wave EEG pattern characteristic of HALO anesthesia. (B) Dose-response curve illustrating the relationship between burst suppression ratio (BSR, left ordinate) and duration of the postburst suppression (right ordinate) as a function of ISO concentration. Each point represents the mean±SEM obtained from 4 to 6 rats. (C) Representative EEG recordings illustrating the effects of increasing concentrations of ISO (left) and HALO (right) on EEG activity in CD-1 mice. (D) Dose-response curve describing the relationship between BSR (left ordinate) and duration of the postburst suppression (right ordinate) as a function of ISO concentration. Each point represents the mean±SEM obtained from 6 mice.
We also recorded cortical EEG in CD-1 mice exposed to increasing concentrations of both anesthetics. As anticipated, ISO anesthesia was associated with a burst suppression EEG pattern qualitatively similar to that observed in rats (Figure 1C, left panel). The BSR increased as a function of ISO dose (Figure 1D, F(4,25)=15.0, P<.001). As in rats, burst duration tended to decrease with increasing doses of ISO (1% ISO: 1.6±0.16 s, 2% ISO: 1.0±0.19 s), but these differences did not reach statistical significance (F(4,25)=2.4, P=.08). However, the duration of the postburst isoelectric interval increased significantly as a function of ISO concentration (Figure 1D; F(4,25)=5.04, P<.005). As illustrated in Figure 1C (right panel), HALO failed to induce burst suppression at any of the concentrations tested.
Isoflurane Prevents the Development of Learned Helplessness in Rats
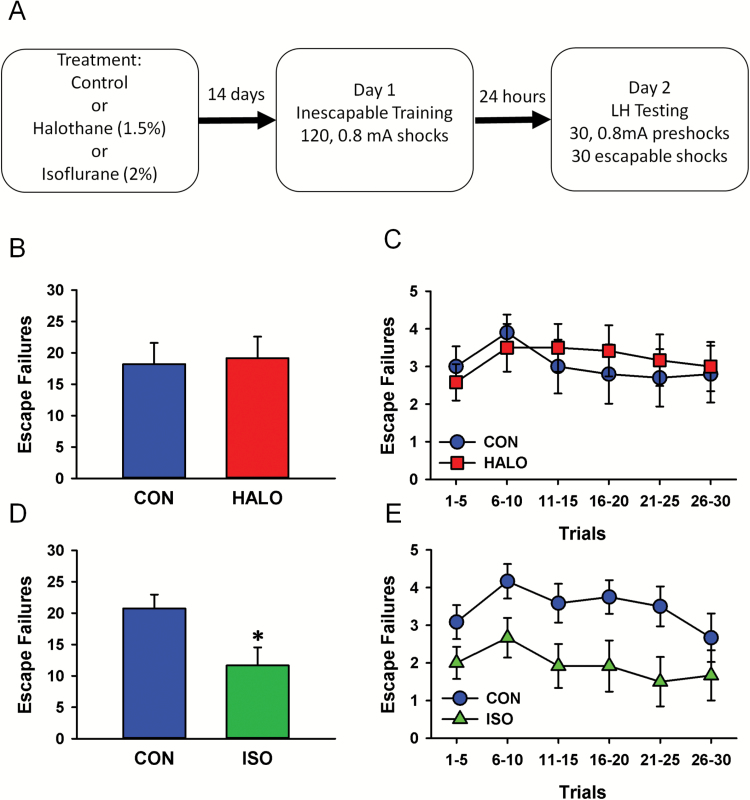
To determine whether prior exposure to burst suppression anesthesia prevents the expression of learned helplessness in rats, 2 cohorts of animals were pretreated with ISO (2.0%) or HALO (1.5%) for 2 hours prior to being returned to their home cage. The concentrations of ISO and HALO were adjusted to account for differences in their respective potencies (Mazze et al., 1985) while maximizing the burst suppression effects of ISO. Two separate groups of rats served as controls and were not treated with any drug. Two weeks later, all rats entered a 2-day learned helplessness procedure consisting of repeated exposure to an unpredictable and uncontrollable foot-shock followed 24 hours later by 30 trials in which subjects could avoid or escape the stressor (Figure 2A). As illustrated in Figure 2B and C, the number of rats that failed to escape the shock when provided the opportunity to do so did not differ between controls and rats that had been previously exposed to HALO anesthesia (F(1,20)=0.041, P=.842). By contrast, rats pretreated with ISO exhibited fewer escape failures overall compared with their naïve counterparts (F(1,22)=5.88, P=.024; Figure 2D). A significant main effect for trial block was also observed with rats exhibiting fewer escape failures as the session progressed (F(5,110)=2.580, P=.030; Figure 2E). There was no significant interaction between treatment and trial block.
Figure 2.
Prior exposure to isoflurane (ISO) but not halothane (HALO) reduces the incidence of learned helplessness in Sprague-Dawley rats. (A) Procedural summary and timeline. (B) Bar graph illustrating the average (mean±SEM) number of escape failures (30 trials total) in untreated rats (n=10) and rats that had been previously anesthetized with HALO (1.5%) for 2 hours (n=12). (C) Line plot illustrating the average number of escape failures (mean±SEM) among naïve controls and HALO-treated rats in blocks of 5 trials for the duration of the test session on Day 2. (D) Bar graph illustrating the average (mean±SEM) number of escape failures in untreated rats (n=12) and rats that had been previously anesthetized with ISO (2%) for 2 hours (n=12). (E) Line plot illustrating the average number of escape failures (mean±SEM) for control and ISO-treated rats in blocks of 5 trials for the duration of the test session (Day2).
Isoflurane Reverses Learned Helplessness in CD-1 Mice
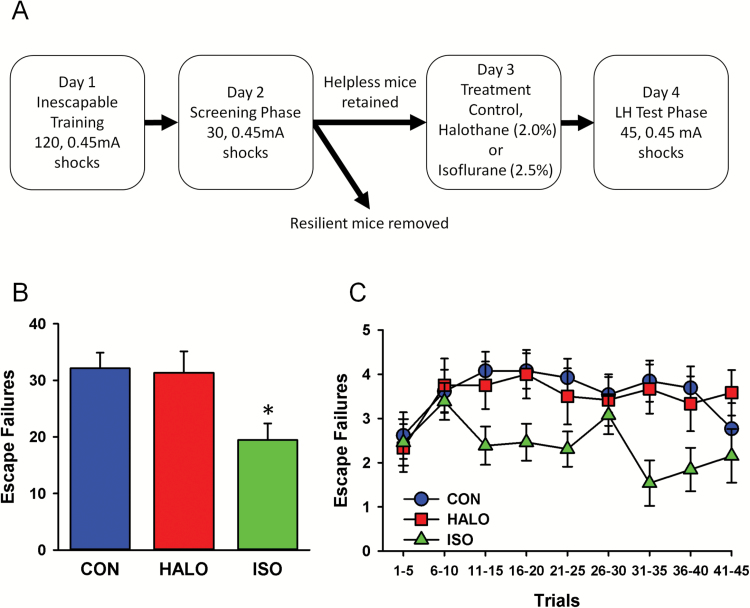
ISO and HALO were also evaluated for their ability to reverse learned helplessness in animals prescreened for expression of this maladaptive behavior. In an effort to increase throughput and to extend our earlier findings to include another species, these studies were conducted using CD-1 mice. A total of 89 mice were trained (Day 1) and subsequently screened (Day 2) in a modified learned helplessness paradigm (Figure 3A and Methods). Of these, 38 mice reached criteria for helpless behavior and were randomly assigned to 1 of 3 treatment groups including cage control (n=13), ISO (n=13), or HALO (n=12). Prior to treatment, the average number of escape failures exhibited by mice in each group was nearly identical (CON: 27.15±0.77; HALO: 26.75±0.98; ISO: 27.15±0.74). Twenty-four hours after screening, mice were induced and exposed to 1 hour of continuous administration of HALO (2.0%) or ISO (2.5%). The concentration of ISO and HALO were adjusted to account for differences in potency while maximizing the burst suppression effects of ISO. Control mice were transported to the treatment room but remained in their home cages for the duration of the anesthetic treatment. On the following day, all mice exhibiting the helpless phenotype were tested to determine the effects of prior anesthetic exposure on the number of escape failures. As illustrated in Figure 3B, a significant main effect was observed for treatment condition (F(2,35)=5.14, P=.01) with mice exposed to ISO showing significantly fewer escape failures than controls or HALO-treated mice.
Figure 3.
Exposure to isoflurane (ISO) but not halothane (HALO) 24 hours prior to testing reverses helpless behavior in CD-1 mice. (A) Procedural summary and timeline. (B) Bar graph illustrating the average number of escape failures (45 trials total) in untreated helpless mice (n=13) and helpless mice that been anesthetized with HALO (2.0%, n=12) or ISO (2.5%, n=13). (C) Line plot illustrating the average number of escape failures for control, HALO-treated, and ISO-treated in blocks of 5 trials for the duration of the retest session (Day 4). Data are the mean±SEM.
Discussion
The results of the present study demonstrate that acute administration of ISO, in doses that produce cortical burst suppression, exerts an antidepressant-like effect in the learned helplessness paradigm, a canonical animal model of a depression phenotype (Maier and Seligman, 2016). In mice exhibiting helpless behavior, a single, 1-hour exposure to ISO reduced escape failures within 24 hours of the initial treatment. The rapid onset of these effects following only a single exposure are consistent with those of the fast-acting antidepressant ketamine (Maeng et al., 2008; Zanos et al., 2015, 2016). Relative to the effects of conventional antidepressant drugs (e.g., fluoxetine), which do not exert effects following a single administration in our mouse learned helplessness paradigm (Zanos et al., 2015), both ketamine and ISO are effective within 24 hours after a single administration. In rats, prior exposure to 2 hours of ISO reduced the incidence of a depression-related maladaptive behavior (failure to escape an electric shock) 2 weeks following treatment, indicating that ISO’s effects are potentially long lasting. Consistent with this, in rats, a single, brief (30-minute) ISO anesthesia administered 24 hours following inescapable shock reduced the number of subsequent escape failures in the learned helplessness paradigm 6 days after administration (Antila et al., 2017). ISO was also found to exhibit antidepressant actions in mice evaluated using the tail suspension and novelty suppressed feeding paradigms 15 to 30 minutes after administration (Antila et al., 2017); however, see also (Yonezaki et al., 2015).
Studies in humans have proposed that EEG burst suppression is required for ISO to exert its antidepressant therapeutic effects (Langer et al., 1995); however, this hypothesis cannot be tested directly in clinical trials. Here, we used an animal model to assess the effects of 2 volatile anesthetics, with markedly different effects on cortical activity on a depression-related behavioral phenotype. EEG recordings established the ability of ISO to elicit sustained burst suppression in both rats and mice (Murrell et al., 2008; Land et al., 2012). These effects were strongly dose dependent and largely attributable to an increase in the duration of the postburst suppression (Hartikainen et al., 1995; Land et al., 2012). Here we show that HALO fails to induce burst suppression in rats or mice at any of the concentrations evaluated. HALO anesthesia also had no effect on learned helplessness paradigm in either rats or mice. These differences are unlikely to reflect a disparity in the degree of anesthesia, since the doses used in the behavioral studies were adjusted to account for the differences in anesthetic potency between ISO and HALO (Mazze et al., 1985; Krasowski and Harrison, 1999) and the known metabolic differences between rats and mice (Kharasch et al., 1999; Martignoni et al., 2006). To the best of our knowledge, this study is the first to directly compare anesthetic agents that differ in their ability to elicit EEG burst suppression on a depression-related phenotype in animals.
Volatile anesthetics including ISO and HALO interact with a host of CNS targets and have the potential to alter neuronal activity in a variety of ways. Thus, it is not certain whether the observed differences in the antidepressant effects of ISO and HALO are completely attributable to their differential effects on cortical EEG. However, despite their structural differences, both drugs exhibit a remarkably similar pharmacodynamic profile and do not differ substantially in their ability to modulate a number of ligand-gated ion channels in the CNS. ISO and HALO are both potent positive allosteric modulators of GABAA, glycine, 5-HT3, and kainate receptors and are equally effective as negative modulators of nicotinic acetylcholine and AMPA receptors (Krasowski and Harrison, 1999). Similarly, both anesthetics only modestly attenuate NMDA receptor activity (Krasowski and Harrison, 1999). ISO and HALO also share similar potencies as activators of 2-pore domain K+ channels, including TASK and TREK-1 (Patel et al., 1999; Luethy et al., 2017), a family of ion channels implicated in the mechanism of action of volatile anesthetics. In addition, both act as inhibitors of inwardly rectifying K+ channels (Sirois et al., 1998) and voltage-gated Na+ currents, which are inhibited in proportion to anesthetic potency (Ouyang et al., 2009). Of particular note, Antila et al. (2017) recently attributed the antidepressant-like behavioral effects of ISO in rodents to an increase in TrkB signaling among parvalbumin interneurons. However, these authors and others (Kang et al., 2017) have shown that both HALO and ISO increase TrkB signaling, implying both anesthetics should exhibit similar antidepressant effects in rodents—a prediction that is not supported by our findings.
In contrast to their reciprocal effects on a number of signal transduction mechanisms, ISO and HALO exert different effects on brain metabolism and cellular energetics. At clinically relevant doses, ISO and HALO have disparate effects on the cerebral metabolic rate of oxygen (CMRO2) (Algotsson et al., 1988; Kuroda et al., 1996), differences that may account for the pronounced dissimilarity in their effects on cortical EEG (Ching et al., 2012; S. Liu and Ching, 2017). Burst suppression, identical to that associated with ISO anesthesia, occurs under a variety of conditions associated with depressed CMRO2 including hypothermia (Stecker et al., 2001; Madhok et al., 2012; Chen et al., 2013) and hypoxic coma (Hofmeijer and van Putten, 2016). The mechanism(s) responsible for the generation of cortical burst suppression are incompletely understood but appear to involve both intrinsic cellular and corticothalamic network properties (Amzica and Kroeger, 2011). The process responsible for the transition from bursting to isoelectric suppression is of particular relevance given that the therapeutic effects of ECT are related to the duration of postictal suppression, the EEG “congener” of ISO-induced postburst isoelectricity. It has been suggested that the transition from burst to isoelectric quiescence is mediated by a Ca2+-induced increase in synaptic transmission or a reduction in neuronal excitability resulting from enhanced charge screening at Na+ channels (Kroeger and Amzica, 2007). More recently, Ching et al. (2012) suggested that bursts transiently deplete intracellular ATP levels, leading to an increase in the activity of ATP-gated K+ channels. A computational model based on this notion recapitulates burst suppression morphology and faithfully predicts the membrane hyperpolarization and increase in pyramidal cell conductance that occurs during the isoelectric phase of burst suppression (Steriade et al., 1994). Since electrically induced seizures increase neuronal energy consumption, it is tempting to speculate that a similar mechanism may contribute to ECT-induced postseizure isoelectricity, a notion that receives some support from studies suggesting that CMRO2 is depressed during the postictal interval (Weiner et al., 1991; Gaines and Rees, 1992).
In summary, our results demonstrate that ISO, but not HALO anesthesia is associated with the rapid emergence of a long-lasting, antidepressant phenotype in the learned helplessness model of despair in depression. While the specific pharmacological mechanism underlying these effects remains unknown, our data are consistent with the involvement of ISO-induced burst suppression. These results provide strong rationale for a well-controlled clinical evaluation of the therapeutic benefits associated with burst suppression anesthesia in major depressive disorder as well as additional mechanistic studies in relevant animal models. In addition to providing a path to an alternative treatment strategy for patients with medication resistant depression, these studies may converge on mechanisms that provide a more explicit and insightful functional link between burst suppression anesthesia and ECT.
Funding
This work was supported by the National Institutes of Health (MH110741 to P.D.S. and G.I.E. and MH107615 to T.D.G.).
Statement of Interest
Dr Shepard has received consulting fees from Takeda Pharmaceuticals U.S.A., Inc. and Eli Lilly and Company during the preceding 3 years. Dr Gould has received consulting fees from Janssen Pharmaceuticals and research funding from Janssen Pharmaceuticals and Roche Pharmaceuticals during the preceding 3 years. All other authors report no financial interest to disclose.
Acknowledgments
The authors gratefully acknowledge the technical assistance provided by Joseph Parise, BS and Cheryl L. Mayo, BS of the Maryland Psychiatric Research Center.
References
- Algotsson L, Messeter K, Nordström CH, Ryding E(1988)Cerebral blood flow and oxygen consumption during isoflurane and halothane anesthesia in man. Acta Anaesthesiol Scand 32:15–20. [DOI] [PubMed] [Google Scholar]
- Amzica F, Kroeger D(2011)Cellular mechanisms underlying EEG waveforms during coma. Epilepsia 52:25–27. [DOI] [PubMed] [Google Scholar]
- Antila H, et al. 2017) Isoflurane produces antidepressant effects and induces TrkB signaling in rodents. Sci Rep 7:7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM(2011)NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma H, Fujita A, Sato K, Arahata K, Otsuki K, Hori M, Mochida Y, Uchida M, Yamada T, Akechi T, Furukawa TA(2007)Postictal suppression correlates with therapeutic efficacy for depression in bilateral sine and pulse wave electroconvulsive therapy. Psychiatry Clin Neurosci 61:168–173. [DOI] [PubMed] [Google Scholar]
- Azuma H, Yamada A, Shinagawa Y, Nakano Y, Watanabe N, Akechi T, Furukawa TA(2011)Ictal physiological characteristics of remitters during bilateral electroconvulsive therapy. Psychiatry Res 185:462–464. [DOI] [PubMed] [Google Scholar]
- Carl C, Engelhardt W, Teichmann G, Fuchs G(1988)Open comparative study with treatment-refractory depressed patients: electroconvulsive therapy–anesthetic therapy with isoflurane (preliminary report). Pharmacopsychiatry 21:432–433. [DOI] [PubMed] [Google Scholar]
- Chen C, Maybhate A, Thakor NV, Jia X(2013)Effect of hypothermia on cortical and thalamic signals in anesthetized rats. Conf Proc IEEE Eng Med Biol Soc 2013:6317–6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching S, Purdon PL, Vijayan S, Kopell NJ, Brown EN(2012)A neurophysiological-metabolic model for burst suppression. Proc Natl Acad Sci U S A 109:3095–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunner DL, Rush AJ, Russell JM, Burke M, Woodard S, Wingard P, Allen J(2006)Prospective, long-term, multicenter study of the naturalistic outcomes of patients with treatment-resistant depression. J Clin Psychiatry 67:688–695. [DOI] [PubMed] [Google Scholar]
- Engelhardt W, Carl G, Hartung E(1993)Intra-individual open comparison of burst-suppression-isoflurane-anaesthesia versus electroconvulsive therapy in the treatment of severe depression. Eur J Anaesthesiol 10:113–118. [PubMed] [Google Scholar]
- Fava M.(2003)Diagnosis and definition of treatment-resistant depression. Biol Psychiatry 53:649–659. [DOI] [PubMed] [Google Scholar]
- Gaines GY 3rd, Rees DI(1992)Anesthetic considerations for electroconvulsive therapy. South Med J 85:469–482. [DOI] [PubMed] [Google Scholar]
- García-Toro M, Segura C, González A, Perelló J, Valdivia J, Salazar R, Tarancón G, Campoamor F, Salva J, De La Fuente L, Romera M(2001)Inefficacy of burst-suppression anesthesia in medication-resistant major depression: a controlled trial. J Ect 17:284–288. [DOI] [PubMed] [Google Scholar]
- García-Toro M, Romera M, Gonzalez A, Ibañez P, Garcia A, Socias L, Rubert C, Rialp G, Salva J, Montes JM(2004)12-hour burst-suppression anesthesia does not relieve medication-resistant major depression. J Ect 20:53–54. [DOI] [PubMed] [Google Scholar]
- Greenberg LB, Gage J, Vitkun S, Fink M(1987)Isoflurane anesthesia therapy: a replacement for ECT in depressive disorders?Convuls Ther 3:269–277. [PubMed] [Google Scholar]
- Hartikainen KM, Rorarius M, Peräkylä JJ, Laippala PJ, Jäntti V(1995)Cortical reactivity during isoflurane burst-suppression anesthesia. Anesth Analg 81:1223–1228. [DOI] [PubMed] [Google Scholar]
- Hofmeijer J, van Putten MJ(2016)EEG in postanoxic coma: prognostic and diagnostic value. Clin Neurophysiol 127:2047–2055. [DOI] [PubMed] [Google Scholar]
- Kang JWM, Keay KA, Mor D(2017)Resolving the contributions of anaesthesia, surgery, and nerve injury on brain derived neurotrophic factor expression in the medial prefrontal cortex of male rats in the CCI model of neuropathic pain. J Neurosci Res 95:2376–2390. [DOI] [PubMed] [Google Scholar]
- Kellner CH, Greenberg RM, Murrough JW, Bryson EO, Briggs MC, Pasculli RM(2012)ECT in treatment-resistant depression. Am J Psychiatry 169:1238–1244. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS, National Comorbidity Survey Replication (2003)The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). Jama 289:3095–3105. [DOI] [PubMed] [Google Scholar]
- Kharasch ED, Hankins DC, Cox K(1999)Clinical isoflurane metabolism by cytochrome P450 2E1. Anesthesiology 90:766–771. [DOI] [PubMed] [Google Scholar]
- Kranaster L, Plum P, Hoyer C, Sartorius A, Ullrich H(2013)Burst suppression: a more valid marker of postictal central inhibition?J Ect 29:25–28. [DOI] [PubMed] [Google Scholar]
- Krasowski MD, Harrison NL(1999)General anaesthetic actions on ligand-gated ion channels. Cell Mol Life Sci 55:1278–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger D, Amzica F(2007)Hypersensitivity of the anesthesia-induced comatose brain. J Neurosci 27:10597–10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal AD, Weiner RD, McCall WV, Shelp FE, Arias R, Smith P(1993)The effects of ECT stimulus dose and electrode placement on the ictal electroencephalogram: an intraindividual crossover study. Biol Psychiatry 34:759–767. [DOI] [PubMed] [Google Scholar]
- Kuroda Y, Murakami M, Tsuruta J, Murakawa T, Sakabe T(1996)Preservation of the ration of cerebral blood flow/metabolic rate for oxygen during prolonged anesthesia with isoflurane, sevoflurane, and halothane in humans. Anesthesiology 84:555–561. [DOI] [PubMed] [Google Scholar]
- Land R, Engler G, Kral A, Engel AK(2012)Auditory evoked bursts in mouse visual cortex during isoflurane anesthesia. PLoS One 7:e49855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer G, Neumark J, Koinig G, Graf M, Schönbeck G(1985)Rapid psychotherapeutic effects of anesthesia with isoflurane (ES narcotherapy) in treatment-refractory depressed patients. Neuropsychobiology 14:118–120. [DOI] [PubMed] [Google Scholar]
- Langer G, Karazman R, Neumark J, Saletu B, Schönbeck G, Grünberger J, Dittrich R, Petricek W, Hoffmann P, Linzmayer L(1995)Isoflurane narcotherapy in depressive patients refractory to conventional antidepressant drug treatment. A double-blind comparison with electroconvulsive treatment. Neuropsychobiology 31:182–194. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS(2010)mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK(2012)Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry 71:996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Ching S(2017)Homeostatic dynamics, hysteresis and synchronization in a low-dimensional model of burst suppression. J Math Biol 74:1011–1035. [DOI] [PubMed] [Google Scholar]
- Luethy A, Boghosian JD, Srikantha R, Cotten JF(2017)Halogenated ether, alcohol, and alkane anesthetics activate TASK-3 tandem pore potassium channels likely through a common mechanism. Mol Pharmacol 91:620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhok J, Wu D, Xiong W, Geocadin RG, Jia X(2012)Hypothermia amplifies somatosensory-evoked potentials in uninjured rats. J Neurosurg Anesthesiol 24:197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK(2008)Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino- 3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 63:349–352. [DOI] [PubMed] [Google Scholar]
- Maier SF, Seligman ME(2016)Learned helplessness at fifty: insights from neuroscience. Psychol Rev 123:349–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martignoni M, Groothuis G, de Kanter R(2006)Comparison of mouse and rat cytochrome P450-mediated metabolism in liver and intestine. Drug Metab Dispos 34:1047–1054. [DOI] [PubMed] [Google Scholar]
- Mazze RI, Rice SA, Baden JM(1985)Halothane, isoflurane, and enflurane MAC in pregnant and nonpregnant female and male mice and rats. Anesthesiology 62:339–341. [DOI] [PubMed] [Google Scholar]
- Murrell JC, Waters D, Johnson CB(2008)Comparative effects of halothane, isoflurane, sevoflurane and desflurane on the electroencephalogram of the rat. Lab Anim 42:161–170. [DOI] [PubMed] [Google Scholar]
- Olchanski N, McInnis Myers M, Halseth M, Cyr PL, Bockstedt L, Goss TF, Howland RH(2013)The economic burden of treatment-resistant depression. Clin Ther 35:512–522. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Herold KF, Hemmings HC Jr(2009)Comparative effects of halogenated inhaled anesthetics on voltage-gated Na+ channel function. Anesthesiology 110:582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Honoré E, Lesage F, Fink M, Romey G, Lazdunski M(1999)Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci 2:422–426. [DOI] [PubMed] [Google Scholar]
- Perera TD, Luber B, Nobler MS, Prudic J, Anderson C, Sackeim HA(2004)Seizure expression during electroconvulsive therapy: relationships with clinical outcome and cognitive side effects. Neuropsychopharmacology 29:813–825. [DOI] [PubMed] [Google Scholar]
- Russell JM, Hawkins K, Ozminkowski RJ, Orsini L, Crown WH, Kennedy S, Finkelstein S, Berndt E, Rush AJ(2004)The cost consequences of treatment-resistant depression. J Clin Psychiatry 65:341–347. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS(2002)Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci 22:3251–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirois JE, Pancrazio JJ, Lynch C 3rd, Bayliss DA(1998)Multiple ionic mechanisms mediate inhibition of rat motoneurones by inhalation anaesthetics. J Physiol 512:851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecker MM, Cheung AT, Pochettino A, Kent GP, Patterson T, Weiss SJ, Bavaria JE(2001)Deep hypothermic circulatory arrest: I. Effects of cooling on electroencephalogram and evoked potentials. Ann Thorac Surg 71:14–21. [DOI] [PubMed] [Google Scholar]
- Steriade M, Amzica F, Contreras D(1994)Cortical and thalamic cellular correlates of electroencephalographic burst-suppression. Electroencephalogr Clin Neurophysiol 90:1–16. [DOI] [PubMed] [Google Scholar]
- Suppes T, Webb A, Carmody T, Gordon E, Gutierrez-Esteinou R, Hudson JI, Pope HG Jr(1996)Is postictal electrical silence a predictor of response to electroconvulsive therapy?J Affect Disord 41:55–58. [DOI] [PubMed] [Google Scholar]
- Vollmayr B, Henn FA(2001)Learned helplessness in the rat: improvements in validity and reliability. Brain Res Brain Res Protoc 8:1–7. [DOI] [PubMed] [Google Scholar]
- Weeks HR 3rd, Tadler SC, Smith KW, Iacob E, Saccoman M, White AT, Landvatter JD, Chelune GJ, Suchy Y, Clark E, Cahalan MK, Bushnell L, Sakata D, Light AR, Light KC(2013)Antidepressant and neurocognitive effects of isoflurane anesthesia versus electroconvulsive therapy in refractory depression. PLoS One 8:e69809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner RD, Coffey CE, Krystal AD(1991)The monitoring and management of electrically induced seizures. Psychiatr Clin North Am 14:845–869. [PubMed] [Google Scholar]
- Yonezaki K, Uchimoto K, Miyazaki T, Asakura A, Kobayashi A, Takase K, Goto T(2015)Postanesthetic effects of isoflurane on behavioral phenotypes of adult male C57BL/6J mice. PLoS One 10:e0122118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Piantadosi SC, Wu HQ, Pribut HJ, Dell MJ, Can A, Snodgrass HR, Zarate CA Jr, Schwarcz R, Gould TD(2015)The prodrug 4-chlorokynurenine causes ketamine-like antidepressant effects, but not side effects, by NMDA/glycineb-site inhibition. J Pharmacol Exp Ther 355:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr, Gould TD(2016)NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]