Abstract
In the Life Span Study cohort of atomic bomb survivors, differences in urbanicity between high-dose and low-dose survivors could confound the association between radiation dose and adverse outcomes. We obtained data on the population distribution in Hiroshima and Nagasaki before the 1945 bombings and quantified the impact of adjustment for population density on radiation risk estimates for mortality (1950–2003) and incident solid cancer (1958–2009). Population density ranged from 4,671 to 14,378 people/km2 in the urban region of Hiroshima and 5,748 to 19,149 people/km2 in the urban region of Nagasaki. Radiation risk estimates for solid cancer mortality were attenuated by 5.1% after adjustment for population density, but those for all-cause mortality and incident solid cancer were unchanged. There was no overall association between population density and adverse outcomes, but there was evidence that the association between density and mortality differed according to age at exposure. Among survivors who were 10–14 years of age in 1945, there was a positive association between population density and risk of all-cause mortality (per 5,000-people/km2 increase, relative risk = 1.053, 95% confidence interval: 1.027, 1.079) and solid cancer mortality (per 5,000-people/km2 increase, relative risk = 1.069, 95% confidence interval: 1.025, 1.115). Our results suggest that radiation risk estimates from the Life Span Study are not sensitive to unmeasured confounding by urban-rural differences.
Keywords: cancer incidence, mortality, population density, radiation
In the Life Span Study of atomic bomb survivors (Hiroshima and Nagasaki, Japan), a survivor’s dose of ionizing radiation was determined primarily by the city in which the survivor was exposed and the ground distance between the bomb’s hypocenter and the survivor’s reported location (1, 2). In Hiroshima, the hypocenter was in the city’s urban center. Therefore, survivors located in urban regions were generally exposed to higher levels of radiation, while survivors located in rural regions were generally exposed to lower levels. Conversely, in Nagasaki, the hypocenter was approximately 3 km northwest of the city’s urban center. Therefore, survivors located in rural regions were generally exposed to higher levels of radiation, while survivors located in urban regions, particularly to the southeast of the hypocenter, were generally exposed to lower levels. Consequently, differences in urbanicity between high-dose and low-dose survivors could confound the association between radiation dose and adverse health outcomes (3).
We previously quantified heterogeneity in the risk of all-cause mortality and incident solid cancer among zero-dose survivors at different distances from the hypocenter (4–6). We hypothesized that these risk differences were driven by confounding that was not accounted for by adjustment for sex and age. We identified location as a key potential confounding factor because radiation dose was determined primarily by location, and location could influence risk through socioeconomic factors, such as education and employment, and environmental factors, such as exposure to pollutants (6). Due to limited information on preexposure individual-level characteristics, we addressed this potential for unmeasured confounding by selecting an appropriate reference group of unexposed individuals and adjusting for risk differences among all unexposed groups. We found that radiation risk estimates for all-cause mortality and incident solid cancer were somewhat sensitive to the choice of the reference group—risk estimates varied within ±10% (5, 6). However, it is possible that our analytical approach did not adequately adjust for confounding because the definition of location—merely a binary indicator of distance from the hypocenter—was too coarse. Therefore, we sought to address this potential for residual confounding by leveraging historical information on population density before the bombings as an adjustment variable to capture urban-rural differences on a finer scale.
A growing body of literature has shown that community-level exposures, including population density, are associated with adverse health outcomes (7–12). In particular, several large cohort studies have documented positive associations between population density and mortality (7–9). These include an analysis of the Japan Public Health Center-based Prospective Study, which showed that population density was strongly associated with all-cause mortality after adjustment for socioeconomic status and behavioral factors (9). In addition, large cohort studies have documented positive associations between population density and cancer incidence (10, 11). Most recently, researchers provided evidence for a biological response to unfavorable community-level exposures by demonstrating that population density was associated with shortened telomere length (12). Taken together, these results support the rationale for considering population density as a potential confounder of the association between radiation dose and adverse health outcomes in the Life Span Study.
Our goals were to determine the population density before the atomic bombings at survivors’ reported locations in Hiroshima and Nagasaki and to quantify the impact of adjustment for population density on radiation risk estimates for mortality and incident solid cancer. First, we used mapping techniques to assign population-density estimates to survivor locations. Second, we reanalyzed Life Span Study data on mortality (13) and solid cancer incidence (14). We focused on radiation excess relative risks (ERRs) and the evidence for curvature in the radiation dose-response.
METHODS
Study cohort
The Life Span Study included 93,741 atomic bomb survivors who reported their location, within 10 km of the hypocenter, at the time of the bombings (15). For these survivors, the Dosimetry System 2002 method (1, 2) was used to estimate Dosimetry System 2002 Revision 1 (DS02R1)-weighted absorbed colon doses in gray (Gy). Weighted doses—the sum of the γ-radiation dose and 10 times the neutron dose—allowed for a greater biological effectiveness of neutrons. Correction for measurement error and truncation of doses >4 Gy was performed (16). We excluded Life Span Study participants who were not in either city during the bombings (i.e., not-in-city residents), because the population density at their location at the time of the bombings was unknown.
This study was approved by the Human Investigation Committee of the Radiation Effects Research Foundation. Hiroshima Prefecture, Nagasaki Prefecture, and the City of Hiroshima approved the linkage of study participants with data from their cancer registries.
Population density
The Joint Commission for the Investigation of the Effects of the Atomic Bomb in Japan sought to determine acute casualty rates in relation to distance from the hypocenter (17). Therefore, the Commission required estimates of the population distribution before the bombings in Hiroshima and Nagasaki. The Commission’s investigation indicated that the most reliable population estimates could be obtained from rice-rationing figures. Japanese cities are divided into districts, which are divided into precincts. During the war, an official in each precinct tracked the number of people entitled to a rice ration and distributed the rations accordingly (18). Precinct officials then reported these numbers to their district office on a monthly basis. In Hiroshima, records from July 1945 were destroyed, but those from June 1945 indicated a population size of 255,260. In Nagasaki, records from July 1945 indicated a population size of 195,290. Based on the rice-rationing figures, the Commission estimated population density within radii of the hypocenter, up to 5 km, in urban regions of Hiroshima and Nagasaki (Web Table 1, available at https://academic.oup.com/aje).
To assign a population density to a survivor in the Life Span Study, we determined whether the survivor was located in an urban region and, if so, the density at that location. We identified urban regions within 5 km of the hypocenters using maps produced by the United States Army Map Service in 1945: polyconic projection, Band 111N, Zone B and C for Hiroshima and Nagasaki, respectively (2). Hiroshima is located on a broad river delta. To identify the urban region of Hiroshima, we used district borders or, for peripheral districts without a defined border, geographical contours of 10–20 m above sea level. Nagasaki is located in several valleys that extend from a large bay. To identify the urban region of Nagasaki, we used district borders along with indicators of manmade features (e.g., residential or industrial structures, roads). In both cities, the urban region extended 50 m offshore. Survivors were positioned on the map according to their reported location at the time of the bombings—recorded in the master sample questionnaire and shielding history (19)—using ArcGIS, version 10.4.1 (Esri, Redlands, California). Survivors located in an urban region within 5 km of the hypocenter were assigned a population density according to their distance from the hypocenter (Web Table 1). Survivors located outside an urban region but within 5 km of the hypocenter, as well as survivors located beyond 5 km, were assigned the average population density in rural Japan according to the February 1944 national census: 116 people/km2 (20, p. 36). Because the population density in nonurban regions of Hiroshima and Nagasaki might have differed from the national average, in sensitivity analyses, survivors located in nonurban regions were assigned a population density of 0 (hypothetical minimum) or 1,000 (hypothetical maximum) people/km2.
Mortality
Mortality analyses were limited to 86,660 survivors with known radiation doses and who were alive at the beginning of follow-up on October 1, 1950 (13). Deaths were identified by the Japanese national family registry system between October 1, 1950, and December 31, 2003. We focused on mortality due to any cause and mortality due to solid cancer, which was classified according to International Classification of Diseases codes (Seventh Revision through Ninth Revision, 1950–1997: codes 140–199; Tenth Revision, 1998–2003: codes C00–C80). Analyses were based on a highly stratified table of case counts and accrued person-years. Details on stratification are provided in the Web Appendix.
Piecewise-constant hazard models related the rate of all-cause and solid cancer mortality to the background rate and an ERR due to radiation exposure. Sex-specific background rates were adjusted for age at exposure—equivalent to birth year because all survivors were exposed in 1945—and attained age. We selected zero-dose survivors within 5 km of the hypocenter as the reference group because population density estimates were available only for urban regions within 5 km of the hypocenter. Thus, the model accounted for risk differences between survivors located outside an urban region but within 5 km of the hypocenter and survivors located beyond 5 km. ERRs were parameterized as a function of weighted absorbed colon dose with effect modification by sex, age at exposure, attained age, and an indicator of total shielded kinetic energy released per unit mass of >4 Gy to account for dose-estimation errors at high doses, which are typically lethal. For both all-cause and solid cancer mortality, we fitted a sex-averaged linear dose-response model. Details on model parameterization are provided in the Web Appendix.
Solid cancer incidence
Analyses of solid cancer incidence were limited to 80,205 survivors with known radiation doses and who were alive and at risk for a first primary solid cancer at the beginning of follow-up on January 1, 1958 (14). Survivors with a diagnosis of cancer documented in medical records before 1958 were excluded. First primary solid cancers were identified by Hiroshima and Nagasaki population-based cancer registries between January 1, 1958, and December 31, 2009. To avoid bias from underrepresentation of cases, person-years were multiplied by the probability of residency in the registries’ catchment regions, with estimated residency probabilities stratified by city, sex, birth year, and calendar year (14, p. 531).
Piecewise-constant hazard models for first primary solid cancer were similar to those for mortality. We considered 2 radiation dose-response models: a sex-averaged linear dose-response model and a sex-specific linear-quadratic dose-response model, because a recent analysis found evidence for curvature among males (14).
Adjustment for population density
All models were fitted with and without adjustment of the background rate for population density. Population density was included as a linear term in the background model because exploratory analyses revealed a linear association across density categories. Because the association between population density and adverse health outcomes could depend on age, a longitudinal measure of density would be preferred to capture density associations at different periods of sensitivity (21). In the absence of a longitudinal measure of population density, we allowed the density association to differ across groups defined by their age when density was measured. Therefore, we also included a term for interaction between population density and a categorical variable for age at exposure (<10 years, 10–14 years, 15–29 years, ≥30 years) in the background model. In particular, we selected age groups to correspond to children, adolescents, younger adults, and older adults.
All analyses were completed using R, version 3.4.0 (R Foundation for Statistical Computing, Vienna, Austria). Estimation was performed using the gnm extension package for generalized non-linear models, assuming a Poisson distribution and log link function (22–24). Likelihood confidence intervals were obtained from the profile likelihood. Statistical hypotheses regarding regression parameters were evaluated using likelihood ratio statistics. Comparisons of model fit were based on the Akaike information criterion (AIC), which penalizes the value of the maximized log-likelihood by the number of estimated parameters (25, 26).
RESULTS
Population density
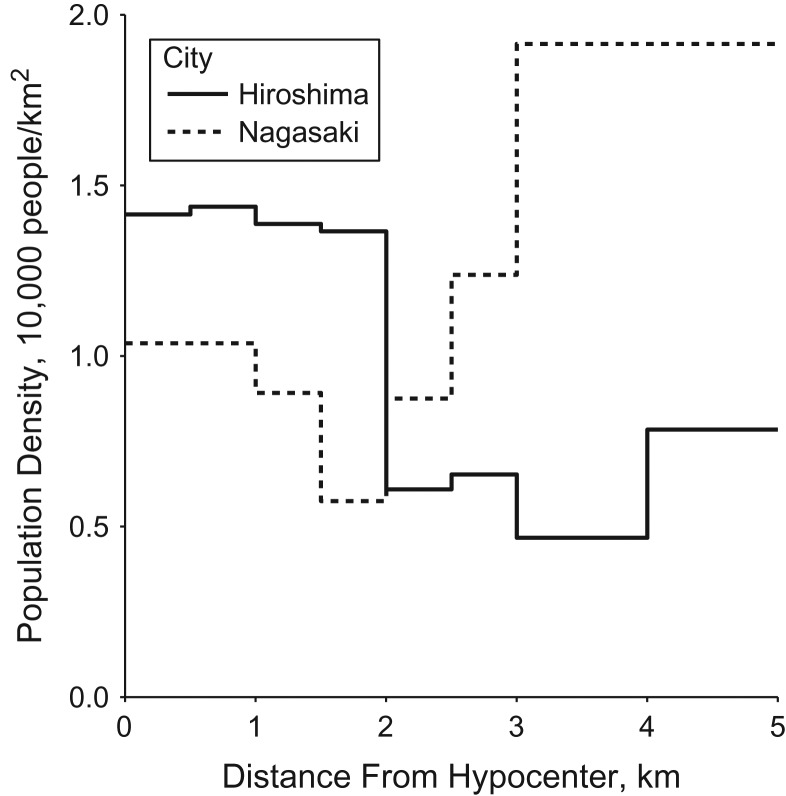
The estimated prebombing population densities in urban regions of Hiroshima and Nagasaki were 8,800 and 13,709 people/km2, respectively (Web Table 1). These estimates were consistent with the geography of each city: Urban regions in Nagasaki were more densely concentrated in valleys, whereas urban regions in Hiroshima were less densely distributed across a flat plain. In Hiroshima, urban regions near the hypocenter were more dense than urban regions far from the hypocenter (Figure 1). Conversely, in Nagasaki, urban regions near the hypocenter were less dense than urban regions far from the hypocenter.
Figure 1.
Population density in urban regions of Hiroshima (solid line) and Nagasaki (dashed line) before the bombings in 1945 according to distance from the hypocenter, obtained from the Joint Commission for the Investigation of the Effects of the Atomic Bomb in Japan.
Among all survivors in the Life Span Study (n = 93,741), 77,487 survivors (82.7%) were located within 5 km of the hypocenter and inside the urban region of either city. For these survivors, density estimates were assigned (Web Figure 1). However, 9,394 survivors (10.0%) were located within 5 km of the hypocenter but outside the urban region of either city, and 6,860 (7.3%) were located beyond 5 km from the hypocenter. For these survivors, population density was set to 116 people/km2. In Hiroshima, there was no difference in the distribution of male and female persons (Web Table 2). The median age was largest among survivors located within 5 km of the hypocenter but outside the city’s urban region. In Nagasaki, there were differences in the distribution of males and females, with the smallest percentage of females among survivors located beyond 5 km from the hypocenter. The median age was largest among survivors located within 5 km of the hypocenter and inside the city’s urban region. However, there was no correlation between population density and age in either city (Web Figure 2).
Among all survivors in the Life Span Study with known radiation doses (n = 86,720), population density and weighted absorbed colon doses were positively correlated in Hiroshima (Spearman rank correlation, 0.83) and negatively correlated in Nagasaki (Spearman rank correlation, −0.34) (Web Figure 3).
Mortality
Among 86,660 survivors with known radiation doses and who were alive in 1950, there were 50,648 deaths due to any cause over a total follow-up time of 3,296,468 person-years; 71,372 survivors (82.4%) were located within 5 km of the hypocenter and inside the urban region of either city (Web Table 3). The sex-averaged ERR per Gy increase in weighted absorbed colon dose was 0.237 (Table 1). Adjustment for population density did not affect the radiation ERR, and model fit was reduced (i.e., larger AIC). There was no overall association between population density and risk of all-cause mortality. There was, however, strong evidence for an interaction between population density and age at exposure (P < 0.001). Of note, among survivors aged 10–14 years in 1945, there was a positive association between population density and risk of all-cause mortality: a 5,000-people/km2 increase in density was associated with a relative risk of 1.053. Inclusion of the density × age interaction improved model fit (i.e., smaller AIC). However, inclusion of the density × age interaction did not affect the radiation ERR.
Table 1.
Radiation Dose and Population Density Associations With Risk of All-Cause Mortality Among 86,660 Atomic Bomb Survivors With Known Radiation Dosesa, Life Span Study, Hiroshima and Nagasaki, Japan, 1950–2003
| Model | Radiation Dose | Population Density | AIC | ||
|---|---|---|---|---|---|
| ERRb | 95% CI | RRc | 95% CI | ||
| Based | 0.237 | 0.188, 0.286 | 104,309.9 | ||
| With density | 0.235 | 0.185, 0.285 | 1.002 | 0.993, 1.011 | 104,311.8 |
| With density × age | 0.239 | 0.189, 0.289 | 104,294.9 | ||
| <10 years | 1.020e | 0.987, 1.054 | |||
| 10–14 years | 1.053e | 1.027, 1.079 | |||
| 15–29 years | 0.993e | 0.976, 1.010 | |||
| ≥30 years | 0.999e | 0.989, 1.009 | |||
Abbreviations: AIC, Akaike information criterion; CI, confidence interval; ERR, excess relative risk; RR, relative risk.
a The Dosimetry System 2002 (1, 2) method was used to estimate Dosimetry System 2002 Revision 1 (DS02R1)-weighted absorbed colon doses.
b Sex-averaged ERR of all-cause mortality per gray increase in weighted absorbed colon dose, among those exposed to a radiation dose with a total shielded kinetic energy released per unit mass of ≤4 gray at age 30 years and who achieved an attained age of 70 years.
c Relative risk of all-cause mortality per 5,000-people/km2 increase in population density.
d Base model adjusted for city, distance >5 km, sex, age at exposure, and attained age as well as effect modification by sex, age at exposure, attained age, and total shielded kinetic energy released per unit mass of >4 gray.
e Likelihood ratio P value for evaluating the null hypothesis of equality in relative risks across age groups (3 degrees of freedom): P < 0.001.
Of the deaths observed during follow-up, 10,940 were due to solid cancer. The sex-averaged ERR per Gy increase in weighted absorbed colon dose was 0.448 (Table 2). Adjustment for population density improved model fit and decreased the radiation ERR by 5.1%. There was a small but nonsignificant association between population density and risk of solid cancer mortality. There was also evidence for an interaction between population density and age at exposure (P = 0.052). Among survivors aged 10–14 years in 1945, a 5,000-people/km2 increase in population density was associated with a relative risk of 1.069. Inclusion of the density × age interaction improved model fit, but did not have an additional impact on the radiation ERR.
Table 2.
Radiation Dose and Population Density Associations With Risk of Solid Cancer Mortality Among 86,660 Atomic Bomb Survivors With Known Radiation Dosesa, Life Span Study, Hiroshima and Nagasaki, Japan, 1950–2003
| Model | Radiation Dose | Population Density | AIC | ||
|---|---|---|---|---|---|
| ERRb | 95% CI | RRc | 95% CI | ||
| Based | 0.448 | 0.340, 0.557 | 43,714.5 | ||
| With density | 0.425 | 0.315, 0.535 | 1.018 | 0.998, 1.039 | 43,713.3 |
| With density × age | 0.428 | 0.318, 0.538 | 43,711.6 | ||
| <10 years | 1.002e | 0.945, 1.062 | |||
| 10–14 years | 1.069e | 1.025, 1.115 | |||
| 15–29 years | 1.031e | 0.999, 1.064 | |||
| ≥30 years | 1.009e | 0.985, 1.033 | |||
Abbreviations: AIC, Akaike information criterion; CI, confidence interval; ERR, excess relative risk; RR, relative risk.
a The Dosimetry System 2002 (1, 2) method was used to estimate Dosimetry System 2002 Revision 1 (DS02R1)-weighted absorbed colon doses.
b Sex-averaged ERR of solid cancer mortality per gray increase in weighted absorbed colon dose, among those exposed to a radiation dose with a total shielded kinetic energy released per unit mass of ≤4 gray at age 30 years and who achieved an attained age of 70 years.
c Relative risk of solid cancer mortality per 5,000-people/km2 increase in population density.
d Base model adjusted for city, distance >5 km, sex, age at exposure, and attained age as well as effect modification by sex, age at exposure, attained age, and total shielded kinetic energy released per unit mass of >4 gray.
e Likelihood ratio P value for evaluating the null hypothesis of equality in relative risks across age groups (3 degrees of freedom): P = 0.052.
Solid cancer incidence
Among 80,205 survivors with known radiation doses, and who were alive and at risk for a first primary solid cancer in 1958, there were 17,316 first primary solid cancers over a total follow-up time of 2,317,884 person-years; 66,084 survivors (82.4%) were located within 5 km of the hypocenter and inside the urban region of either city (Web Table 4). The sex-averaged ERR per Gy increase in weighted absorbed colon dose was 0.476 (Table 3). Adjustment for population density did not have an impact on the radiation ERR, and model fit was reduced. There was no overall association between population density and risk of first primary solid cancer. In addition, there was no evidence for an interaction between population density and age at exposure. Inclusion of the density × age interaction did not affect the radiation ERR, and model fit was further reduced. Adjustment for population density did not affect the strong evidence for curvature in the radiation dose-response among male persons (Web Table 5).
Table 3.
Radiation Dose (Linear Dose-Response Model) and Population Density Associations With Risk of First Primary Solid Cancer Among 80,205 Atomic Bomb Survivors With Known Radiation Dosesa, Life Span Study, Hiroshima and Nagasaki, Japan, 1958–2009
| Model | Radiation Dose | Population Density | AIC | ||
|---|---|---|---|---|---|
| ERR | 95% CI | RR | 95% CI | ||
| Base | 0.476 | 0.389, 0.566 | 91,045.5 | ||
| With density | 0.468 | 0.380, 0.560 | 1.007 | 0.991, 1.023 | 91,046.9 |
| With density × age | 0.465 | 0.376, 0.558 | 91,051.0 | ||
| <10 years | 0.997 | 0.966, 1.030 | |||
| 10–14 years | 1.018 | 0.991, 1.046 | |||
| 15–29 years | 1.005 | 0.985, 1.025 | |||
| ≥30 years | 1.008 | 0.987, 1.029 | |||
Abbreviations: AIC, Akaike information criterion; CI, confidence interval; ERR, excess relative risk; RR, relative risk.
a The Dosimetry System 2002 (1, 2) method was used to estimate Dosimetry System 2002 Revision 1 (DS02R1)-weighted absorbed colon doses.
b Sex-averaged ERR of first primary solid cancer per gray increase in weighted absorbed colon dose, among those exposed to a radiation dose with a total shielded kinetic energy released per unit mass of ≤4 gray at age 30 years and who achieved an attained age of 70 years.
c Relative risk of first primary solid cancer per 5,000-people/km2 increase in population density.
d Base model adjusted for city, distance >5 km, sex, age at exposure, and attained age as well as effect modification by sex, age at exposure, attained age, and total shielded kinetic energy released per unit mass of >4 gray.
e Likelihood ratio P value for evaluating the null hypothesis of equality in relative risks across age groups (3 degrees of freedom): P = 0.59.
Survivors located in nonurban regions comprised 17.6% of the analysis cohort for both mortality and solid cancer incidence. We obtained identical results when we assigned a population density of 0 or 1,000 people/km2 to these survivors.
DISCUSSION
From the earliest analyses of the data from the Life Span Study of atomic bomb survivors, researchers have been concerned about the potential influence of unmeasured confounding (27). We obtained historical data on the prebombing population distributions in Hiroshima and Nagasaki. Using modern mapping techniques, we assigned population density estimates to survivors in the Life Span Study. We found that adjustment for population density had no substantial impact on radiation risk estimates for mortality and incident solid cancer, likely due to the lack of a strong association between density and these outcomes. If we assume that population density is a surrogate measure of socioeconomic and environmental factors that differ between urban and rural regions, then these results suggest a lack of residual confounding by location.
Although there was no overall association between population density and adverse health outcomes, there was evidence that the association between density and mortality differed by age at exposure. In particular, among survivors aged 10–14 years in August 1945 (i.e., born during 1930–1935), there was a positive association between population density and risk of all-cause and solid cancer mortality. We hypothesize that this association was driven by wartime deprivation in urban regions, to which developing children and adolescents were particularly sensitive. Disparate social conditions existed between urban and rural regions in Japan during the war (28). In particular, lower- and middle-class individuals in dense, urban regions experienced widespread food shortages throughout the war, even with the support of rations, whereas individuals in rural regions were able to grow their own food or to trade with local farmers. In addition, during later years of the war, adolescents in urban regions were conscripted into work groups. Associations of adverse social conditions during childhood and adolescence with adult mortality are well documented (29, 30). Our results are also consistent with age-period-cohort mortality trends in urban Japan, which showed a cohort effect among those born during 1925–1940 (31).
Among survivors aged 10–14 years in 1945, population density was associated with both all-cause and solid cancer mortality but not with incident solid cancer. This disparity could be due to different time horizons between the analyses. Because follow-up for cancer incidence began in 1958, Life Span Study participants who died during 1950–1957 were not included in the cancer incidence analysis. In a post-hoc analysis, we stratified the model for all-cause mortality by calendar year (Web Table 6; AIC, 104,266.9). We found significant differences according to calendar year (P < 0.001), such that population density was more strongly associated with early mortality. Therefore, it is plausible that survivors who were exposed in more dense regions were informatively censored from the cancer incidence analysis, which attenuated the association between population density and risk of first primary solid cancer (32). Note that even after stratifying the density association by calendar year, the radiation ERR was unchanged (0.241, 95% confidence interval: 0.190, 0.291) compared with the estimates provided in Table 1.
There are several strengths of our analysis. First, prior analyses of the Life Span Study data have adjusted for risk factors such as smoking, alcohol consumption, and dietary habits. However, the collection of these risk factors occurred after radiation exposure and was limited to subgroups of study participants, such that rates of missing data were approximately 50% (33). In our analysis, population density was defined prior to radiation exposure and for all survivors. Second, our analysis leveraged the natural experiment that arose from between-city differences in the location of the hypocenter relative to the city’s urban center. Thus, we exploited both within- and between-city heterogeneity in the population distribution to adjust radiation risk estimates for population density. Third, the large sample size of the Life Span Study and sufficient number of outcomes allowed us to adjust for population density overall and among age subgroups.
We acknowledge the following limitations. First, we used ecological data on prebombing population density as a surrogate measure of individual-level socioeconomic and environmental factors that differed between urban and rural regions, and we were unable to elucidate the impact of different factors. Second, we used density estimates from a single time point, which did not capture the evolution of socioeconomic and environmental factors over time. However, we allowed the density association to differ by age and calendar time. Third, estimation of density associations could have been influenced by measurement error. We assigned density values to survivors based on their reported locations at the time of exposure, which might not have been their homes. Survivors’ density values could therefore have been affected by their movement, either in the process of their regular daily activities or to seek shelter following air-raid warnings. The small relative risks for population density could be particularly sensitive to measurement error.
In summary, we observed age-specific associations of population density with mortality, but not with incident solid cancer, in the Life Span Study of atomic bomb survivors. Adjustment for population density did not have a substantial impact on radiation risk estimates for mortality or incident solid cancer. Future work could focus on specific cancer sites, particularly those with established urban-rural disparities, such as breast cancer (11, 34).
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Statistics, Radiation Effects Research Foundation, Hiroshima, Japan (Benjamin French, Sachiyo Funamoto, John Cologne, Harry M. Cullings); Department of Epidemiology, Radiation Effects Research Foundation, Hiroshima, Japan (Hiromi Sugiyama, Ritsu Sakata); Radiation Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland (Kiyohiko Mabuchi); and Hirosoft International Corporation, Eureka, California (Dale L. Preston).
The Radiation Effects Research Foundation, Hiroshima and Nagasaki, Japan, is a public interest incorporated foundation funded by the Japanese Ministry of Health, Labour and Welfare, and the US Department of Energy. The research was also funded in part through the Department of Energy and National Academy of Sciences (award DE-HS0000031) and the National Cancer Institute (contract HHSN261201400009C), with additional support from the Division of Cancer Epidemiology and Genetics in the National Cancer Institute Intramural Research Program.
This publication was supported by Radiation Effects Research Foundation Research Protocols 1-75 and 18-61. The views of the authors do not necessarily reflect those of the two governments.
We thank the staff of the Radiation Effects Research Foundation Tumor and Tissue Registry Office and the Master File Section for their support.
Conflict of interest: none declared.
Abbreviations
- AIC
Akaike information criterion
- ERR
excess relative risk
- Gy
gray
REFERENCES
- 1. Cullings HM, Fujita S, Funamoto S, et al. Dose estimation for atomic bomb survivor studies: its evolution and present status. Radiat Res. 2006;166(1 Pt 2):219–254. [DOI] [PubMed] [Google Scholar]
- 2. Cullings HM, Grant EJ, Egbert SD, et al. DS02R1: improvements to atomic bomb survivors’ input data and implementation of Dosimetry System 2002 (DS02) and resulting changes in estimated doses. Health Phys. 2017;112(1):56–97. [DOI] [PubMed] [Google Scholar]
- 3. Grant EJ, Ozasa K, Ban N, et al. A report from the 2013 international symposium: the evaluation of the effects of low-dose radiation exposure in the Life Span Study of atomic bomb survivors and other similar studies. Health Phys. 2015;108(5):551–556. [DOI] [PubMed] [Google Scholar]
- 4. Cologne JB, Preston DL. Longevity of atomic-bomb survivors. Lancet. 2000;356(9226):303–307. [DOI] [PubMed] [Google Scholar]
- 5. Cologne JB, Preston DL. Impact of comparison group on cohort dose response regression: an example using risk estimation in atomic-bomb survivors. Health Phys. 2001;80(5):491–496. [DOI] [PubMed] [Google Scholar]
- 6. French B, Cologne J, Sakata R, et al. Selection of reference groups in the Life Span Study of atomic bomb survivors. Eur J Epidemiol. 2017;32(12):1055–1063. [DOI] [PubMed] [Google Scholar]
- 7. Chaix B, Rosvall M, Lynch J, et al. Disentangling contextual effects on cause-specific mortality in a longitudinal 23-year follow-up study: impact of population density or socioeconomic environment? Int J Epidemiol. 2006;35(3):633–643. [DOI] [PubMed] [Google Scholar]
- 8. Meijer M, Kejs AM, Stock C, et al. Population density, socioeconomic environment and all-cause mortality: a multilevel survival analysis of 2.7 million individuals in Denmark. Health Place. 2012;18(2):391–399. [DOI] [PubMed] [Google Scholar]
- 9. Nakaya T, Honjo K, Hanibuchi T, et al. Associations of all-cause mortality with census-based neighbourhood deprivation and population density in Japan: a multilevel survival analysis. PLoS One. 2014;9(6):e97802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meijer M, Bloomfield K, Engholm G. Neighbourhoods matter too: the association between neighbourhood socioeconomic position, population density and breast, prostate and lung cancer incidence in Denmark between 2004 and 2008. J Epidemiol Community Health. 2013;67(1):6–13. [DOI] [PubMed] [Google Scholar]
- 11. Fei X, Wu J, Kong Z, et al. Urban-rural disparity of breast cancer and socioeconomic risk factors in China. PLoS One. 2015;10(2):e0117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lynch SM, Mitra N, Ravichandran K, et al. Telomere length and neighborhood circumstances: evaluating biological response to unfavorable exposures. Cancer Epidemiol Biomarkers Prev. 2017;26(4):553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ozasa K, Shimizu Y, Suyama A, et al. Studies of the mortality of atomic bomb survivors, Report 14, 1950–2003: an overview of cancer and noncancer diseases. Radiat Res. 2012;177(3):229–243. [DOI] [PubMed] [Google Scholar]
- 14. Grant EJ, Brenner A, Sugiyama H, et al. Solid cancer incidence among the Life Span Study of atomic bomb survivors: 1958–2009. Radiat Res. 2017;187(5):513–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beebe GW, Usagawa M. The Major ABCC Samples Hiroshima, Japan: Atomic Bomb Causualty Commission; 1968. (ABCC Technical Report 12-68). [Google Scholar]
- 16. Pierce DA, Stram DO, Vaeth M. Allowing for random errors in radiation dose estimates for the atomic bomb survivor data. Radiat Res. 1990;123(3):275–284. [PubMed] [Google Scholar]
- 17. Oughterson AW, Warren S. Medical Effects of the Atomic Bomb in Japan. New York, NY: McGraw-Hill International Book Co.; 1956. [Google Scholar]
- 18. Havens TRH. Valley of Darkness: The Japanese People and World War Two. New York, NY: W.W. Norton and Company, Inc.; 1978. [Google Scholar]
- 19. Ozasa K, Grant EJ, Cullings HM, et al. Invited commentary: missing doses in the Life Span Study of Japanese atomic bomb survivors. Am J Epidemiol. 2013;177(6):569–573. [DOI] [PubMed] [Google Scholar]
- 20. Bureau of Statistics, Office of the Prime Minister Population census of 1950: volume 1: total population. https://www.e-stat.go.jp/en/stat-search/files?page=1&layout=datalist&toukei=00200521&tstat=000001036869&cycle=0&tclass1=000001037373&second=1&second2=1 Published November 20, 1950. Updated June 10, 2014. Accessed January 4, 2018.
- 21. Diez Roux AV. Investigating neighborhood and area effects on health. Am J Public Health. 2001;91(11):1783–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Turner H, Firth D. Generalized nonlinear models in R: An overview of the gnm package. R package version 1.0-8. Warwick, UK: University of Warwick; 2015. [Google Scholar]
- 23. Holford TR. The analysis of rates and of survivorship using log-linear models. Biometrics. 1980;36(2):299–305. [PubMed] [Google Scholar]
- 24. Laird N, Olivier D. Covariance analysis of censored survival data using log-linear analysis techniques. J Am Stat Assoc. 1981;76(374):231–240. [Google Scholar]
- 25. Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19(6):716–723. [Google Scholar]
- 26. Burnham KP, Anderson DR. Multimodel inference: understanding AIC and BIC in model selection. Sociol Methods Res. 2004;33(2):261–304. [Google Scholar]
- 27. Beebe GW, Ishida M, Jablon S. Studies of the mortality of A-bomb survivors. I. Plan of study and mortality in the medical subsample (selection 1), 1950–1958. Radiat Res. 1962;16:253–280. [PubMed] [Google Scholar]
- 28. Yamashita SH. Daily Life in Wartime Japan, 1940–1945. Lawrence, KS: University Press of Kansas; 2015. [Google Scholar]
- 29. Elo IT, Preston SH. Effects of early-life conditions on adult mortality: a review. Popul Index. 1992;58(2):186–212. [PubMed] [Google Scholar]
- 30. Galobardes B, Lynch JW, Davey Smith G. Childhood socioeconomic circumstances and cause-specific mortality in adulthood: systematic review and interpretation. Epidemiol Rev. 2004;26(1):7–21. [DOI] [PubMed] [Google Scholar]
- 31. Tango T, Kurashina S. Age, period and cohort analysis of trends in mortality from major diseases in Japan, 1955 to 1979: peculiarity of the cohort born in the early Showa Era. Stat Med. 1987;6(6):709–726. [DOI] [PubMed] [Google Scholar]
- 32. Pierce DA, Vaeth M, Shimizu Y. Selection bias in cancer risk estimation from A-bomb survivors. Radiat Res. 2007;167(6):735–741. [DOI] [PubMed] [Google Scholar]
- 33. Grant EJ, Ozasa K, Preston DL, et al. Effects of radiation and lifestyle factors on risks of urothelial carcinoma in the Life Span Study of atomic bomb survivors. Radiat Res. 2012;178(1):86–98. [DOI] [PubMed] [Google Scholar]
- 34. Robert SA, Strombom I, Trentham-Dietz A, et al. Socioeconomic risk factors for breast cancer: distinguishing individual- and community-level effects. Epidemiology. 2004;15(4):442–450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.