Abstract
Frailty is an age-related clinical syndrome of decreased resilience to stressors. Among numerous assessments of frailty, the frailty phenotype (FP) scale proposed by Fried et al. has been the most widely used. We aimed to develop a continuous frailty scale that could overcome limitations facing the categorical FP scale and to evaluate its construct validity, predictive validity, and measurement properties. Data were from the Cardiovascular Health Study (n = 4,243) and Health and Retirement Study (n = 7,600), both conducted in the United States. Frailty was conceptualized as a continuous construct, assessed by 5 measures used in the FP scale: gait speed, grip strength, exhaustion, physical activity, and weight loss. We used confirmatory factor analysis to investigate the relationship between the 5 indicators and the latent frailty construct. We examined the association of the continuous frailty scale with mortality and disability. The unidimensional model fit the data satisfactorily; similar factor structure was observed across 2 cohorts. Gait speed and weight loss were the strongest and weakest indicators, respectively; grip strength, exhaustion, and physical activity had similar strength in measuring frailty. In each cohort, the continuous frailty scale was strongly associated with mortality and disability and continued to be associated with outcomes among robust and prefrail persons classified by the FP scale.
Keywords: confirmatory factor analysis, construct validity, frailty, older adults, predictive validity
Frailty is a clinical syndrome characterized by decreased resilience to stressors and is a consequence of dysregulation in multiple physiological systems (1). Frailty is prevalent in elderly persons and is associated with a wide range of adverse outcomes (2–5). In the absence of a gold standard, there is a lack of consensus on the operational definition of frailty (6). In 2001, Fried et al. (5) developed the frailty phenotype (FP) scale using gait speed, grip strength, exhaustion, physical activity, and weight loss. Since its emergence, the FP scale has been repeatedly validated and widely used in assessing frailty (2, 4, 7–9). However, the FP scale, like all other frailty assessments, has limitations. First, because sample-specific cutoff points were used to divide continuously measured variables into dichotomous criteria (e.g., slow gait speed and weak grip strength), precision may be lost. Additionally, all frailty indicators in the FP scale are assumed to be of equal importance in measuring frailty. Moreover, the FP scale is very effective in identifying the frailest elders but has limited ability to differentiate robust persons (10). In the Cardiovascular Health Study (CHS), approximately half of the participants did not meet any of the 5 frailty criteria and were therefore classified as robust (5). It is questionable whether these participants have the same level of frailty, however.
A more finely graded frailty scale may have the following advantages compared with the categorical FP scale: 1) providing a greater differentiation of the frailty syndrome; 2) further stratifying risk of outcomes among robust, prefrail, and frail persons identified by the FP scale; and 3) increasing power for identifying genetic and nongenetic associations with frailty.
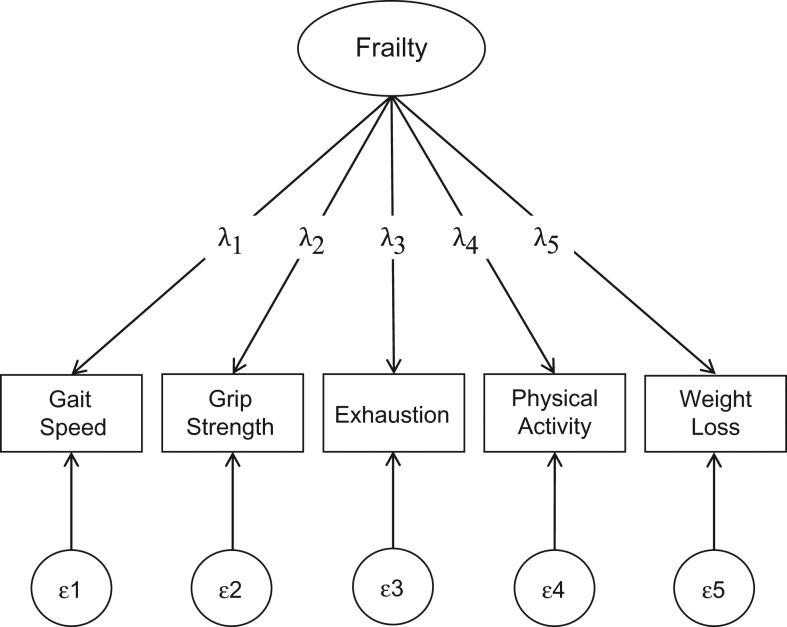
The purpose of this study is 4-fold. First, we used confirmatory factor analysis (CFA) to examine the factor structure of frailty, conceptualized as a continuous latent construct, among the CHS cohort (Figure 1 displays the conceptual framework). Second, we investigated the relative importance of the 5 indicators in measuring frailty. Third, we developed a continuous frailty scale, examined its relationship with the FP scale, and assessed its associations with mortality and disability. Last, we examined the association of the continuous frailty scale with outcomes among robust, prefrail, and frail persons identified by the FP scale. We validated the factor structure and predictive validity using data from the Health and Retirement Study (HRS).
Figure 1.
Hypothesized causal relationship between the latent frailty construct and 5 observed indicators. The terms λ1–λ5 represent factor-loading estimates, quantifying the association of 5 observed indicators (gait speed, grip strength, exhaustion, physical activity, and weight loss) with the latent construct—frailty. The terms ε1–ε5 denote residual errors of indicators not accounted for by the latent factor (i.e., accounted for by other factors and/or random error). The oval represents the latent construct; squares represent the observed measures; and circles represent the variance of observed measures not accounted for by the latent construct.
METHODS
Cardiovascular Health Study
The CHS is a cohort study of 5,888 community-dwelling men and women, aged ≥65 years, in the United States. Participants in the CHS were randomly sampled from Medicare eligibility lists in 4 communities. A total of 5,201 participants were enrolled in 1989–1990 (original cohort), and an additional sample of 687 black participants was recruited in 1992–1993 (new cohort). All participants were asked to provide blood samples and to complete an interview, health questionnaire, and comprehensive physical examination at enrollment and annually through 1999–2000. Institutional review boards at each site approved the study protocol; all participants signed informed consent.
We used data from the 1992–1993 and 1996–1997 examinations, when physical activity was assessed, and direct calculation of weight loss between 2 consecutive visits was possible. Examinations in 1992–1993 and 1996–1997 served as baseline for the original and new cohorts, respectively. The analytical sample was limited to participants with data on all 5 frailty indicators (n = 4,243).
Health and Retirement Study
The HRS is a cohort study of a nationally representative sample of noninstitutionalized residents in the contiguous United States. In the 2006–2007 wave, approximately half of the HRS participants were randomly selected to participate in an enhanced face-to-face interview. Functional performance was assessed during these interviews. The other half was selected to complete the functional measures in the 2008–2009 wave. Ethical approval was obtained from the institutional review board at the University of Michigan; all participants signed informed consent.
We used pooled data from the 2006–2007 and 2008–2009 waves, when physical functioning measures were available. The analytical sample included 7,600 participants who were aged ≥65 years and had data on all frailty indicators in the 2006–2007 or 2008–2009 wave (baseline for the HRS).
Frailty measures
Gait speed
Gait speed was assessed by converting the amount of time required to walk a 15-foot (4.6-m) course in CHS and a 98.5-inch (2.5-m) course in HRS at usual speed into meters per second. For each cohort, linear models were fitted regressing gait speed on height separately for men and women; residuals were computed, representing sex- and height-adjusted gait speed (4).
Grip strength
Grip strength of both hands was measured 3 times using a handheld dynamometer in both cohorts. We used the average reading of the dominant hand. Participants who had pain, injury, or recent surgery were not asked to perform the test (and here treated as missing). For each cohort, we used the above-mentioned residual approach to adjust for sex and body mass index.
Exhaustion
CHS participants reported the frequency they felt “I could not get going” and “I felt that everything I did was an effort” during the prior week: “rarely/none of the time; less than 1 day” (coded 0), “some or a little of the time; 1 to 2 days” (coded 1.5), “a moderate amount of time; 3 to 4 days” (coded 3.5), or “most of the time; 5 to 7 days” (coded 6). The sum score indicated exhaustion. Responses were coded according to the severity/duration of each symptom. HRS participants answered “yes” or “no” to whether they had experienced each question for much of the time during the prior week. “yes” and “no” were scored 1 and 0, respectively; the sum score ranged from 0 to 2.
Physical activity
In the CHS, physical activity was assessed using a modification of the Minnesota Leisure Time Activities Questionnaire (11). Participants reported the frequency as well as duration of 18 activities in the prior 2 weeks. Total kilocalories were calculated.
In the HRS, physical activity was measured by frequency of vigorous, moderate, and light physical activities. Vigorous, moderate, and light physical activities were scored 8, 4, and 2, respectively, according to the metabolic equivalent of task (12). We computed a weighted-sum score for each participant, representing the total energy cost of physical activities, accounting for intensity and frequency. Weights were determined by the frequency of physical activity; “every day,” “more than once a week,” “once a week,” “1–3 times a month,” and “hardly ever” were scored 7, 4, 1, 0.5, and 0, respectively.
Weight loss
Body weight was measured in the CHS and was self-reported in the HRS. Percentage weight loss was calculated as (weight in previous visit minus current measured weight) ÷ (weight in previous visit) × 100%. In the CHS, a zero was assigned to persons who reported that diet or exercise was a major factor in weight change.
Covariates
Age was calculated by the difference between the visit date and birth date; sex, education, and race/ethnicity were self-reported. Education was categorized as less than high school completion, high school completion or equivalent, and more than high school. Race/ethnicity was dichotomized as white versus other. Smoking status was categorized as current, former, or never smoker. Body mass index was calculated as weight (kg) divided by height (m) squared, and was categorized as underweight (<18.5), normal (18.5–24.9), overweight (25.0–30.0), or obese (>30.0).
History of cardiac disease, stroke, hypertension, diabetes, cancer (excluding minor skin cancer), and arthritis was assessed based on self-reported physician diagnosis in both cohorts. Cognitive function was measured by a modified Mini-Mental State Examination (13) in the CHS and by a modified Telephone Interview for Cognitive Status (14) in the HRS. Disability was assessed by difficulty in 6 activities of daily living (dressing, eating, toileting, bathing, transferring, and walking across a room) and was dichotomized as none versus any. Biomarkers included systolic and diastolic blood pressure (mm Hg), C-reactive protein (mg/dL), cystatin C (mg/L), and total cholesterol (mg/dL).
Validation outcomes
Mortality
Mortality data in the CHS were obtained according to review of obituaries, medical records, death certificates, the Centers for Medicare and Medicaid Services health-care utilization database for hospitalizations, and from household contacts (15). Mortality data in the HRS were ascertained based on a variable recording participants’ year of death taken from an exit interview or a spouse/partner’s core interview. Because the overall follow-up period differed substantially between the 2 cohorts, we examined 5-year mortality to facilitate comparability.
Disability
We examined incident disability among initially nondisabled persons. Of the 3,807 initially nondisabled CHS participants, 3,281 were alive and had complete measurements of activities of daily living in the 2 years following baseline; 181 participants who died and 345 who were lost to follow-up were excluded from the analysis for disability. Of the 6,454 initially nondisabled HRS participants, 5,949 had measurements of activities of daily living in the following 2 years; 194 participants who died and 311 who were lost to follow-up were excluded.
Statistical analysis
We compared the baseline characteristics between the 2 cohorts using t tests for continuous variables and tests for categorical variables. We also compared the characteristics between the persons with all 5 frailty indicators and those with ≥1 indicator missing within each cohort.
Factor analysis
We first created a variance-covariance matrix between the 5 frailty indicators and examined their internal consistency using Cronbach’s α (16). We then fitted exploratory factor analysis using maximum likelihood with an oblique (geomin) rotation and determined the appropriate number of factors to be extracted in subsequent analyses using the Kaiser-Guttman rule (17) and the scree test (18). Subsequently, we fitted a unidimensional CFA to examine the latent factor structure among the CHS participants. CFA is widely used to account for the correlations among a set of indicators and to identify the association of observed indicators with latent constructs. The association of each indicator with the latent frailty construct was quantified by the item’s factor loading. Overall model fit was evaluated using the test, the root-mean-square-error of approximation, the comparative fit index, and the Tucker-Lewis index. Ideal model fit was determined based on the following criteria: test not statistically significant, root-mean-square-error of approximation ≤ 0.05, comparative fit index ≥ 0.95, and Tucker-Lewis index ≥ 0.95; acceptable model fit was identified with root-mean-square-error of approximation ≤ 0.08, comparative fit index ≥ 0.90, and Tucker-Lewis index ≥ 0.90 (19). Local goodness-of-fit was inspected using 1) standardized residuals, representing discrepancies between observed and estimated values; and 2) modification indices (20), reflecting the estimated reduction in the overall statistic if a fixed or constrained parameter is freely estimated. The initial CFA model assumed the residuals of the 5 indicators were independent. We respecified the model by correlating residuals if the initial model did not reach satisfactory fit. Once an ideal fit was achieved, we generated standardized loading estimates.
We examined the factor structure in the HRS cohort following the same procedure described above. Standardized factor loadings were generated and compared between the 2 cohorts.
Measurement properties
Once a satisfactory model was identified, we examined the relative importance of the 5 frailty indicators. First, we reverse-coded exhaustion and weight loss so that a high value indicated a lower frailty level for all indicators. Then, we performed a series of CFAs with 2 standardized factor loadings constrained to be equal, and we compared each of them with the initial CFA. Because there were 10 comparisons, we used a Bonferroni-corrected threshold (α = 0.05/10 = 0.005) to determine whether the statistic was significant. A significant test implied that 2 indicators that were constrained to have the same factor loading did not have equal strength in measuring frailty.
Scale construction
We computed standardized values for each indicator by dividing the difference between observed value and sample mean by sample standard deviation. Subsequently, we added individual component scores, weighted by the standardized factor loadings estimated in CFA (see Web Appendix 1, available at https://academic.oup.com/aje for Stata (StataCorp LLC, College Station, Texas) code to construct the continuous frailty scale). Frailty score for individual i is:
where represent standardized values for individual i, respectively; indicate standardized factor loadings.
Predictive validity
We calculated the death rate per 1,000 person-years for all participants, as well as numbers and proportions of initially nondisabled persons who had incident disability across quintiles of the continuous frailty scale. Cox models were used to determine the association of frailty with mortality. Poisson models with robust variance estimates were used to identify the association of frailty with disability among initially nondisabled participants. We first adjusted for clinical site (only for the CHS), age, sex, race/ethnicity, and education. We added smoking status, body mass index, chronic conditions, cognitive status, disability (only for modeling mortality), and biomarkers in full-adjustment models. The continuous frailty scale was modeled both continuously and in quintiles. Analyses were conducted separately for 2 cohorts.
To evaluate whether the new scale provided additional value in risk stratification beyond the categorical FP scale, we assessed the association of frailty with outcomes among robust, prefrail, and frail participants identified by the FP scale. The 5 criteria were:
Slowness: gait speed in the lowest height- and sex-adjusted 20%.
Weakness: grip strength in the lowest body mass index– and sex-adjusted 20%.
Exhaustion: reporting “a moderate amount of the time” or “most of the time” to either of the 2 exhaustion questions, or responding “yes” to ≥1 exhaustion question in the HRS.
Inactivity: total caloric expenditure (metabolic equivalent of task in the HRS) in the lowest sex-specific 20%.
Shrinking: loss of ≥10 pounds or ≥5% of body weight in prior year.
We classified persons as “robust” (0 criteria), “prefrail” (1–2 criteria), or “frail” (3–5 criteria).
We evaluated the discrimination performance of the continuous frailty score and the original FP scale using the C statistic. C statistics from different frailty assessments were compared using bootstrapped confidence intervals based on 1,000 replications. Sociodemographic factors (age, sex, race/ethnicity, and education) were included as covariates.
We performed several sensitivity analyses. First, we used absolute weight loss instead of percentage weight loss in the CFA. Additionally, we assessed whether missingness in frailty indicators biased the results by fitting additional CFA using participants with ≥1 frailty indicator measured. Third, we refitted CFA in the HRS cohort with exhaustion (coded 0, 1, or 2) modeled as an ordered categorical indicator with a robust weighted least-squares estimator. Fourth, we repeated the analyses of the association between frailty and outcomes, by including participants who were unable to perform tests of gait speed or grip strength; their scores on the FP scale and the continuous frailty scale were imputed as 1 (meeting the specific frailty criterion) and 2 standard deviations below the mean, respectively.
All tests were 2-sided with a significance level of P < 0.05. Statistical analyses other than CFA were conducted in Stata, version 13.1 (StataCorp LLC), and R, version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria). CFA was performed using maximum likelihood estimation with robust standard error in Mplus 7.2 (http://www.statmodel.com/) to account for nonnormality of continuous indicators and nonindependence of observations in the HRS (participants nested in households). Missing data in frailty indicators were handled using full information maximum-likelihood estimation (for sensitivity analyses only). Under the missing-at-random assumption, the full information maximum-likelihood parameter estimates are unbiased and more efficient than other missing data techniques (e.g., listwise deletion, mean imputation) (21).
RESULTS
Descriptive statistics
The average age was 72.1 years in the CHS; the HRS participants were slightly older, with an average age of 74.9 years (Table 1). The CHS cohort had lower prevalence of chronic conditions and disability than the HRS cohort. Additionally, CHS participants had higher levels of C-reactive protein and total cholesterol but lower cystatin C. Participants with complete data on frailty indicators were younger, were more likely to be male and white, and had a higher prevalence of chronic conditions, lower level of cognitive function, and unhealthier biomarkers than those who had ≥1 frailty indicator missing (Web Tables 1 and 2).
Table 1.
Characteristics of Participants in the Cardiovascular Health Study (1989–1993) and the Health and Retirement Study (2006–2009), United Statesa
| Characteristic | Cardiovascular Health Study (n = 4,243) | Health and Retirement Study (n = 7,600) | P Valueb | ||||
|---|---|---|---|---|---|---|---|
| No. | % | Mean (SD) | No. | % | Mean (SD) | ||
| Age, years | 72.1 (5.0) | 74.9 (6.9) | <0.001 | ||||
| Male sex | 1,788 | 42.1 | 3,315 | 43.6 | 0.067 | ||
| White (vs. other) | 3,683 | 86.8 | 6,763 | 89.0 | 0.123 | ||
| Education | <0.001 | ||||||
| Did not complete high school | 1,058 | 24.9 | 1,838 | 24.2 | |||
| High school completion | 1,212 | 28.6 | 2,729 | 35.9 | |||
| Beyond high school | 1,964 | 46.3 | 3,032 | 39.9 | |||
| Smoking status | 0.036 | ||||||
| Never | 1,882 | 44.4 | 3,279 | 43.1 | |||
| Former | 1,888 | 44.5 | 3,575 | 47.0 | |||
| Current | 392 | 9.2 | 702 | 9.2 | |||
| Body mass indexc | <0.001 | ||||||
| <25.0 | 1,589 | 37.4 | 2,009 | 26.4 | |||
| 25.0–30.0 | 1,793 | 42.3 | 2,856 | 37.6 | |||
| >30.0 | 861 | 20.3 | 2,735 | 36.0 | |||
| Cardiac diseased | 1,237 | 25.1 | 2,341 | 30.8 | <0.001 | ||
| Stroke | 209 | 4.9 | 518 | 6.9 | <0.001 | ||
| Hypertension | 2,370 | 55.9 | 4,850 | 63.9 | <0.001 | ||
| Diabetes | 620 | 15.1 | 1,653 | 21.8 | <0.001 | ||
| Cancere | 600 | 14.2 | 1,453 | 19.1 | <0.001 | ||
| Arthritis | 1,929 | 46.6 | 5,196 | 68.4 | <0.001 | ||
| Disabilityf | 430 | 10.2 | 1,146 | 21.0 | <0.001 | ||
| Systolic BP, mm Hg | 135.6 (21.2) | 134.4 (20.8) | 0.213 | ||||
| Diastolic BP, mm Hg | 70.7 (11.2) | 78.4 (11.6) | <0.001 | ||||
| CRP, μg/L | 5.2 (9.7) | 4.3 (8.6) | <0.001 | ||||
| Cystatin C, mg/L | 1.1 (0.3) | 1.2 (0.5) | <0.001 | ||||
| Total cholesterol, mg/dL | 208.2 (38.5) | 197.9 (41.7) | <0.001 | ||||
Abbreviations: BP, blood pressure; CRP, C-reactive protein; SD, standard deviation.
a Values in subgroups for education and smoking status variables do not sum to the total due to a small amount of missing data.
bP values were obtained from generalized linear regression with clustered sandwich estimator for comparison between the Cardiovascular Health Study and the Health and Retirement Study participants.
c Weight (kg)/height (m)2.
d Coronary heart disease and heart failure were included in the Cardiovascular Health Study; myocardial infarction, coronary heart disease, angina, heart failure, or other heart problems were included in the Health and Retirement Study.
e Nonmelanoma skin cancer was excluded.
f Having difficulty in any activities of daily living: dressing, eating, toileting, bathing, transferring, and walking across a room.
Factor structure
The variance-covariance matrix between frailty indicators is presented in Web Table 3. The Cronbach’s α was 0.50 and 0.59 for the CHS and HRS cohorts, respectively. In both cohorts, we retained only 1 factor from the exploratory factor analysis, based on the Kaiser-Guttman rule and the scree plot (Web Figure 1). Fitting a unidimensional CFA model of frailty in the CHS cohort yielded a nonsignificant test (P = 0.061; Web Table 4), indicating that the hypothesized unidimensional factor structure reproduced the variance-covariance matrix of the 5 indicators. Each of the other goodness-of-fit indices suggested that the unidimensional model fit the data satisfactorily. In the HRS cohort, with the exception of a significant test (P < 0.001), all goodness-of-fit indices indicated an adequate model fit. Refitting the CFA treating exhaustion as an ordered categorical indicator generated similar model-fit indices and factor-loading estimates (Web Table 5). In both cohorts, inspection of the standardized residuals and modification indices showed no localized points of ill fit (Web Table 6). No model respecification was needed; all standardized loadings were statistically significant and all directions were as expected.
Results were robust to sensitivity analyses. First, there were virtually no differences in factor-loading estimates or goodness-of-fit indices between models using different operational definitions of weight loss (Table 2 and Web Table 7). Additionally, missing data in frailty indicators had minimal impact on the estimates of factor-loading estimates and goodness-of-fit indices.
Table 2.
Standardized Factor Loadings (Standard Errors) of 5 Indicators (Sensitivity Analysis) for Participants in the Cardiovascular Health Study (1989–1993) and the Health and Retirement Study (2006–2009), United States
| Indicator | Cardiovascular Health Study | Health and Retirement Study | ||||||
|---|---|---|---|---|---|---|---|---|
| 5 Indicators Measured (n = 4,243) | ≥1 Indicator Unmeasured (n = 4,938) | 5 Indicators Measured (n = 7,600) | ≥1 Indicator Unmeasured (n = 9,221) | |||||
| % Weight Loss | Weight Difference | % Weight Loss | Weight Difference | % Weight Loss | Weight Difference | % Weight Loss | Weight Difference | |
| Gait speeda | −0.55 (0.03) | −0.55 (0.03) | −0.58 (0.03) | −0.59 (0.03) | −0.61 (0.02) | −0.61 (0.02) | −0.60 (0.02) | −0.60 (0.02) |
| Grip strengthb | −0.33 (0.02) | −0.33 (0.02) | −0.34 (0.02) | −0.34 (0.02) | −0.43 (0.01) | −0.44 (0.01) | −0.45 (0.01) | −0.46 (0.01) |
| Exhaustionc | 0.37 (0.02) | 0.37 (0.02) | 0.44 (0.02) | 0.44 (0.02) | 0.40 (0.02) | 0.40 (0.02) | 0.43 (0.01) | 0.43 (0.01) |
| Physical activityd | −0.33 (0.02) | −0.33 (0.02) | −0.33 (0.02) | −0.33 (0.02) | −0.47 (0.02) | −0.47 (0.02) | −0.50 (0.01) | −0.50 (0.01) |
| Weight loss | 0.09 (0.02) | 0.09 (0.02) | 0.12 (0.02) | 0.12 (0.02) | 0.15 (0.02) | 0.14 (0.02) | 0.15 (0.02) | 0.14 (0.02) |
a Gait speed (meters/second) was measured over a 4.6-meter and a 2.5-meter course in the Cardiovascular Health Study and the Health and Retirement Study, respectively.
b Grip strength (kilograms) was measured by a hand dynamometer in both cohorts.
c Exhaustion was measured by 2 items from the Center for Epidemiologic Studies–Depression Scale (“I could not get going” and “I felt that everything I did was an effort”); the total score ranged from 0–12 in the Cardiovascular Health Study and from 0–2 in the Health and Retirement Study.
d Physical activity was measured by self-reported total energy expenditure in the Cardiovascular Health Study and by self-reported frequency of light, moderate, and vigorous activities in the Health and Retirement Study.
Relative importance
In the CHS cohort, grip strength, exhaustion, and physical activity did not have significantly different strengths in measuring frailty (Web Table 4). Gait speed (loading: −0.55) was significantly more strongly associated with frailty than were the other 4 indicators, while weight loss had the smallest contribution (loading: 0.09; Web Table 8). We observed a similar pattern in the HRS cohort; gait speed was the strongest indicator of frailty (loading: 0.61), weight loss was the weakest (loading: 0.15), and grip strength, exhaustion, and physical activity had similar strength.
Distribution
In both cohorts, the continuous frailty score was approximately normally distributed (Web Figure 2); mean scores were considerably different between robust, prefrail, and frail adults (Web Table 9). The overlap was large between distributions of frailty scores among robust, prefrail, and frail persons, especially for those who were robust or frail (Web Figures 3 and 4). For robust persons, only 4 CHS participants and none of the HRS participants had a continuous score in the highest quintile (Web Table 10). For CHS and HRS participants who were classified as frail, 92.7% and 95.3% had a continuous score in the highest quintile, respectively.
Association with mortality
In a demographic-adjusted model, mortality was 57% higher per unit of the continuous frailty scale among CHS participants (Table 3). The continuous frailty scale continued to be associated with mortality after adjusting for additional covariates. We observed similar results in the HRS cohort. After adjustment, mortality was 67% higher per unit of the continuous frailty scale. Estimates were robust against multiple sensitivity analyses (Web Table 11).
Table 3.
Association of Frailty With 5-Year Mortality Among Participants in the Cardiovascular Health Study (1989–1993) and the Health and Retirement Study (2006–2009), United States
| Frailty Score | Cardiovascular Health Study (n = 4,243) | Health and Retirement Study (n = 7,519a) | ||||||
|---|---|---|---|---|---|---|---|---|
| Demographic-Adjustedb | Fully Adjustedc | Demographic-Adjustedb | Fully Adjustedd | |||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Continuous score | 1.57 | 1.46, 1.68 | 1.24 | 1.12, 1.36 | 1.65 | 1.57, 1.73 | 1.48 | 1.40, 1.57 |
| Quintilee | ||||||||
| 2 | 1.25 | 0.94, 1.69 | 1.04 | 0.75, 1.44 | 1.39 | 1.05, 1.85 | 1.32 | 0.99, 1.75 |
| 3 | 1.40 | 1.05, 1.87 | 1.07 | 0.78, 1.48 | 1.91 | 1.46, 2.49 | 1.72 | 1.32, 2.26 |
| 4 | 2.17 | 1.65, 2.84 | 1.44 | 1.06, 1.96 | 3.56 | 2.78, 4.56 | 2.80 | 2.17, 3.62 |
| 5 | 3.94 | 3.03, 5.12 | 1.85 | 1.35, 2.53 | 5.63 | 4.40, 7.20 | 3.98 | 3.06, 5.18 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Mortality data were not available for 91 persons in the Health and Retirement Study.
b Adjusted for clinic site (only for the Cardiovascular Health Study), age, sex, race/ethnicity, and education.
c Adjusted for clinic site, age, sex, race/ethnicity, education, smoking status, body mass index, and disability in activities of daily living; history of coronary heart disease, heart failure, stroke, hypertension, diabetes, cancer, and arthritis; and cognitive function, systolic and diastolic blood pressure, C-reactive protein, cystatin C, and total cholesterol.
d Adjusted for age, sex, race/ethnicity, education, smoking status, body mass index, and disability in activities of daily living; history of cardiac disease, stroke, hypertension, diabetes, cancer, and arthritis; and cognitive function, blood pressure, C-reactive protein, cystatin C, and total cholesterol.
e Quintile 1 was the reference group.
The continuous frailty score had better discrimination performance for 5-year mortality than the FP scale in the CHS cohort (Harrell’s C statistic: 0.73 vs. 0.70, ∆ = 0.03, 95% confidence interval: 0.02, 0.05) and the HRS cohort (Harrell’s C statistic: 0.76 vs. 0.74, ∆ = 0.02, 95% confidence interval: 0.01, 0.03).
Association with mortality in robust, prefrail, and frail persons
After adjustment for sociodemographic factors, the continuous frailty score was significantly associated with higher mortality among the robust and prefrail but not the frail (Table 4). Among persons in the prefrail category, the greatest risk of death was observed for those in the quintiles 4 or 5 of the continuous frailty score. The continuous frailty scale was more strongly associated with death among robust, prefrail, and frail persons in the HRS cohort. After adjusting for sociodemographic factors, each unit increase in the continuous frailty score was associated with 54%, 55%, and 47% higher mortality among robust, prefrail, and frail persons, respectively (Table 4). Estimates did not change substantially when we included persons who were unable to perform the gait speed or grip strength test (Web Table 12).
Table 4.
Association of Frailty With 5-Year Mortality Among Participants Classified as Robust, Prefrail, and Frail According to the Frailty Phenotype Scale, Cardiovascular Health Study (1989–1993) and Health and Retirement Study (2006–2009), United States
| Frailty Score | Cardiovascular Health Study (n = 4,243) | Health and Retirement Study (n = 7,519a) | ||||||
|---|---|---|---|---|---|---|---|---|
| Demographic-Adjustedb | Fully Adjustedc | Demographic-Adjustedb | Fully Adjustedd | |||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Robuste | ||||||||
| Continuous frailty score | 1.35 | 1.07, 1.71 | 1.12 | 0.87, 1.44 | 1.54 | 1.28, 1.85 | 1.44 | 1.19, 1.73 |
| Quintilef | ||||||||
| 2 | 1.22 | 0.85, 1.76 | 1.10 | 0.73, 1.67 | 1.40 | 1.01, 1.94 | 1.30 | 0.94, 1.81 |
| 3 | 1.57 | 1.07, 2.30 | 1.05 | 0.68, 1.61 | 1.86 | 1.32, 2.61 | 1.71 | 1.21, 2.43 |
| 4 and 5 | 1.66 | 0.94, 2.91 | 1.26 | 0.74, 2.14 | 3.45 | 2.27, 5.25 | 2.87 | 1.86, 4.43 |
| Prefraile | ||||||||
| Continuous frailty score | 1.56 | 1.34, 1.81 | 1.26 | 1.07, 1.50 | 1.55 | 1.40, 1.71 | 1.41 | 1.27, 1.57 |
| Quintilef | ||||||||
| 2 | 1.19 | 0.69, 2.06 | 1.16 | 0.62, 2.17 | 1.02 | 0.54, 1.93 | 0.96 | 0.51, 1.80 |
| 3 | 1.06 | 0.63, 1.77 | 0.87 | 0.47, 1.61 | 1.36 | 0.76, 2.42 | 1.24 | 0.70, 2.21 |
| 4 | 1.65 | 1.02, 2.67 | 1.17 | 0.66, 2.08 | 2.44 | 1.39, 4.27 | 1.95 | 1.11, 3.42 |
| 5 | 2.62 | 1.60, 4.30 | 1.51 | 0.84, 2.71 | 3.02 | 1.71, 5.33 | 2.34 | 1.32, 4.13 |
| Fraile | ||||||||
| Continuous frailty score | 1.06 | 0.82, 1.37 | 0.94 | 0.69, 1.27 | 1.47 | 1.26, 1.73 | 1.31 | 1.11, 1.56 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Mortality data were not available for 91 persons in the Health and Retirement Study.
b Adjusted for clinic site (only for the Cardiovascular Health Study), age, sex, race/ethnicity, and education.
c Adjusted for clinic site, age, sex, race (white, black), education (less than high school completion, high school completion or equivalent, and more than high school), smoking status (current, former, and never), and body mass index (<25.0, 25.0–30.0, and >30.0); history of coronary heart disease, heart failure, stroke, hypertension, diabetes, cancer, and arthritis; and cognitive function measured by a modified Mini-Mental State Examination, disability in activities of daily living, systolic and diastolic blood pressure, C-reactive protein, cystatin C, and total cholesterol.
d Adjusted for age, sex, race (white, black, other), education (less than high school completion, high school completion or equivalent, and more than high school), smoking status (current, former, and never), and body mass index (<25.0, 25.0–30.0, and >30.0); history of cardiac disease (heart attack, coronary heart disease, angina, heart failure, or other heart problems), stroke, hypertension, diabetes, cancer, and arthritis; and cognitive function measured by the Telephone Interview for Cognitive Status, disability in activities of daily living, systolic and diastolic blood pressure, C-reactive protein, cystatin C, and total cholesterol.
e Participants were identified as robust, prefrail, and frail based on the frailty phenotype scale (separately for the 2 cohorts). In both cohorts, >90% of frail persons identified by the physical frailty phenotype scale were in the quintile 5 of the continuous frailty scale.
f Quintile 1 was the reference group.
Association with disability
The continuous frailty scale stratified the CHS participants by risk of disability (Table 5). In a demographic-adjusted model, persons with the highest continuous frailty scores (quintile 5) had 4.61-fold higher disability risk than those with the lowest scores (quintile 1). In the HRS cohort, persons with the highest continuous frailty scores (quintile 5) had 6.72-fold higher risk of disability than those with the lowest scores (quintile 1). The continuous frailty scale continued to be associated with disability after adjusting for additional covariates (Table 5). Estimates were robust against multiple sensitivity analyses (Web Table 11).
Table 5.
Association of Frailty With 2-Year Incident Disability in Activities of Daily Livinga Among Participants in the Cardiovascular Health Study (1989–1993) and the Health and Retirement Study (2006–2009), United States
| Frailty Score | Cardiovascular Health Study (n = 3,281) | Health and Retirement Study (n = 5,949) | ||||||
|---|---|---|---|---|---|---|---|---|
| Demographic-Adjustedb | Fully Adjustedc | Demographic-Adjustedb | Fully Adjustedd | |||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | |
| Continuous frailty score | 1.71 | 1.60, 1.82 | 1.53 | 1.39, 1.68 | 1.72 | 1.65, 1.79 | 1.50 | 1.40, 1.61 |
| Quintilee | ||||||||
| 2 | 1.47 | 1.10, 1.97 | 1.36 | 1.00, 1.87 | 1.43 | 1.09, 1.87 | 1.33 | 0.96, 1.84 |
| 3 | 1.95 | 1.47, 2.58 | 1.45 | 1.05, 1.98 | 2.19 | 1.71, 2.81 | 1.85 | 1.37, 2.52 |
| 4 | 2.99 | 2.29, 3.89 | 2.13 | 1.57, 2.88 | 3.56 | 2.81, 4.50 | 2.45 | 1.82, 3.31 |
| 5 | 4.61 | 3.54, 6.00 | 2.96 | 2.17, 4.02 | 6.72 | 5.33, 8.45 | 3.85 | 2.83, 5.25 |
Abbreviations: CI, confidence interval; RR, risk ratio.
a Participants who reported having difficulty in any of 6 activities of daily living (dressing, eating, toileting, bathing, transferring or getting out of bed, and walking across a room) were considered disabled.
b Adjusted for clinic site (only for the Cardiovascular Health Study), age, sex, race/ethnicity, and education.
c Adjusted for clinic site, age, sex, race/ethnicity, education, smoking status, and body mass index; history of coronary heart disease, heart failure, stroke, hypertension, diabetes, cancer, and arthritis; cognitive function, systolic and diastolic blood pressure, C-reactive protein, cystatin C, and total cholesterol.
d Adjusted for age, sex, race/ethnicity, education, smoking status, and body mass index; history of cardiac disease, stroke, hypertension, diabetes, cancer, and arthritis; and cognitive function, blood pressure, C-reactive protein, cystatin C, and total cholesterol.
e Quintile 1 was the reference group.
The continuous frailty score had better discrimination performance for 2-year incident disability (activities of daily living) than the FP scale in the CHS cohort (C statistic: 0.70 vs. 0.68, ∆ = 0.02, 95% confidence interval 0.01, 0.04) and the HRS cohort (C statistic: 0.74 vs. 0.71, ∆ = 0.03, 95% confidence interval 0.02, 0.05).
Association with disability in robust, prefrail, and frail persons
In demographic-adjusted models, risk of disability was 85%, 63%, and 38% greater per unit of the continuous frailty scale among robust, prefrail, and frail persons, respectively (Table 6). In the HRS, each higher unit of the continuous frailty score was associated with 55%, 62%, and 38% greater risk of disability among robust, prefrail, and frail persons, respectively (Table 6). Estimates did not change appreciably when persons not completing the gait speed or grip strength test were included (Web Table 12).
Table 6.
Association of Frailty With 2-Year Incident Disability in Activities of Daily Livinga Among Participants Classified as Robust, Prefrail, and Frail Using the Frailty Phenotype Scale, Cardiovascular Health Study (1989–1993) and Health and Retirement Study (2006–2009), United States
| Frailty Score | Cardiovascular Health Study (n = 3,281) | Health and Retirement Study (n = 5,949) | ||||||
|---|---|---|---|---|---|---|---|---|
| Demographic-Adjustedb | Fully Adjustedc | Demographic-Adjustedb | Fully Adjustedd | |||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | |
| Robuste | ||||||||
| Continuous frailty score | 1.85 | 1.42, 2.42 | 1.57 | 1.19, 2.07 | 1.55 | 1.30, 1.86 | 1.34 | 1.08, 1.66 |
| Quintilef | ||||||||
| 2 | 1.35 | 0.94, 1.94 | 1.30 | 0.88, 1.93 | 1.25 | 0.91, 1.71 | 1.13 | 0.78, 1.64 |
| 3 | 1.62 | 1.09, 2.40 | 1.15 | 0.74, 1.79 | 1.78 | 1.29, 2.47 | 1.40 | 0.96, 2.05 |
| 4 and 5 | 4.15 | 2.75, 6.26 | 2.58 | 1.65, 4.05 | 3.32 | 2.22, 4.97 | 2.56 | 1.58, 4.17 |
| Prefraile | ||||||||
| Continuous frailty score | 1.63 | 1.42, 1.86 | 1.44 | 1.23, 1.69 | 1.62 | 1.49, 1.76 | 1.43 | 1.26, 1.62 |
| Quintilef | ||||||||
| 2 | 1.37 | 0.82, 2.30 | 1.16 | 0.66, 2.05 | 1.29 | 0.73, 2.26 | 2.18 | 0.79, 5.98 |
| 3 | 1.59 | 0.98, 2.59 | 1.16 | 0.68, 1.99 | 1.63 | 0.96, 2.74 | 3.00 | 1.14, 7.90 |
| 4 | 1.97 | 1.24, 3.15 | 1.34 | 0.80, 2.26 | 2.26 | 1.36, 3.75 | 3.44 | 1.31, 9.00 |
| 5 | 2.93 | 1.82, 4.74 | 1.92 | 1.13, 3.25 | 3.60 | 2.16, 5.99 | 4.73 | 1.78, 12.54 |
| Fraile | ||||||||
| Continuous frailty score | 1.38 | 1.08, 1.76 | 1.40 | 0.90, 2.20 | 1.38 | 1.25, 1.53 | 1.55 | 1.26, 1.90 |
Abbreviations: CI, confidence interval; RR, risk ratio.
a Participants who reported having difficulty in any of 6 activities of daily living (dressing, eating, toileting, bathing, transferring or getting out of bed, and walking across a room) were considered disabled.
b Adjusted for clinic site (only for the Cardiovascular Health Study), age, sex, race/ethnicity, and education.
c Adjusted for clinic site, age, sex, race (white, black), education (less than high school completion, high school completion or equivalent, and more than high school), smoking status (current, former, and never), and body mass index (<25.0, 25.0–30.0, and >30.0); history of coronary heart disease, heart failure, stroke, hypertension, diabetes, cancer, and arthritis; and cognitive function measured by a modified Mini-Mental State Examination, systolic and diastolic blood pressure, C-reactive protein, cystatin C, and total cholesterol.
d Adjusted for age, sex, race (white, black, other), education (less than high school completion, high school completion or equivalent, and more than high school), smoking status (current, former, and never), and body mass index (<25.0, 25.0–30.0, and >30.0); history of cardiac disease (heart attack, coronary heart disease, angina, heart failure, or other heart problems), stroke, hypertension, diabetes, cancer, and arthritis; and cognitive function measured by the Telephone Interview for Cognitive Status, systolic and diastolic blood pressure, C-reactive protein, cystatin C, and total cholesterol.
e Participants were identified as robust, prefrail, and frail based on the frailty phenotype scale (separately for the 2 cohorts). In both cohorts, >90% of frail persons identified by the physical frailty phenotype scale were in the quintile 5 of the continuous frailty scale.
f Quintile 1 was the reference group.
DISCUSSION
Using data from 2 large US cohorts, we developed a continuous frailty scale and evaluated its construct validity, measurement properties, and predictive validity. Five major findings warrant comment. First, frailty—as assessed by gait speed, grip strength, exhaustion, physical activity, and weight loss—is a valid continuous construct with a similar factor structure across 2 studies. Second, gait speed contributes the most to the measurement of frailty in both studies. Third, the concordance between the continuous frailty scale and the original FP scale was high. Fourth, the continuous frailty scale was strongly associated with mortality and disability among older adults and had better discrimination performance than the FP scale. Finally, the continuous frailty scale was associated with mortality and disability beyond the categorical FP scale, especially among robust and prefrail persons.
To our knowledge, this is the first application of CFA to empirically validate the frailty construct as measured by the 5 indicators originally proposed by Fried et al. (5). We conceptualized frailty as a continuous latent construct, and our approach is different from previous work by Bandeen-Roche et al. (2), in which a latent class analysis was applied to examine the construct validity of frailty, operationalized as a discrete syndrome. Both latent variable–based methods are useful to investigate the construct validity of frailty, a clinical syndrome that is not directly measurable. CFA is more appropriate than latent class analysis for constructs conceptualized as a spectrum rather than a discrete phenomenon. Our findings showed that a unidimensional factor structure fit the data satisfactorily in 2 cohorts.
In both studies, gait speed contributes the most to the measurement of frailty. Gait speed, a quick, easy, and inexpensive physical performance measure, is an integrative measure of health and a well-documented risk factor for adverse outcomes among older adults (22–27). Additionally, gait speed has been advocated for by the Geriatric Advisory Panel of the International Academy of Nutrition and Aging task force as the most suitable single-item measure of frailty in clinical practice (28).
The continuous frailty scale was able to provide additional risk stratification for mortality and disability beyond the FP scale, especially at the lower end to middle of the frailty continuum. These results suggest that robust and prefrail persons are 2 heterogeneous groups with different risks of developing unfavorable outcomes. Among the prefrail group, the increase in risk observed in the highest quintile suggests the possibility of a threshold effect. Our findings were consistent with a prior study showing that the frailty index, which counts the presence of deficits, was associated with poor self-rated health and high health-care utilization among robust persons identified by the FP scale (29). However, the frailty index includes a long checklist of comorbidities and disability and is therefore not specific for frailty (30). We demonstrated that the continuous frailty scale, developed based on the same 5 indicators used in the FP scale, can achieve the same purpose.
The continuous frailty scale and the FP scale may serve different purposes. The FP scale classifies persons into 3 categories; this discrete nature is clinic-friendly and may facilitate the implementation of frailty assessment in clinical practice (31). On the other hand, the continuous frailty scale provides a more sensitive measure of frailty than the FP scale; the new instrument may be a more useful assessment to evaluate the effectiveness of preventive or therapeutic interventions for frailty. Additionally, the continuous frailty scale is a potentially more powerful tool for identifying biomarkers that have clinically meaningful but small effects on frailty. The continuous frailty scale may likewise increase power in genetic studies aimed at discovering the genetic underpinnings of frailty. Frailty is an exceedingly complex phenotype that involves dysregulations of multiple physiological systems (32); any genetic effects of frailty are expected to be modest and difficult to detect. Furthermore, the continuous frailty scale may offer benefits to describe the trajectories of frailty over time and allow interventions at an earlier stage. Although frailty is dynamic, the likelihood of transitioning from being at an end stage of frailty to robust is extremely low (33); these results emphasize the importance of designing interventions for persons who are vulnerable but not yet frail.
We acknowledge several limitations. First, although the 5 frailty indicators were measured similarly across the CHS and HRS cohorts, nuanced differences still exist. Gait speed, for example, was measured over a 15-foot course in the CHS, while a 98.5-inch course was adopted in the HRS. However, factor-loading estimates were similar across the 2 cohorts, suggesting that the factor structure was robust to nuanced differences in assessment of indicators. Second, weight loss, calculated by weight measure in 2 visits at least 1 year apart, may be more susceptible to measurement error than are other indicators, which may account for the fact that weight loss was the weakest indicator for frailty. Third, factor-loading estimates may be biased in sensitivity analyses including persons with missing frailty indicators if the missing-at-random assumption is violated. Last, differences in birth cohort, age structure, and health characteristics may have affected results of the outcome analyses and led to different calibrations for the 2 cohorts.
Our findings provide evidence that frailty is a valid continuous construct with a unidimensional factor structure, robust to nuanced differences in measurement of indicators. Not all indicators had the same strength in measuring frailty, with gait speed contributing the most. Additionally, we demonstrated the validity of the new scale for predicting mortality and disability. Moreover, the continuous frailty scale was able to provide risk stratification among robust and prefrail persons identified by the FP scale. Future research should examine the ability of this new frailty instrument to predict older adults’ ability to recover from stressors (e.g., disability)—a defining feature of frailty. Future research can also help identify the utility of this new assessment in detecting small genetic effects and evaluating effectiveness of interventions.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Public Health, School of Health Sciences and Practice, New York Medical College, Valhalla, New York (Chenkai Wu); School of Biological and Population Health Sciences, Oregon State University, Corvallis, Oregon (Chenkai Wu, Michelle C. Odden); School of Social and Behavioral Health Sciences, Oregon State University, Corvallis, Oregon (G. John Geldhof); Department of Medicine, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Qian-Li Xue); Center on Aging and Health, Johns Hopkins Medical Institutions, Baltimore, Maryland (Qian-Li Xue); Division of Gerontology, Beth Israel Deaconess Medical Center, Harvard University, Boston, Massachusetts (Dae H. Kim); and Department of Epidemiology, University of Pittsburgh, Pittsburgh, Pennsylvania (Anne B. Newman). C.W. is currently at the Global Health Research Center, Duke Kunshan University, Kunshan, Jiangsu, China.
This research was supported by the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke (contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114). Additional support was provided by the National Institute on Aging (grants R01AG023629 (M.C.O.), R03AG048541 (Q.-L.X.), K08AG051187 (D.H.K.), and P30AG013679 (D.H.K.)). D.H.K. is supported by the American Federation for Aging Research, the John A. Hartford Foundation, and the Atlantic Philanthropies. A full list of principal Cardiovascular Health Study investigators and institutions can be found at CHS-NHLBI.org.
D.H.K. provides paid consultative services to Alosa Health, a nonprofit educational organization with no relationship to any drug or device manufacturers.
Abbreviations
- CFA
confirmatory factor analysis
- CHS
Cardiovascular Health Study
- FP
frailty phenotype
- HRS
Health and Retirement Study
REFERENCES
- 1. Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–263. [DOI] [PubMed] [Google Scholar]
- 2. Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61(3):262–266. [DOI] [PubMed] [Google Scholar]
- 3. Ensrud KE, Ewing SK, Cawthon PM, et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. 2009;57(3):492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu C, Smit E, Xue QL, et al. Prevalence and correlates of frailty among community-dwelling Chinese older adults: the China Health and Retirement Longitudinal Study. J Gerontol A Biol Sci Med Sci. 2017;73(1):102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. [DOI] [PubMed] [Google Scholar]
- 6. Aguayo GA, Donneau AF, Vaillant MT, et al. Agreement between 35 published frailty scores in the general population. Am J Epidemiol. 2017;186(4):420–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70(11):1427–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xue QL, Tian J, Fried LP, et al. Physical frailty assessment in older women: can simplification be achieved without loss of syndrome measurement validity? Am J Epidemiol. 2016;183(11):1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Widagdo IS, Pratt N, Russell M, et al. Construct validity of four frailty measures in an older Australian population: a Rasch analysis. J Frailty Aging. 2016;5(2):78–81. [DOI] [PubMed] [Google Scholar]
- 10. Sanders JL, Boudreau RM, Fried LP, et al. Measurement of organ structure and function enhances understanding of the physiological basis of frailty: the Cardiovascular Health Study. J Am Geriatr Soc. 2011;59(9):1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taylor HL, Jacobs DR Jr, Schucker B, et al. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31(12):741–755. [DOI] [PubMed] [Google Scholar]
- 12. Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25(1):71–80. [DOI] [PubMed] [Google Scholar]
- 13. Teng EL, Chui HC. The Modified Mini-Mental State examination (3MS). J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 14. Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1(2):111–117. [Google Scholar]
- 15. Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):278–285. [DOI] [PubMed] [Google Scholar]
- 16. Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16(3):297–334. [Google Scholar]
- 17. Fabrigar LR, Wegener DT, MacCallum RC, et al. Evaluating the use of exploratory factor analysis in psychological research. Psychol Methods. 1999;4(3):272–299. [Google Scholar]
- 18. Cattell RB. The scree test for the number of factors. Multivariate Behav Res. 1966;1(2):245–276. [DOI] [PubMed] [Google Scholar]
- 19. Marsh HW, Hau KT, Wen Z. In search of golden rules: comment on hypothesis-testing approaches to setting cutoff values for fit indexes and dangers in overgeneralizing Hu and Bentler’s (1999) findings. Struct Equ Modeling. 2004;11(3):320–341. [Google Scholar]
- 20. Sörbom D. Model modification. Psychometrika. 1989;54(3):371–384. [Google Scholar]
- 21. Enders C, Bandalos D. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Struct Equ Modeling. 2001;8(3):430–457. [Google Scholar]
- 22. Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people—results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53(10):1675–1680. [DOI] [PubMed] [Google Scholar]
- 23. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10):881–889. [DOI] [PubMed] [Google Scholar]
- 25. Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295(17):2018–2026. [DOI] [PubMed] [Google Scholar]
- 26. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–M231. [DOI] [PubMed] [Google Scholar]
- 27. Dumurgier J, Elbaz A, Ducimetière P, et al. Slow walking speed and cardiovascular death in well functioning older adults: prospective cohort study. BMJ. 2009;339:b4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abellan van Kan G, Rolland Y, Bergman H, et al. The I.A.N.A. Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging. 2008;12(1):29–37. [DOI] [PubMed] [Google Scholar]
- 29. Blodgett J, Theou O, Kirkland S, et al. Frailty in NHANES: comparing the frailty index and phenotype. Arch Gerontol Geriatr. 2015;60(3):464–470. [DOI] [PubMed] [Google Scholar]
- 30. Walston JD, Bandeen-Roche K. Frailty: a tale of two concepts. BMC Med. 2015;13:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cesari M, Gambassi G, van Kan GA, et al. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. 2014;43(1):10–12. [DOI] [PubMed] [Google Scholar]
- 32. Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62(7):731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gill TM, Gahbauer EA, Allore HG, et al. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166(4):418–423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.