Abstract
Background and Aims
Understanding root traits and their trade-off with other plant processes is important for understanding plant functioning in natural ecosystems as well as agricultural systems. The aim of the present study was to determine the relationship between root morphology and the hydraulic characteristics of several orders of fine roots (<2 mm) for species differing in shade tolerance (low, moderate and high).
Methods
The morphological, anatomical and hydraulic traits across five distal root orders were measured in species with different levels of shade tolerance and life history strategies. The species studied were Acer negundo, Acer rubrum, Acer saccharum, Betula alleghaniensis, Betula lenta, Quercus alba, Quercus rubra, Pinus strobus and Pinus virginiana.
Key Results
Compared with shade-tolerant species, shade-intolerant species produced thinner absorptive roots with smaller xylem lumen diameters and underwent secondary development less frequently, suggesting that they had shorter life spans. Shade-tolerant species had greater root specific hydraulic conductance among these roots due to having larger diameter xylems, although these roots had a lower calculated critical tension for conduit collapse. In addition, shade-intolerant species exhibited greater variation in hydraulic conductance across different root growth rings in woody transport roots of the same root order as compared with shade-tolerant species.
Conclusions
Plant growth strategies were extended to include root hydraulic properties. It was found that shade intolerance in trees was associated with conservative root hydraulics but greater plasticity in number of xylem conduits and hydraulic conductance. Root traits of shade-intolerant species were consistent with the ability to proliferate roots quickly for rapid water uptake needed to support rapid shoot growth, while minimizing risk in uncertain environments.
Keywords: Hydraulic conductance, root morphology, shade tolerance, t/d, trait plasticity, xylem diameter
INTRODUCTION
Canopy gaps and light availability are important factors determining plant community composition and structure (Valladers, 2003; Zavala et al., 2007; Gravel et al., 2010). At the initial stages of stand development, shade-intolerant species often grow in high density and typically allocate a large amount of biomass to fast-growing shoots to compete effectively for light (Casper and Jackson, 1997; Schenk, 2006). A higher level of soil nutrient availability is generally found in early successional forests as compared with late successional forests, which benefits early seral (early successional status), shade-intolerant species typified by fast growth and relatively high above-ground biomass allocation (Walker and del Moral, 2003; Schenk, 2006; Brassard et al., 2009; Freschet et al., 2015). Shade-intolerant species have also been found to be more deeply rooted than shade-tolerant species (Persson et al., 1995; Finér et al., 1997; Chen and Brassard, 2013) with greater ability to absorb soil resources when resource availability is high (Comas et al., 2002; Comas and Eissenstat, 2004; Chen and Brassard, 2013). In contrast, shade-tolerant species may be better able to acquire nutrients located near the soil surface and also may more effectively suppress the encroachment of neighbours with their relatively shallow, dense root system.
By adapting to more stable environments but longer establishment periods, shade-tolerant species may benefit by having roots with less plasticity, greater structural reinforcement and longer life spans (Chen and Brassard, 2013). This may be reflected in thicker root diameter in more shade-tolerant species (Comas et al., 2002; Comas and Eissenstat, 2004), which is a trait associated with greater root longevity (McCormack et al., 2012), less proliferation in nutrient hot spots and greater dependence on mycorrhizal fungi (Comas et al., 2014; Eissenstat et al., 2015; Liu et al., 2015; Chen et al., 2016; Cheng et al., 2016). While trade-offs in root traits have been considered in terms of nutrient acquisition (e.g. Comas et al., 2002) and interactions with mycorrhizal fungi (e.g. Brundrett, 2002; Comas et al., 2014; Liu et al., 2015), the relationships of root diameter and root order to axial hydraulic conductance and to resistance to loss of hydraulic function have yet to be thoroughly investigated. According to the Hagen–Poiseuille law, if smaller diameter roots are associated with smaller diameter xylem, then roots of fast-growing, early seral species may also exhibit reduced hydraulic conductance compared with shade-tolerant, late seral species. Larger xylem conduits with greater hydraulic conductance often come at a cost, with increased risk of cavitation (Hacke et al., 2000; Lopez et al., 2005; Gleason et al., 2016). Although high cavitation resistance may be beneficial for roots with a shallow distribution (Sperry and Hacke, 2002; Lopez et al., 2005), shade-tolerant species may experience lower levels of whole-plant water stress, despite being shallow rooted, due to their lower stomatal conductance and slower rates of soil water depletion relative to shade-intolerant species (Hastwell and Facelli, 2003; Poorter et al., 2012).
Considering the biological consequences of the morphological and anatomical properties, there is a need to study root traits and their links to plant adaptations to the environment. Root functioning (absorption and transport) is generally strongly influenced by root position within a branching hierarchy. Absorptive roots, which are typically the first 2–3 root branch orders, function in nutrient and water uptake; while transport roots with a developed vascular cambium and phellem (secondary growth) are found higher in the branching hierarchy and primarily play a structural and transport role with limited or no capacity for water and nutrient absorption (Pregitzer et al., 2002; Guo et al., 2008; Xia et al., 2010). The anatomy of transport roots should theoretically be linked to hydraulic conductance. Few studies, however, have examined hydraulic conductance and cavitation resistance with respect to root position in the branching hierarchy (Long et al., 2013; Kong et al., 2014), where the most distal root orders (absorptive roots) are characterized by the highest rate of water uptake and higher root orders (transport roots), involved in water transport and hydraulic redistribution, play an important role in plant adaptation to water deficits (Rewald et al., 2011). Although there has been increased interest in root traits associated with shade tolerance (Kotowska et al., 2015), limited information exists on xylem traits across different root orders in species that differ in shade tolerance. Moreover, mycorrhizal colonization intensity – related to root position in the branching hierarchy – may have several implications on our understanding of the hydraulic conductance of absorptive roots (Kong et al., 2017). Furthermore, as temperature and water availability vary among years, examining the characteristics of xylem conduits formed in different years offers an opportunity to examine year to year plasticity in root traits at the anatomical scale.
In the present study, genetically based trait differences were examined among species grown in a common garden in order to standardize environmental variation across species. Several root traits within the same root order were evaluated, including root diameter, development of primary or secondary growth, number of annual growth rings (as a proxy for root age), size and number of xylem conduits (per cross-section), and mycorrhizal colonization. The objective of this study was to determine whether the various root traits were associated with differences in shade tolerance using congeneric contrasts of species in four genera, Acer, Betula, Quercus and Pinus. We tested the hypotheses that, compared to shade-tolerant species, shade-intolerant species (1) have greater potential to conduct water; (2) have reduced cell wall structural strength in xylem conducting elements and thus have increased risk of hydraulic failure in roots; (3) have thinner roots with a greater level of branching that is absortive; and (4) have xylem conducting elements with greater variation in size (diameter) in different years and, thus, exhibit more plasticity in regards to conductance.
MATERIALS AND METHODS
Study site
The study site was located at the Russell E. Larson Agricultural Research Center, near State College, Pennsylvania, USA (40.8°N, 77.9°W). The common garden consisted of 16 species of trees, each planted in eight blocks in 1996 using a randomized complete block design, with the exception for Betula trees which were planted in 2004. Plants were obtained as 1-year-old liners from local native-plant nurseries, except for individuals of Acer negundo, which were collected in early spring as seedlings from around State College. In each block, each species was planted in a group of six trees in a double row of three trees with a spacing of 3 m between trees within the plot. A 9 m spacing was used between the six-tree plots. Thus, each species was represented by a total of 48 trees. The soil in the study site was a relatively fertile, well-drained, Hagerstown silt loam with a pH ranging from 6.1 to 6.5. Some areas of the soil were high in calcium. Prior to 1996, the site was used as a grass hayfield and the entire area was fenced to keep out deer. Blocking was used to control for variation in soil characteristics (primarily the depth of the A and B horizons). In the present study, only roots of Acer negundo, Acer rubrum, Acer saccharum, Betula alleghaniensis, Betula lenta, Quercus alba, Quercus rubra, Pinus strobus and Pinus virginiana were examined. These species are known to vary widely in shade tolerance, mainly in seedling and sapling growth stages (Table 1; Burns and Honkala, 1990). This life history strategy is thus linked to their place in succession. The selected species represent genera within the Sapindaceae (Acer), Betulaceae (Betula), Fagaceae (Quercus) and Pinaceae (Pinus).
Table 1.
Life history characteristics of the nine North America tree species sampled in this study
| Family | Species | Common name | Shade tolerance | Successional status | Tree longevity | Growth rate |
|---|---|---|---|---|---|---|
| Sapindaceae | ||||||
| Acer negundo | Boxelder | Low | Early | <100 years | Fast | |
| Acer rubrum | Red maple | Moderate | Midseral | <150 years | Intermediate | |
| Acer saccharum | Sugar maple | High | Late | >350 years | Slow | |
| Betulaceae | ||||||
| Betula lenta | Sweet birch | Low | Early | <150 years | Fast | |
| Betula alleghaniensis | Yellow birch | Moderate | Midseral | >300 years | Moderately fast | |
| Fagaceae | ||||||
| Quercus rubra | Red oak | Low-moderate | Early | <250 years | Intermediate | |
| Quercus alba | White oak | Moderate | Midseral | >400 years | Slow | |
| Pinaceae | ||||||
| Pinus virginiana* | Virginia pine | Low | Midseral | <120 years | Fast | |
| Pinus strobus † | White pine | Moderate | Midseral | >400 years | Moderately fast |
Species contrasted in shade tolerance, successional status, tree longevity and growth rate.
*Often found on ridgetops and other less fertile but open canopy conditions.
†Common on hill slopes in mid-successional mixed hardwood forests.
Root sampling
Two trees from each of three plots for each species were randomly selected in September 2008. Ten intact root samples were collected from each plot. Samples were collected by carefully digging out plant litter and soil to find the distal root tip, and then gently unearthing subsequent root branches with brushes and spatulas. Roots were confirmed to be from an individual tree by tracing the roots back to the trunks of the selected trees. Roots were sampled up to 1 m from the trunk. Intact samples were cut off from the rest of the root system with scissors and placed on a paper towel moistened with deionized water and then placed into a zippered plastic bag. Soil particles adhering to the root samples were not removed at the time of collection. Root samples were stored in the refrigerator (approx. 5 °C) until further processing. During storage (up to 1 week), the plastic bags were opened and the moistened towels were replaced to prevent any drying and deterioration of the roots.
Root samples were large enough to include at least five levels of branching. The designation of root branch orders followed the topological nomenclature developed in the literature, which identifies root tips as first-order branches, second order as the roots from which first-order roots branch, and so on (Pregitzer et al., 2002). Once removed from plastic bags, root samples were gently washed in a water bath and divided into individual branch orders using a steel scalpel. All roots were kept wet during the dissection and separation process. Only live roots, as determined by texture and visual appearance, were used for further analysis. Ten roots from each branch order were selected from each of the three plots (30 roots of each branch order; 150 roots from each species).
Anatomical analysis
Excised root orders were immediately fixed in FAA (5 mL of formaldehyde + 5 mL of acetic acid + 90 mL of 70 % ethanol, v/v/v) for 48 h. The entire root of lower orders (first, second and third orders) was fixed, while higher root orders were cut into a 10 mm length when the root orders exceeded 15 mm. After 48 h in FAA, each root order was washed twice in 70 % ethyl alcohol, and then dehydrated in an ethyl alcohol series (90, 96 and 100 %) for 1 h at each concentration. Further dehydration was performed in a mixture of ethyl alcohol:butyl alcohol (3:1, 1:1 and 3:1, v/v) for 20 min and subsequently with pure butyl alcohol for 0.5 h. Samples were then left overnight at room temperature in fresh butyl alcohol. Samples were subsequently infiltrated with and embedded in Paraplast Plus using the protocol described by Bagniewska-Zadworna et al. (2012). Cross-sections (10 µm thick) were obtained using a microtome (Leica RM2265) (Leica, Germany) and stained in 0.5 % toluidine blue O in 1 % sodium tetraborate. Observations of cross-sections were made using a Carl Zeiss Axioskop 20 (Carl Zeiss, Germany) light microscope with photographs taken at ×5–40 magnification with an AxioCam (Carl Zeiss) using AxioVision software (Carl Zeiss).
Root analysis
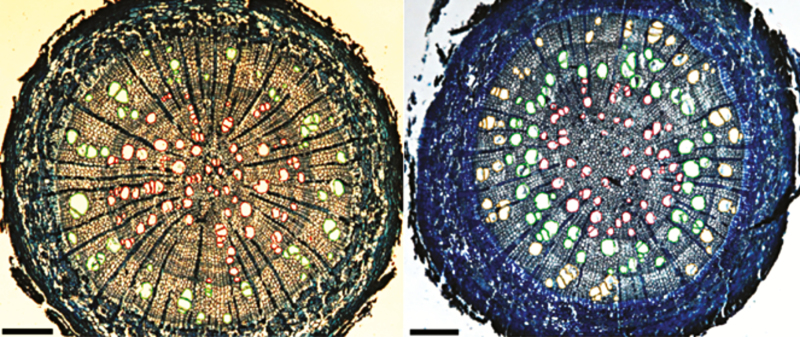
Digital images (Fig. 1) were used to measure various root parameters. Root diameter was measured using AxioVision software (Carl Zeiss). Each sample was classified as having primary or secondary development based on the presence of anatomical features such as a vascular cambium and phellem (cork cambium). In temperate climates, growth rings in roots can develop with changes in the seasons and during a period of winter dormancy. The number of growth rings in roots with secondary development can therefore be used to estimate root age (Fayle, 1968). Since woody roots frequently form discontinuous rings, only complete rings were counted for consistency, which yielded a conservative estimate of root age.
Fig. 1.
Transverse section of A. rubrum (left panel) and A. saccharum (right panel) fifth-order roots showing vessels within two distinct years of xylem growth (annual rings) in A. rubrum and three years in A. saccharum – first ring (red colour), second ring (green colour) and third ring (yellow colour). Scale bars = 200 µm.
To assess if shade tolerance was related to root water transport ability of a given root order, transverse (cross-section) images of roots were used to measure various parameters of xylem conduits (vessel or tracheids) with AxioVision 4 software. Data were collected from roots of each branch order (ten roots per branch order) for each species. Conduits from each root and growth ring were counted and measured separately. A hydraulic weighted mean conduit diameter (d) was estimated by using each lumen radius (r) across all conduits of a cross-section or growth ring, and applying the following equation: d = 2(Σr5/Σr4) (Sperry et al., 1994). The cell wall was not included in the diameter. In the case of coniferous species, P. strobus and P. virginiana, the fact that tracheid lumens are square was taken into account and following correction d = (area)1/2 was used. The theoretical/calculated root-specific hydraulic conductance for individual roots within each root order was estimated using the Hagen–Poiseuille equation as previously described (Valenzuela-Estrada et al., 2009):
where Lx (m3s–1 MPa–1 cm–2) is the theoretical/calculated root hydraulic conductance, nv is the mean number of xylem conduits per root, Ra (m) is the average weighted conduit radius, η (1 × 10–9 MPa s at 20 ºC) is the viscosity of water, and SA (cm2) is root cross-sectional area.
Five representative conduits were used within each growth ring (if secondary growth was present) or cross-section (if only primary growth was present) to estimate the resistance against implosion, and additional anatomical measurements were taken on these conduits. Conduit cell wall thickness (t) was measured on at least five conduits per growth ring or cross-section.
Euler buckling theory suggests that the critical tension resulting in conduit collapse is constant when t scales proportionately with d (Hacke et al., 2001; Blackman et al., 2010); as such, the ratio of t/d (assuming proportional scaling) represents the premium a species places on avoiding conduit collapse. We compared (t/d)2 among species to evaluate the potential differences in the critical tension that would result in conduit collapse (Hacke et al., 2001), as well as the possible trade-off between t/d and hydraulic capacity [i.e. (t/d)2 ~ Lx].
Mycorrhizal colonization
The fungal colonization intensity of individual roots was assessed with individual root cross-sections classified as one of five categories of intensity based on the proportion of cortical cells colonized by mycorrhizal fungi for arbuscular mycorrhizae or the proportion of cortical cells with Hartig’s network for ectomycorrhizae. A transect line was randomly drawn across each cross-section to estimate colonization, and the number of colonized cells along each transect line was counted. Roots were considered to be colonized by ectomycorrhizal fungi based on the presence of a Hartig net and mantle (Peterson et al., 2004), and as being associated with endomycorrhizas by the presence of arbuscules or vesicles. The following categories of colonization intensity were used: 0, no colonization; I, 1–25 % colonization; II, 25–50 % colonization; III, 50–75 % colonization; and IV, 75–100 % colonization.
Statistical analysis
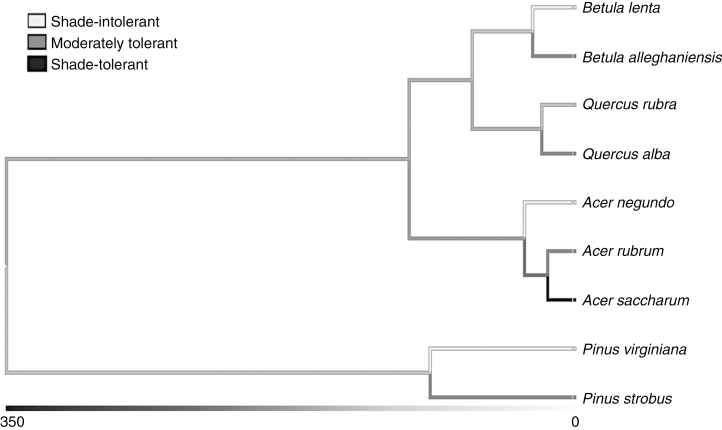
A phylogenetic tree was assembled based upon current evaluations of plant species diversification (Fig. 2; Wikstrom et al., 2001; Magallon and Sanderson, 2005; Gernandt et al., 2008). These evaluations used recent methods to date species divergences, including relaxed-clock analyses and fossil-based calibrations, and estimated branch lengths with maximum likelihood. Branch lengths were unavailable for species divergences within Betula, Quercus and Acer. As a result, these tips were estimated as half-splits from their divergence from their closest relative.
Fig. 2.
A phylogenetic tree with shade tolerance mapped was assembled from literature on plant species diversification (Wikstrom et al., 2001; Magallon and Sanderson, 2005; Gernandt et al., 2008). Branch lengths of the phylogenetic tree indicate time since divergence from a shared ancestor in units of million years.
Analyses of phylogenetic trait patterns were conducted on a species average by root order, separately for each root order and by averages of root functional classes, i.e. absorptive vs. transport (McCormack et al., 2015). Root orders were classified as absorptive or transport roots based on anatomical traits. Specifically, first-, second- and almost all third-order roots were considered as absorptive roots. On the other hand, third- (when >75 % showed secondary development), fourth- and fifth-order roots were classified as transport roots.
Correlations between shade tolerance and root traits were assessed using phylogenetically independent contrasts (PICs) (Felsenstein, 1985) to account for phylogenetic sampling of species. PICs have been found to give robust estimates of the correlation between characters with as few as eight terminal taxa (Oakley and Cunningham, 2000). PIC analyses were computed with the PDAP:PDTREE Package version 1.15 (Midford et al., 2010) within the Mesquite system (Maddison and Maddison, 2010) for phylogenetic computing using the fully resolved phylogenetic tree described above.
Overall effects of shade tolerance, family relationship and root order on root traits were analysed with a general linearized model (GLM), and a hierarchical analysis of variance (ANOVA) using mixed effects where shade tolerance (random variable) was nested within taxonomic contrasts. Taxonomic contrast and species within contrast were fixed terms. Root traits were dependent variables. The pooled standard error for individual traits was calculated from the square root of the mean square error in the hierarchical ANOVA. All analyses were conducted using Statistica version 8.0 (StatSoft Tulsa, OK, USA) software.
RESULTS
Hydraulic conductance and likelihood of failure
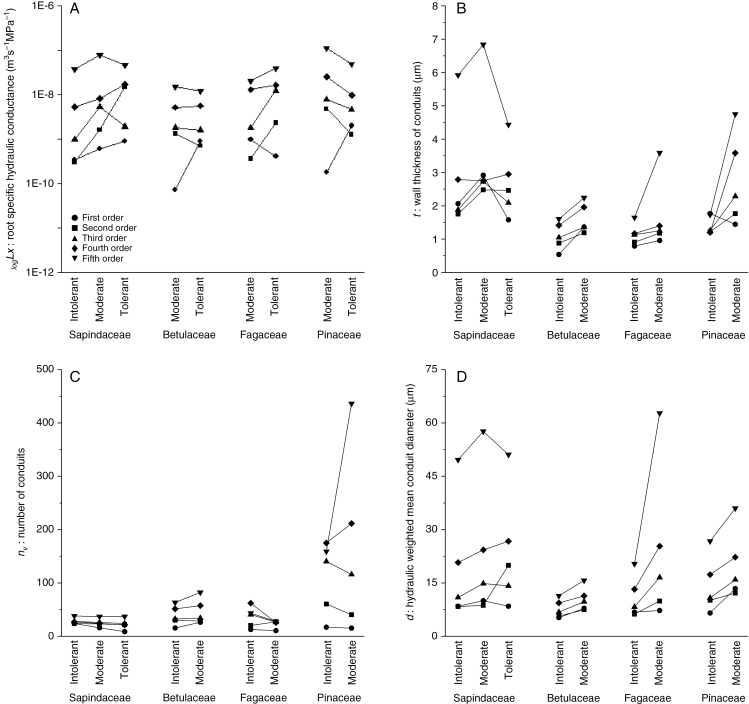
Contrary to the initial hypotheses, root-specific hydraulic conductance (Lx) generally increased with increasing shade tolerance when traits were compared across species of four genera using PIC analyses (second-order, fouth-order and pooled absorptive roots, trend for first order; Table 2). There was no general relationship between Lx and shade tolerance in third- and fifth-order roots or in pooled transport root PICs (Table 2). Within comparisons of Acer, Betula and Quercus, Lx was approx. 140 % higher in moderately shade-tolerant than in shade-intolerant species across all root orders, but in Pinus, except for first-order roots, the trend was reversed and species of moderate shade tolerance had 200 % lower Lx than intolerant species (Fig. 3A).
Table 2.
Pearson correlation coefficient (r) between pairs of traits among phylogenetically independent contrasts (PICs) analysed separately for each root order (first to fifth order), and by root functional classes, i.e. absorptive (average of first to third order) vs. transport (average of fourth to fifth root order)
| n v | Lx (m3 s–1 MPa–1 cm–2) | t | d | (t/d)2 | RAD (mm) | Develop | Rings | Colonization | |
|---|---|---|---|---|---|---|---|---|---|
| First order | |||||||||
| Shade tolerance | –0.45 | 0.56 | –0.21 | 0.29 | –0.41 | 0.62* | – | – | 0.16 |
| n v | 0.03 | 0.58* | 0.32 | 0.56 | –0.18 | – | – | –0.65** | |
| Lx (m3 s–1 MPa–1 cm–2) | –0.05 | 0.62* | –0.34 | 0.37 | – | – | –0.26 | ||
| t | 0.57# | 0.93† | 0 | – | – | –0.60* | |||
| d | 0.24 | 0.46 | – | – | –0.20 | ||||
| (t/d)2 | –0.22 | – | – | –0.63* | |||||
| RAD (mm) | – | – | 0.23 | ||||||
| Develop | – | – | |||||||
| Rings | – | ||||||||
| Second order | |||||||||
| Shade tolerance | –0.21 | 0.75** | 0.68** | 0.84*** | –0.51 | 0.61* | 0.18 | – | 0.6* |
| n v | 0 | –0.19 | –0.05 | –0.03 | –0.37 | 0.04 | – | 0.18 | |
| Lx (m3 s–1 MPa–1 cm–2) | 0.31 | 0.96† | –0.85*** | 0.14 | 0.68** | – | 0.54* | ||
| t | 0.42 | 0.13 | 0.73** | –0.07 | – | 0.40 | |||
| d | –0.84*** | 0.35 | 0.54 | – | 0.68** | ||||
| (t/d)2 | 0.01 | –0.60* | – | –0.49 | |||||
| RAD (mm) | –0.40 | – | 0.24 | ||||||
| Develop | – | – | |||||||
| Rings | – | ||||||||
| Third order | |||||||||
| Shade tolerance | . | 0.14 | 0.15 | 0.54 | –0.59* | 0.56 | 0.46 | 0.55 | ‡ |
| n v | § | § | § | § | § | § | § | § | |
| Lx (m3 s–1 MPa–1 cm–2) | 0.29 | 0.84*** | –0.46 | 0.03 | 0.17 | –0.15 | ‡ | ||
| t | 0.48 | 0.45 | 0.57* | –0.33 | –0.54 | ‡ | |||
| d | –0.53 | 0.53 | 0.20 | –0.05 | ‡ | ||||
| (t/d)2 | –0.1 | –0.54 | –0.60** | ‡ | |||||
| RAD (mm) | –0.12 | –0.12 | ‡ | ||||||
| Develop | 0.66** | ‡ | |||||||
| Rings | ‡ | ||||||||
| Fourth order | |||||||||
| Shade tolerance | –0.16 | 0.60* | 0.48 | 0.59* | –0.04 | 0.35 | – | 0.66** | – |
| n v | 0.28 | 0.09 | –0.57* | 0.45 | –0.07 | – | –0.39 | – | |
| Lx (m3 s–1 MPa–1 cm–2) | 0.25 | 0.46 | –0.66* | –0.17 | – | 0.86*** | – | ||
| t | 0.40 | 0.56 | 0.81*** | – | 0.12 | – | |||
| d | –0.42 | 0.69** | – | 0.54 | – | ||||
| (t/d)2 | 0.16 | – | –0.54 | – | |||||
| RAD (mm) | – | 0.05 | – | ||||||
| Develop | 0.06 | – | |||||||
| Rings | – | ||||||||
| Fifth order | |||||||||
| Shade tolerance | 0.18 | –0.14 | –0.12 | 0.28 | –0.42 | 0.28 | – | 0.71** | – |
| n v | –0.42 | 0.21 | –0.09 | 0.50 | 0.3 | – | –0.12 | – | |
| Lx (m3 s–1 MPa–1 cm–2) | 0.50 | 0.49 | –0.16 | 0.21 | – | –0.01 | – | ||
| t | 0.70** | 0.33 | 0.75** | – | 0.12 | – | |||
| d | –0.33 | 0.91† | – | 0.60* | – | ||||
| (t/d)2 | –0.10 | – | –0.55 | – | |||||
| RAD (mm) | – | 0.52 | – | ||||||
| Develop | – | – | |||||||
| Rings | – | ||||||||
| Absorptive | |||||||||
| Shade tolerance | –0.34 | 0.83† | 0.16 | 0.89*** | –0.53 | 0.62* | 0.72** | ¶ | 0.27 |
| n v | –0.42 | 0.09 | –0.35 | 0.33 | –0.13 | –0.42 | ¶ | –0.58* | |
| Lx (m3 s–1 MPa–1 cm–2) | –0.17 | 0.84*** | –0.76** | 0.30 | 0.94† | ¶ | 0.64** | ||
| t | 0.24 | 0.72** | 0.53 | –0.35 | ¶ | –0.31 | |||
| d | –0.48 | 0.71** | 0.68** | ¶ | 0.53 | ||||
| (t/d)2 | –0.05 | –0.82*** | ¶ | –0.59* | |||||
| RAD (mm) | 0.21 | ¶ | 0.05 | ||||||
| Develop | ¶ | – | |||||||
| Rings | – | ||||||||
| Transport | |||||||||
| Shade tolerance | 0.13 | –0.18 | 0.06 | 0.37 | –0.28 | 0.32 | –0.02 | 0.74** | – |
| n v | –0.36 | 0.25 | –0.22 | 0.56 | 0.21 | –0.14 | –0.25 | – | |
| Lx (m3 s–1 MPa–1 cm–2) | 0.44 | 0.50 | –0.26 | 0.24 | 0.30 | –0.12 | – | ||
| t | 0.65* | 0.29 | 0.81*** | 0.17 | 0.06 | – | |||
| d | –0.42 | 0.87*** | 0.39 | 0.58* | – | ||||
| (t/d)2 | –0.07 | –0.68** | –0.54 | – | |||||
| RAD (mm) | 0.31 | 0.40 | – | ||||||
| Develop | 0.16 | – | |||||||
| Rings | – |
Traits included the number of conduits (nv), root-specific hydraulic conductance (Lx), thickness of individual conduit walls (t), hydraulic weighted mean conduit (lumen) diameter (d), the critical tension for conduit collapse (t/d)2, root average diameter (RAD), proportion of the sample under secondary development (Develop), number of growth rings (Rings) and mycorrhizal colonization (Colonization).
Statistical significance is indicated by *P < 0.1, **P < 0.05, ***P < 0.01 and †P < 0.001
– Indicates that analyses were not run. For example, first-order roots do not undergo secondary development, and thus do not form growth rings and periderm. All species had fourth- and fifth-order roots with secondary development, thus this trait was the same among all species so analyses were not run.
‡There was only colonization on third-order roots of Q. alba so analyses were not run.
¶Analyses were not run because only two species (P. virginiana and A. saccharum) formed rings on third-order roots.
§Analyses of nv in third-order roots were excluded because these analyses did not pass assumptions for the statistical tests.
Fig. 3.
Root hydraulic traits among species and root orders. Traits include root-specific hydraulic conductance, Lx (A); wall thickness of conduits, t (B); number of conduits, nv (C); and hydraulic weighted mean conduit (lumen) diameter, d (D) given separately for first-, second-, third-, fourth- and fifth-order roots among species with low (Intolerant), moderate (Moderate) and high (Tolerant) shade tolerance in Sapindaceae, Betulaceae, Fagaceae and Pinaceae. Species are given in Table 1. Mean square error = 8.E-09 for Lx; 0.48 for t; 41.78 for nv; and 7.54 for d.
Also in contrast to our hypotheses, no general pattern was found between root hydraulic mechanical strength (t) and shade tolerance assessed by PICs among root orders, or root functional classes, except among second-order roots (Table 2). There was, however, a general pattern of moderately shade-tolerant species in all four families having thicker cell walls (t) (Fig. 3B) and a tendency for t to be thicker with increased Lx in second- to fifth-order roots and in transport roots, suggesting that roots with greater Lx were more heavily fortified (Table 2). The critical tension for conduit collapse (t/d)2 decreased as Lx increased in second-order, fourth-order and absorptive roots, with tendencies in the others, when assessed with PICs (Table 2).
Root morphology, anatomy and mycorrhizal colonization
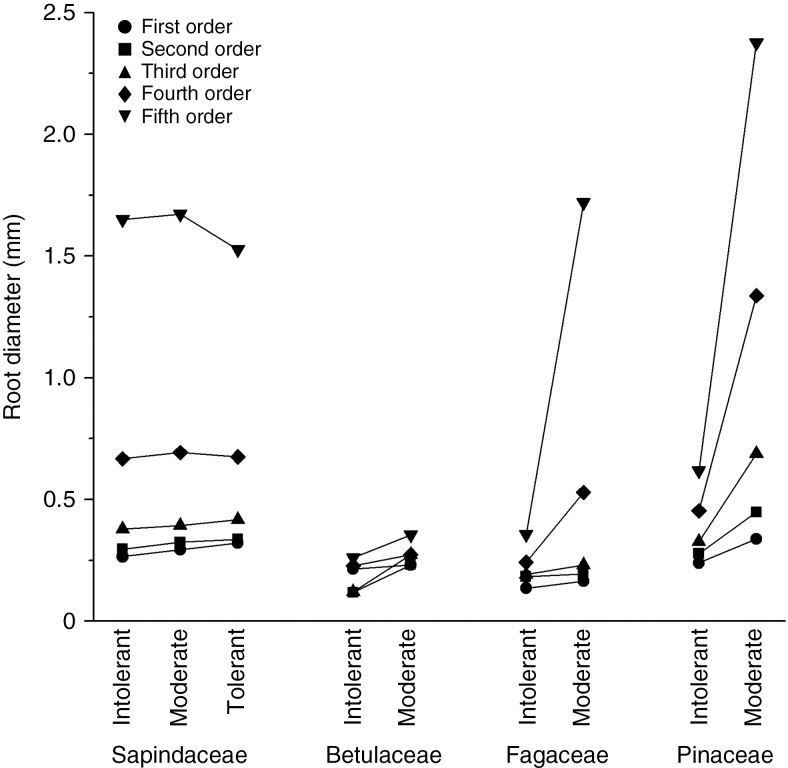
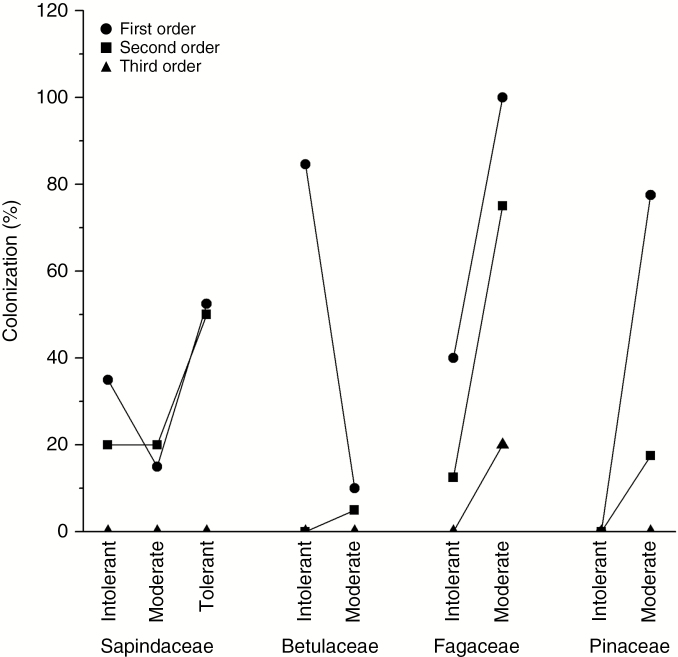
Root diameter increased with shade tolerance across first- and second-order roots and absorptive roots (Table 2). Across all root classes, diameters of moderate and high shade-tolerant species were thicker than root diameter of shade-intolerant species, with differences increasing in higher root orders (Fig. 4; 50–70 % greater in absorptive and approx. 140 % greater in transport roots). Overall differences in root diameter (P < 0.001) were larger in some families than in others, which led to a significant interaction between root diameter and shade tolerance nested within a family × root order (P = 0.019). Differences were smaller among Acer species (P > 0.53) than among species within the other three genera (P < 0.001). Roots with thicker diameter had a thicker xylem lumen diameter (d) or a tendency towards it across all root orders and functional classes (Table 2). This relationship was probably the primary driver of the relationship between Lx and shade tolerance as there was no relationship between the number of conduits and shade tolerance (Table 2; Figs 3A, C, D and 4). The thicker roots in species with greater shade tolerance tended to be more intensely colonized by mycorrhizal fungi than species with moderate or low shade tolerance (approx. 35, 28 and 16 % calculated as a mean of first, second and third orders, respectively) (Fig. 5). This relationship held true in all but one family, the Betulaceae; thus, relationships showed trends but were either only marginally significant (P < 0.10) or not statistically significant (P > 0.10) when assessed with PICs across root orders (Table 2). There was also increased Lx associated with mycorrhizal colonization among second-order and absorptive functional class roots when assessed with PICs (Table 2).
Fig. 4.
Root diameter (mm) of first-, second-, third-, fourth- and fifth-order roots among species with low (Intolerant), moderate (Moderate) and high (Tolerant) shade tolerance in Sapindaceae, Betulaceae, Fagaceae and Pinaceae. Species are given in Table 1. Mean square error = 0.11 for root diameter.
Fig. 5.
Mycorrhizal colonization (%) of first-, second- and third-order roots among species with low (Intolerant), moderate (Moderate) and high (Tolerant) shade tolerance in Sapindaceae, Betulaceae, Fagaceae and Pinaceae. Species are given in Table 1. Mycorrhizal colonization was determined from the proportion of cortical cells colonized by mycorrhizal fungi for arbuscular mycorrhizae or the proportion of cortical cells with Hartig’s network for ectomycorrhizae. Mean square error = 0.79 for mycorrhiza colonization.
Secondary development was hypothesized to occur to a greater degree in lower root branching orders in shade-tolerant species, presumably because shade-intolerant species produce a more highly branched absorptive root system. First- and second-order roots are generally believed to be ephemeral and rarely showed secondary development among any of the species. Similarly, fourth- and fifth-order roots were mainly woody across the nine species. The main variation occurred in the third-order roots that were primarily absorptive in some species but exhibited more of a role in transport in others. There was no consistent pattern among the four contrasts. Shade-tolerant species had a greater degree of secondary development in third-order roots among Acer (Sapindaceae) and Quercus (Fagaceae), but an opposite trend was observed in Betula species (Betulaceae) and no difference was observed between Pinus species (Pinaceae) (Table 3).
Table 3.
Percentage of roots with secondary development and number of growth rings for individual root orders (I–V) across nine North American tree species growing in a common garden contrasting in shade tolerance
| Family | Species | Shade tolerance | I | II | III | IV | V | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Secondary development | No. of rings | Secondary development | No. of rings | Secondary development | No. of rings | Secondary development | No. of rings | Secondary development | No. of rings | |||
| Sapindaceae | ||||||||||||
| Acer negundo | Low | – | – | – | – | – | – | 100 | 0.6 | 100 | 2.3 | |
| Acer rubrum | Moderate | – | – | – | – | 30 | – | 100 | 0.5 | 100 | 1.9 | |
| Acer saccharum | High | – | – | 20 | – | 100 | 0.3 | 100 | 1.5 | 100 | 3 | |
| Betulaceae | ||||||||||||
| Betula lenta | Low | – | – | 29 | – | 87 | – | 100 | – | 100 | – | |
| Betula alleghaniensis | Moderate | – | – | – | – | 10 | – | 90 | – | 100 | 1.5 | |
| Fagaceae | ||||||||||||
| Quercus rubra | Low–moderate | – | – | – | – | 20 | – | 100 | – | 100 | 0.8 | |
| Quercus alba | Moderate | – | – | – | – | 60 | – | 100 | 0.4 | 100 | 2.2 | |
| Pinaceae | ||||||||||||
| Pinus virginiana | Low | – | – | – | – | 100 | 0.4 | 100 | 0.7 | 100 | 1.3 | |
| Pinus strobus | Moderate | – | – | – | – | 100 | – | 100 | – | 100 | 1 | |
Ten roots from each branch order from plots were selected for observations.
(–) indicates that no roots in the designated root order had secondary development or growth rings. Mean square error was ±0.16 for secondary development based on percentile data and ±0.32 for no. of rings.
Examining the number of growth rings as a proxy for root age, we found that the number of rings generally increased with shade tolerance in higher root orders (fourth and fifth order) and the collective category of transport roots (rPIC= 0.66–0.74, P = 0.01–0.05; Table 2), suggesting that higher order roots of moderately and highly shade-tolerant species were generally older (had more growth rings) than roots of shade-intolerant species. This relationship was primarily driven by patterns within Acer, Betula and Quercus (Table 3). The Pinus species showed the opposite trend.
Year to year hydraulic plasticity
The number of growth rings nested into root order in shade-intolerant species showed a tendency for xylem conduit number to vary among different years (P = 0.08), suggesting an enhanced level of plasticity, while the number of conduits in growth rings of moderately and highly shade-tolerant species did not vary from year to year, suggesting a low level of plasticity (P = 0.21 and P = 0.37, respectively) (Supplementary Data Fig. S1). Likewise, the root specific hydraulic conductance (Lx) differed in shade-intolerant and moderately tolerant species among different years (P < 0.02 and P < 0.01, respectively), while no significant relationship was observed in Lx among growth rings in highly shade-tolerant species (P = 0.27) (Supplementary Data Fig. S1). However, the hydraulically weighted mean conduit diameter, d, did not differ in growth rings of shade-intolerant species (P = 0.1) but differed in those of moderately and highly shade-tolerant species (P < 0.001), suggesting that plasticity in Lx of shade-intolerant species was due to variation in xylem conduit number (Supplementary Data Fig. S1).
DISCUSSION
In the present study, we analysed root traits and strategies for hydraulic conductance associated with shade tolerance among tree species from different taxonomic families. Root samples were collected from species grown in a common garden where environmental conditions, especially light interception, were similar for all individuals. Thus, trait variation should be due to the adaptation of these species to their natural habitat, or reflect phylogenetic inertia. Counter to hypotheses, high Lx was generally associated with shade tolerance rather than shade intolerance among absorptive roots, potentially fitting with the adaptation of shade-intolerant species to fluctuating and uncertain water status, rather than high conductance associated with fast growth. Increases in Lx across all roots were generally associated with increases in xylem conduit diameter (d), with d also generally increasing with root average diameter (RAD) across all roots. Interestingly, mycorrhizal colonization also increased with Lx in absorptive roots, suggesting coupled traits for resource acquisition (nutrient and water). Fitting with our initial hypotheses, thickness of the conduit walls, t, showed a tendency to increase with Lx in transport roots across all species, consistent with needs for greater hydraulic structural support with increased Lx. However, d generally increased with increased Lx more quickly than t, such that the critical tension needed for conduit collapse, (t/d)2, generally decreased with increased Lx. The results suggested that species with greater shade tolerance had root traits associated with longer root life spans, including roots with greater RAD, fewer levels of branching among the absorptive roots (i.e. third-order roots more frequently had secondary development) and transport roots that were older (as evidenced by having fourth- and fifth-order roots with more growth rings). Finally, shade-intolerant species generally had transport roots with more plasticity in conduit numbers and Lx among growth rings, suggesting further adaptation to fluctuating and uncertain water status.
The initial hypothesis that shade-intolerant plants evolved greater hydraulic conductance in their root systems than shade-tolerant tree species was proposed based on the fast growth of early seral species and high water requirements anticipated to support this faster growth, as the above-ground plant physiological response should be related to root system development (Norby et al., 2001; Trubat et al., 2006). For example, grasses were found to increase vessel root area with decreasing light supply in order to support sufficient hydraulic conductance (Wahl et al., 2001). As discussed by Hubbard et al. (2001) or Lopez et al. (2005), in order to provide the amount of water needed to maintain carbon assimilation and evaporative demands, the root system in shade-tolerant species may be designed for high water conductance (Cochard et al., 1997; Maherali et al., 1997) as their leaf dark respiration rate is higher in a standardized light environment (Lusk and Reich, 2000). Shade-tolerant species often have thinner leaves with lower transpiration efficiency (Wyka et al., 2012; Giuliani et al., 2013), and thus may have higher water requirements, congruent with patterns of higher root-specific water conductance (Lx) when grown in a common garden. We found general patterns of both thicker root average diameters and greater mycorrhizal colonization associated with shade tolerance, similar to observations in the literature (Comas and Eissenstat, 2004; Chen et al., 2013; Kong et al., 2014). Interestingly, the association of shade tolerance with both increased Lx and mycorrhizal colonization, and increased Lx with increased mycorrhizal colonization among absorptive roots functional classes is in accordance with literature results that demonstrated increased hydraulic conductivity with greater mycorrhizal colonization (Muhsin and Zwiazek, 2002). Additionally, shade-tolerant species are suggested to increase their capacity for water absorption through promoting increased external mycelium of long-distance transport types (Lehto and Zwiazek, 2011).
From a mechanical perspective, species with high hydraulic conductance may benefit from mechanisms that decrease the risk of cavitation (Wagner et al., 1998; Lens et al., 2011). Protection of xylem conduits from cavitation can be achieved in two ways: increased cell wall fortification, or reduction in conduit diameter. We showed a tendency of increased t with Lx in transport roots, as well as increased t with increased d with shade tolerance, supporting the idea that risk of hydraulic failure drives adaptation of xylem anatomy of transport roots to confer protection against embolism (Hacke et al., 2001, 2007; Jacobsen et al., 2007). However, d increased more rapidly than t in association with greater Lx, thus roots with greater Lx generally had lower (t/d)2 as often found in the literature (Hacke et al., 2000; Lopez et al., 2005; Gleason et al., 2016). Smaller d in shade-intolerant species may reflect a strategy of conservative hydraulic regulation by limiting risk of cavitation under water-limiting environment.
Examining relationships between conduit number and Lx across growth rings, our data suggested greater plasticity in traits among years in shade-intolerant species. Greater plasticity in conduit number and Lx in shade-intolerant species may be advantageous for growing in early seral habitats, which may be unpredictable and require swift adjustment to prevailing conditions between years for plants to compete for water and nutrients. Moreover, control of conduit number and Lx may be a means to increase xylem safety in a cost-effective way for handling periodic drought. Inversely, less trait variation in shade-tolerant species may reflect adaptation to growth in an environment with less fluctuation from year to year. Such conservation might, however, be a disadvantage when roots foraging in the soil encounter resource-rich patches. These patterns of root trait plasticity are in agreement with previous observations that species adapted to higher light requirements exhibit greater phenotypic plasticity in morphological and physiological traits than those adapted to shade (Henry and Aarssen, 1997; Sánchez-Gómez et al., 2006a). In contrast, the low plasticity of shade-tolerant species growing under more predictable conditions may reflect a conservative strategy of resource use and greater reliance on a sufficient water supply (Abrams and Mostoller, 1995; Grime and Mackey, 2002; Niinemets and Valladares, 2006; Sánchez-Gómez et al., 2006a,b).
Root traits such as diameter and secondary development impact nutrient uptake. Trait variation in this study aligns with the idea that species with thinner roots are often capable of fast soil foraging, although it was previously considered that thin roots are characterized by high specific hydraulic conductance (Huang and Eissenstat, 2000; Hernández et al., 2010), which was not found here for axial conductance. The association of shade intolerance with smaller root diameter, a more branched absorptive root system and a lower number of growth rings (lower average age) of their transport roots suggests strategies of rapidly proliferating roots with a short life span (sensuEissenstat and Yanai, 1997; Comas et al., 2002). However, nutrient acquisition is, importantly, also influenced by the developmental status of roots, where roots with primary growth (absorptive roots) reflect the ability to take up resources, and roots with secondary development (transport roots) reflect the ability to transport water and resources (Eissenstat and Achor, 1999; Gambetta et al., 2013). Generally, absorptive roots are within the first to third root orders, and roots located higher in the branching hierarchy are transport roots (Pregitzer et al., 2002; Guo et al., 2008; Xia et al., 2010). Roots with secondary development generally occurred more frequently in the lower branching orders in more shade-tolerant species within the Sapindaceae and Fagaceae than in shade-intolerant species, although the opposite trend was observed within the Betulaceae. The patterns observed within the Betulaceae may be partially accounted for by the growth habitat of the examined species. Betula alleghaniensis grows in cooler regions with a shorter growing season than B. lenta (Burns and Honkala, 1990). Maintaining absorptive roots without secondary growth is a common attribute in species that grow in cold soils (Chapin, 1974; Zadworny et al., 2016). Additionally, although B. alleghaniensis is considered a moderately shade-tolerant species, during the early stages of its life cycle, it also grows rapidly as a pioneer species (Burns and Honkala, 1990). From a root system perspective, secondary development of roots minimizes water loss to dry soils, also referred to as reverse water flow, i.e. from the root to the soil (Gambetta et al., 2013).
In conclusion, results here expand ideas of plant growth strategies to include hydraulic strategies of root systems. This study supports previous ideas of plant growth strategies suggesting that species adapted to high light and resource-rich environments produce thinner roots with shorter life span (Walters and Reich, 1999), and that thinner roots are less reliant on mycorrhizal fungi, in contrast to thicker, extensively colonized roots (Baylis, 1975; Brundrett, 2002; Comas et al., 2012, 2014; Kong et al., 2014; Eissenstat et al., 2015; Liu et al., 2015). We also show that thinner roots of shade-intolerant species also had thinner xylem and lower hydraulic conductance, potentially limiting the risk of cavitation under low water conditions, which may occur with greater frequency under conditions of early succession where competition for water rather than light might be greater. Moreover, our results showed that shade-intolerant species had greater plasticity in number of xylem conduits and hydraulic conductance across different root growth rings of the same root order as compared with shade-tolerant species, also potentially tied to greater fluctuation of water availability in early successional habitats. Expanded species comparisons are needed to improve understanding of how universally these plant life history strategies are linked to hydraulic strategies and interactions with mycorrhizal fungi.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of Fig. S1: root hydraulic traits by growth ring and shade tolerance.
ACKNOWLEDGEMENTS
This work was supported by grants from the Polish Ministry of Science and Higher Education (project no. 11/MOB/2007/0), the Institute of Dendrology of the Polish Academy of Sciences and the US National Science Foundation (projects no. IOS 07-19259, OEI 0613832). We thank Sean Gleason, anonymous reviewers and the editor for helpful comments that greatly improved this paper.
LITERATURE CITED
- Abrams MD, Mostoller SA. 1995. Gas exchange, leaf structure and nitrogen in contrasting successional tree species growing in open and understory sites during a drought. Tree Physiology 15: 361–370. [DOI] [PubMed] [Google Scholar]
- Bagniewska-Zadworna A, Byczyk J, Eissenstat DM, Oleksyn J, Zadworny M. 2012. Avoiding transport bottlenecks in an expanding root system: xylem vessel development in fibrous and pioneer roots under field conditions. American Journal of Botany 99: 1417–1426. [DOI] [PubMed] [Google Scholar]
- Baylis GTS. 1975. The magnolioid mycorrhiza and mycotrophy in root systems derived from it. In: Sanders FE, Mosse B, Tinker PB, eds. Endomycorrhizas. New York: Academic Press, 373–389. [Google Scholar]
- Blackman CJ, Brodribb TJ, Jordan GJ. 2010. Leaf hydraulic vulnerability is related to conduit dimensions and drought resistance across a diverse range of woody angiosperms. New Phytologist 188: 1113–1123. [DOI] [PubMed] [Google Scholar]
- Brassard BW, Chen HYH, Bergeron Y. 2009. Influence of environmental variability on root dynamics in northern forests. Critical Reviews in Plant Sciences 28: 179–197. [Google Scholar]
- Brundrett MC. 2002. Coevolution of roots and mycorrhizas of land plants. New Phytologist 154: 275–304. [DOI] [PubMed] [Google Scholar]
- Burns RM, Honkala BH. 1990. Silvics of North America. Washington, DC: US Department of Agriculture Forest Service. [Google Scholar]
- Casper BB, Jackson RB. 1997. Plant competition underground. Annual Review of Ecology and Systematics 28: 545–570. [Google Scholar]
- Chapin FS. 1974. Morphological and physiological mechanisms of temperature compensation in phosphate absorption along a latitudinal gradient. Ecology 55: 1180–1198. [Google Scholar]
- Chen HYH, Brassard BW. 2013. Intrinsic and extrinsic controls of fine root life span. Critical Reviews in Plant Sciences 32: 151–161. [Google Scholar]
- Chen W, Zeng H, Eissenstat DM, Guo DL. 2013. Variation of first-order root traits across climatic gradients and evolutionary trends in geological time. Global Ecology and Biogeography 22: 846–856. [Google Scholar]
- Chen W, Koide RT, Adams TS, DeForest JL, Cheng L, Eissenstat DM. 2016. Root morphology and mycorrhizal symbioses together shape nutrient foraging strategies of temperate trees. Proceedings of the National Academy of Sciences, USA 113: 8741–8746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Chen W, Adams TS et al. . 2016. Mycorrhizal fungi and roots are complementary in foraging within nutrient patches. Ecology 97: 2815–2823. [DOI] [PubMed] [Google Scholar]
- Cochard H, Peiffer M, LeGall K, Granier A. 1997. Developmental control of xylem hydraulic resistances and vulnerability to embolism in Fraxinus excelsior L: impacts on water relations. Journal of Experimental Botany 48: 655–663. [Google Scholar]
- Comas LH, Eissenstat DM. 2004. Linking fine root traits to maximum potential growth rate among 11 mature temperate tree species. Functional Ecology 18: 388–397. [Google Scholar]
- Comas LH, Bouma TJ, Eissenstat DM. 2002. Linking root traits to potential growth rate in six temperate tree species. Oecologia 132: 34–43. [DOI] [PubMed] [Google Scholar]
- Comas LH, Mueller KE, Taylor LL, Midford PE, Callahan HS, Beerling DJ. 2012. Evolutionary patterns and biogeochemical significance of angiosperm root traits. International Journal of Plant Sciences 173: 584–595. [Google Scholar]
- Comas LH, Callahan HS, Midford PE. 2014. Patterns in root traits of woody species hosting arbuscular and ectomycorrhizas: implications for the evolution of belowground strategies. Ecology and Evolution 4: 2979–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenstat DM, Achor DS. 1999. Anatomical characteristics of roots of citrus rootstocks that vary in specific root length. New Phytologist 141: 309–321. [DOI] [PubMed] [Google Scholar]
- Eissenstat DM, Yanai RD. 1997. The ecology of root lifespan. In: Advances in ecological research, Vol 27 London: Academic Press Ltd/Elsevier Science Ltd, 1–60. [Google Scholar]
- Eissenstat DM, Kucharski JM, Zadworny M, Adams TS, Koide RT. 2015. Linking root traits to nutrient foraging in arbuscular mycorrhizal trees in a temperate forest. New Phytologist 208: 114–124. [DOI] [PubMed] [Google Scholar]
- Fayle DCF. 1968. Radial growth in tree roots: distribution, timing, anatomy. Technical report 9 Toronto: Faculty of Forestry, University of Toronto. [Google Scholar]
- Felsenstein J. 1985. Phylogenies and the comparative method. American Naturalist 125: 1–15. [DOI] [PubMed] [Google Scholar]
- Finér L, Messier C, DeGrandpre L. 1997. Fine-root dynamics in mixed boreal conifer–broad-leafed forest stands at different successional stages after fire. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestiere 27: 304–314. [Google Scholar]
- Freschet GT, Swart EM, Cornelissen JHC. 2015. Integrated plant phenotypic responses to contrasting above- and below-ground resources: key roles of specific leaf area and root mass fraction. New Phytologist 206: 1247–1260. [DOI] [PubMed] [Google Scholar]
- Gambetta GA, Fei J, Rost TL et al. . 2013. Water uptake along the length of grapevine fine roots: developmental anatomy, tissue-specific aquaporin expression, and pathways of water transport. Plant Physiology 163: 1254–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernandt DS, Magallon S, Lopez GG, Flores OZ, Willyard A, Liston A. 2008. Use of simultaneous analyses to guide fossil-based calibrations of Pinaceae phylogeny. International Journal of Plant Sciences 169: 1086–1099. [Google Scholar]
- Giuliani R, Koteyeva N, Voznesenskaya E, Evans MA, Cousins AB, Edwards GE. 2013. Coordination of leaf photosynthesis, transpiration, and structural traits in rice and wild relatives (genus Oryza). Plant Physiology 162: 1632–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason SM, Westoby M, Jansen S et al. . 2016. Weak tradeoff between xylem safety and xylem-specific hydraulic efficiency across the world’s woody plant species. New Phytologist 209: 123–136. [DOI] [PubMed] [Google Scholar]
- Gravel D, Canham CD, Beaudet M, Messier C. 2010. Shade tolerance, canopy gaps and mechanisims of coexistence of forest trees. Oikos 119: 475–484. [Google Scholar]
- Grime JP, Mackey JML. 2002. The role of plasticity in resource capture by plants. Evolutionary Ecology 16: 299–307. [Google Scholar]
- Guo DL, Xia MX, Wei X, Chang WJ, Liu Y, Wang ZQ. 2008. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytologist 180: 673–83. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS, Pittermann J. 2000. Drought experience and cavitation resistance in six shrubs from the Great Basin, Utah. Basic and Applied Ecology 1: 31–41. [Google Scholar]
- Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloch KA. 2001. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126: 457–461. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS, Feild TS, Sano Y, Sikkema EH, Pittermann J. 2007. Water transport in vesselless angiosperms: conducting efficiency and cavitation safety. International Journal of Plant Sciences 168: 1113–1126. [Google Scholar]
- Hastwell GT, Facelli JM. 2003. Differing effects of shade-induced facilitation on growth and survival during the establishment of a chenopod shrub. Journal of Ecology 91: 941–950. [Google Scholar]
- Henry HAL, Aarssen LW. 1997. On the relationship between shade tolerance and shade avoidance strategies in woodland plants. Oikos 80: 575–582. [Google Scholar]
- Hernández EI, Vilagrosa A, Pausas JG, Bellot J. 2010. Morphological traits and water use strategies in seedlings of Mediterranean coexisting species. Plant Ecology 207: 233–244. [Google Scholar]
- Huang B, Eissenstat DM. 2000. Linking root hydraulic conductivity to anatomy in citrus rootstocks that vary in specific root length. Journal of the American Society for Horticultural Science 125: 260–265. [Google Scholar]
- Hubbard RM, Ryan MG, Stiller V, Sperry JS. 2001. Stomatal conductance and photosynthesis vary linearly with plant hydraulic conductance in ponderosa pine. Plant, Cell and Environment 24: 113–121. [Google Scholar]
- Jacobsen AL, Pratt RB, Ewers FW, Davis SD. 2007. Cavitation resistance among 26 chaparral species of southern California. Ecological Monographs 77: 99–115. [Google Scholar]
- Kong D, Wang J, Zeng H et al. . 2017. The nutrient absorption–transportation hypothesis: optimizing structural traits in absorptive roots. New Phytologist 213: 1569–1572. [DOI] [PubMed] [Google Scholar]
- Kong DL, Ma CG, Zhang Q et al. . 2014. Leading dimensions in absorptive root trait variation across 96 subtropical forest species. New Phytologist 203: 863–872. [DOI] [PubMed] [Google Scholar]
- Kotowska MM, Hertel D, Abou Rafab Y, Barus H, Schuldt B. 2015. Patterns in hydraulic architecture from roots to branches in six tropical tree species from cacao agroforestry and their relation to wood density and stem growth. Frontiers in Plant Science 6: e16. doi.org/10.3389/fpls.2015.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto T, Związek JJ. 2011. Ectomycorrhizas and water relations of trees: a review. Mycorrhiza 21: 71–90. [DOI] [PubMed] [Google Scholar]
- Lens F, Sperry JS, Christman MA, Choat B, Rabaey D, Jansen S. 2011. Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus Acer. New Phytologist 190: 709–723. [DOI] [PubMed] [Google Scholar]
- Liu BT, Li HB, Zhu BA, Koide RT, Eissenstat DM, Guo DL. 2015. Complementarity in nutrient foraging strategies of absorptive fine roots and arbuscular mycorrhizal fungi across 14 coexisting subtropical tree species. New Phytologist 208: 125–136. [DOI] [PubMed] [Google Scholar]
- Long YQ, Kong DL, Chen ZX, Zeng H. 2013. Variation of the linkage of root function with root branch order. PLoS One 8: e11. doi.org/10.1371/journal.pone.0057153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez OR, Kursar TA, Cochard H, Tyree MT. 2005. Interspecific variation in xylem vulnerability to cavitation among tropical tree and shrub species. Tree Physiology 25: 1553–1562. [DOI] [PubMed] [Google Scholar]
- Lusk CH, Reich PB. 2000. Relationships of leaf dark respiration with light environment and tissue nitrogen content in juveniles of 11 cold-temperate tree species. Oecologia 123: 318–329. [DOI] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR. 2010. Mesquite: a modular system for evolutionary analysis.Version 2.74 http://mesquiteproject.org
- Magallon SA, Sanderson MJ. 2005. Angiosperm divergence times: the effect of genes, codon positions, and time constraints. Evolution 59: 1653–1670. [DOI] [PubMed] [Google Scholar]
- Maherali H, DeLucia EH, Sipe TW. 1997. Hydraulic adjustment of maple saplings to canopy gap formation. Oecologia 112: 472–480. [DOI] [PubMed] [Google Scholar]
- McCormack ML, Adams TS, Smithwick EAH, Eissenstat DM. 2012. Predicting fine root lifespan from plant functional traits in temperate trees. New Phytologist 195: 823–831. [DOI] [PubMed] [Google Scholar]
- McCormack ML, Dickie IA, Eissenstat DM et al. . 2015. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytologist 207: 505–518. [DOI] [PubMed] [Google Scholar]
- Midford PE, Garland T, Maddison WP. 2010. PDAP: PDTREE package of Mesquite.Version 1.15 http://mesquiteproject.org/pdap_mesquite/
- Muhsin TM, Zwiazek JJ. 2002. Ectomycorrhizas increase apoplastic water transport and root hydraulic conductivity in Ulmus americana seedlings. New Phytologist 153: 153–158. [Google Scholar]
- Niinemets Ü, Valladares F. 2006. Tolerance to shade, drought, and waterlogging of temperate Northern Hemisphere trees and shrubs. Ecological Monographs 76: 521–547. [Google Scholar]
- Norby RJ, Ogle K, Curtis PS et al. . 2001. Aboveground growth and competition in forest gap models: an analysis for studies of climatic change. Climatic Change 51: 415–447. [Google Scholar]
- Oakley TH, Cunningham CW. 2000. Independent contrasts succeed where ancestor reconstruction fails in a known bacteriophage phylogeny. Evolution 54: 397–405. [DOI] [PubMed] [Google Scholar]
- Persson H, Von Fircks Y, Majdi H, Nilsson LO. 1995. Root distribution in a Norway spruce (Picea abies (L.) Karst.) stand subjected to drought and ammonium-sulphate application. Plant and Soil 168: 161–165. [Google Scholar]
- Peterson RL, Massicotte HB, Melville LH. 2004. Mycorrhizas: anatomy and cell biology. Ottawa: NRC Research Press. [Google Scholar]
- Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L. 2012. Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytologist 193: 30–50. [DOI] [PubMed] [Google Scholar]
- Pregitzer KS, DeForest JL, Burton AJ, Allen MF, Ruess RW, Hendrick RL. 2002. Fine root architecture of nine North American trees. Ecological Monographs 72: 293–309. [Google Scholar]
- Rewald B, Ephrath JE, Rachmilevitch S. 2011. A root is a root is a root? Water uptake rates of Citrus root orders. Plant, Cell and Environment 34: 33–42. [DOI] [PubMed] [Google Scholar]
- Sánchez-Gómez D, Valladares F, Zavala MA. 2006a Functional traits and plasticity in response to light in seedlings of four Iberian forest tree species. Tree Physiology 26: 1425–1433. [DOI] [PubMed] [Google Scholar]
- Sánchez-Gómez D, Valladares F, Zavala MA. 2006b Performance of seedlings of Mediterranean woody species under experimental gradients of irradiance and water availability: trade-offs and evidence for niche differentiation. New Phytologist 170: 795–805. [DOI] [PubMed] [Google Scholar]
- Schenk HJ. 2006. Root competition: beyond resource depletion. Journal of Ecology 94: 725–739. [Google Scholar]
- Sperry JS, Hacke UG. 2002. Desert shrub water relations with respect to soil characteristics and plant functional type. Functional Ecology 16: 367–378. [Google Scholar]
- Sperry JS, Nichols KL, Sullivan JEM, Eastlack SE. 1994. Xylem embolism in ring-porous, diffuse-porous, and coniferous trees of Northern Utah and Interior Alaska. Ecology 75: 1736–1752. [Google Scholar]
- Trubat R, Cortina J, Vilagrosa A. 2006. Plant morphology and root hydraulics are altered by nutrient deficiency in Pistacia lentiscus (L.). Trees-Structure and Function 20: 334–339. [Google Scholar]
- Valenzuela-Estrada LR, Richards JH, Diaz A, Eissensat DM. 2009. Patterns of nocturnal rehydration in root tissues of Vaccinium corymbosum L. under severe drought conditions. Journal of Experimental Botany 60: 1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladers F. 2003. Light heterogeneity and plants: from ecophysiology to species coexistence and biodiversity. In: Eser K, Lütge U, Beyschlag W, Hellwig F, eds. Progress in botany. Heidelberg: Springer-Verlag, 439–471. [Google Scholar]
- Wagner KR, Ewers FW, Davis SD. 1998. Tradeoffs between hydraulic efficiency and mechanical strength in the stems of four co-occurring species of chaparral shrubs. Oecologia 117: 53–62. [DOI] [PubMed] [Google Scholar]
- Wahl S, Ryser P, Edwards PJ. 2001. Phenotypic plasticity of grass root anatomy in response to light intensity and nutrient supply. Annals of Botany 88: 1071–1078. [Google Scholar]
- Walker LR, del Moral R. 2003. Primary succession and ecosystem rehabilitation. Cambridge: Cambridge University Press. [Google Scholar]
- Walters MB, Reich PB. 1999. Low-light carbon balance and shade tolerance in the seedlings of woody plants: do winter deciduous and broad-leaved evergreen species differ?New Phytologist 143: 143–154. [Google Scholar]
- Wikstrom N, Savolainen V, Chase MW. 2001. Evolution of the angiosperms: calibrating the family tree. Proceedings of the Royal Society B: Biological Sciences 268: 2211–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyka TP, Oleksyn J, Żytkowiak R, Karolewski P, Jagodzinski AM, Reich PB. 2012. Responses of leaf structure and photosynthetic properties to intra-canopy light gradients: a common garden test with four broadleaf deciduous angiosperm and seven evergreen conifer tree species. Oecologia 170: 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia MX, Guo DL, Pregitzer KS. 2010. Ephemeral root modules in Fraxinus mandshurica. New Phytologist 188: 1065–1074. [DOI] [PubMed] [Google Scholar]
- Zadworny M, McCormack ML, Mucha J, Reich PB, Oleksyn J. 2016. Scots pine fine roots adjust along a 2000-km latitudinal climatic gradient. New Phytologist 212: 389–399. [DOI] [PubMed] [Google Scholar]
- Zavala MA, Angulo O, de la Parra RB, Lopez-Marcos JC. 2007. An analytical model of stand dynamics as a function of tree growth, mortality and recruitment: the shade tolerance–stand structure hypothesis revisited. Journal of Theoretical Biology 244: 440–450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.