Abstract
A decade after the alarming association of cabergoline-associated valvulopathy (CAV) in Parkinson disease, only two confirmed cases have occurred in patients with prolactinoma. Routine screening for CAV by echocardiography has not proved to be of diagnostic utility, has several limitations, and is not widely practiced. We have previously highlighted the value of annual cardiovascular examination as a screening tool for CAV in patients with prolactinoma. We present a case, now the third confirmed case of CAV, to highlight the value of the cardiovascular examination. A 52-year-old woman with a 25-year history of macroprolactinoma had received multimodal treatment, including surgery, radiosurgery, and medical therapy. Her medical therapy initially consisted of bromocriptine, followed by cabergoline. The cabergoline dose was 6 mg weekly. In 2009, the cumulative dose was 3272 mg when an echocardiogram showed no evidence of valvular disease. A routine cardiovascular examination in the clinic detected a new murmur in 2016. The echocardiogram demonstrated new-onset mild to moderate aortic regurgitation, with a thickened and restricted valve consistent with CAV. The cumulative dose of cabergoline at that point was 4192 mg. Follow-up echocardiography at 6-month intervals showed progression to moderate to severe aortic regurgitation, which has since stabilized. Cabergoline therapy was weaned and stopped completely in April 2017. An annual cardiovascular examination is the best screening test for CAV and can change the course of a patient’s treatment. Echocardiograms should be reserved for patients with a new-onset cardiac murmur or a high cumulative dose of cabergoline.
Keywords: cabergoline, prolactinoma, valvular heart disease
Screening for cabergoline-associated valvulopathy by echocardiography has not been shown to be useful for patients with prolactinoma. The cardiovascular examination is, however, a useful screening tool.
Indisputable evidence exists for the causal relationship of ergot-derived dopamine agonists (bromocriptine and cabergoline) and cardiac valve disease in Parkinson disease related to high cumulative doses. During the past decade, several studies have examined the risk of valvulopathy in patients with prolactinoma taking cabergoline. In our 2015 systematic review, the risk was found to be extremely low (only two confirmed and one possible case), most likely owing to the lower cumulative doses used in treating prolactinoma [1]. At the time of that review, 19 studies and 2 case reports pertaining to cabergoline and valvular heart disease had been reported. Since then, three further reported studies have not shown an increased prevalence of cabergoline-associated valvulopathy (CAV): now totaling 2000 cases of prolactinoma studied [2–4]. Studies of serial echocardiograms in patients with prolactinoma have not shown new cases of CAV [2, 5, 6]; however, in reality, few patients are undergoing serial echocardiograms [3].
Given the low prevalence of CAV in patients with prolactinoma, we believe that optimal screening should consist of an annual cardiovascular examination for all treated patients, with echocardiograms directed at those with a murmur or high cumulative doses of cabergoline.
We present the case of a patient to emphasize the value of the cardiovascular examination, because this is now the third confirmed case of CAV. The patient provided written informed consent for the reporting of her case.
1. Case Report
A 52-year-old woman had originally presented in 1989 at age 22 years with headaches, secondary amenorrhea, and galactorrhea. The prolactin level was elevated to >6000 mIU/L (undiluted), and a CT scan showed a 3-cm adenoma extending into the sphenoid sinus. She was initially treated with bromocriptine from 1990 to 1992 with some initial shrinkage to 2.5 cm on subsequent scans, with a prolactin level of 10,000 mIU/L (reference range <615 mIU/L) after serial dilution. Because of the limited response to bromocriptine with the lesion abutting the optic apparatus, she subsequently underwent two debulking microscopic transsphenoidal surgeries in 1992. However, her prolactin level remained elevated at 7000 mIU/L, with residual disease in the right cavernous sinus. Bromocriptine was started again, until cabergoline became available in 1997. Cabergoline was begun at 2 mg weekly and subsequently increased to 6 to 7 mg weekly over time, with failure to ever reduce the prolactin level to <1000 mIU/L. After initially declining radiotherapy in 1999, the patient subsequently underwent stereotactic radiosurgery in 2004 and continued with cabergoline at high doses.
The patient had panhypopituitarism and was taking cortisone acetate and thyroxine. She had long-standing hypogonadism but had been variably compliant with estrogen and progestogen replacement. She developed osteopenia and experienced a low trauma fracture of the fibular in January 2017, thereafter beginning antiresorptive therapy.
The patient underwent echocardiography in 2009, which showed no evidence of valvular disease. The weekly dose of cabergoline was 6 mg, and the cumulative dose was 3272 mg. Her prolactin level at this time was at its nadir of 900 to 1000 mIU/L. In 2012, a repeat MRI scan had shown a substantial reduction in the residual adenoma, after which the dose of cabergoline had been weaned at the rate of 0.5 mg every 6 months.
A routine cardiovascular examination detected a new murmur in 2016. The patient was asymptomatic. The echocardiogram demonstrated new-onset mild to moderate aortic regurgitation, with a thickened and restricted valve—the three cardinal echocardiographic features of CAV. The cumulative dose of cabergoline at that point was 4192 mg, with a weekly dose of 3 mg. Follow-up echocardiograms at 6-month intervals showed progression to moderate to severe aortic regurgitation, which at the last follow-up examination had stabilized (Fig. 1). Cabergoline was progressively weaned and was stopped in April 2017. Her prolactin level increased to 4706 mIU/L, but no interval tumor growth was seen on MRI.
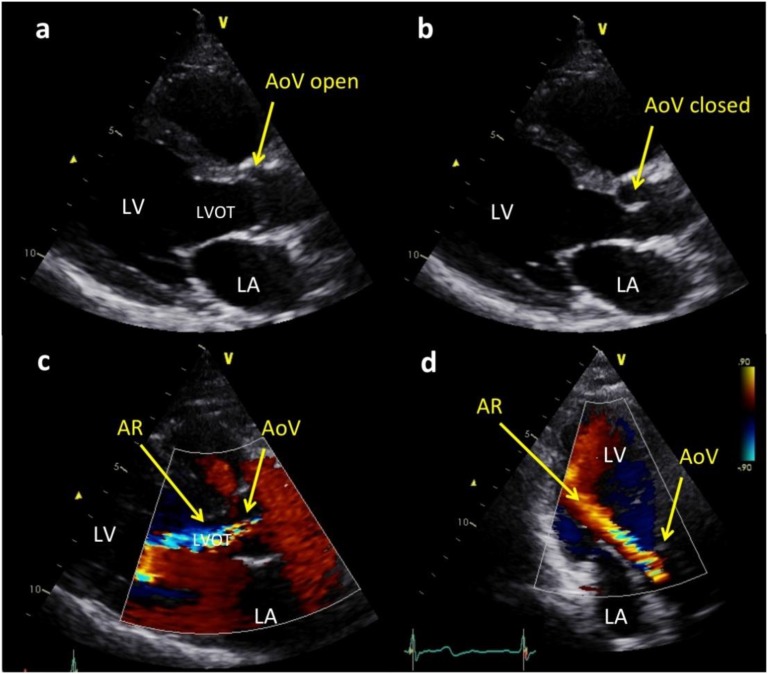
Figure 1.
Echocardiographic images of a thickened and mildly restricted aortic valve (AoV) with resultant moderately severe aortic regurgitation (AR). (a) Parasternal long axis views in which the AoV leaflets are mildly thickened and show mild doming owing to incomplete opening in systole. (b) The AoV leaflet tips are mildly thickened when the valve is closed. (c) Parasternal long axis view showing color Doppler image of a broad jet of AR almost filling the left ventricular outflow tract (LVOT). (d) Apical long axis view of the color Doppler jet of AR reaching the left ventricular apex. LA, left atrium; LV, left ventricle.
2. Discussion
Our patient’s case represents the third confirmed case of CAV in >2000 cases of prolactinoma treated with ergot-derived dopamine agonists reported since 2008. Although other studies have shown evidence of various cardiac valvular abnormalities, CAV represents a specific pathology characterized by three salient echocardiographic features: (1) moderate or severe regurgitation, (2) valve thickening, and (3) valve restriction. Furthermore, the absence of calcification and myxomatous changes distinguishes CAV from age-related sclerosis and myxomatous valve disease, respectively. If a valvular lesion is found, it is crucial to distinguish among age-related changes, myxomatous changes, and CAV to determine whether cabergoline should be weaned or ceased. In our patient, the three salient features of CAV were present on the echocardiogram, leading to cessation of cabergoline therapy as recommend by the Food and Drug Administration (FDA) [7]. Although measurement of the tricuspid valve tethering area and mitral valve tenting is useful in comparing defined patient groups to quantify the degree of valve restriction, these are subject to user and machine variability. They are not reliably reproducible and are only surrogate markers of subclinical changes in the valve [1]. In clinical practice, tethering and tenting are not echocardiographic markers that would change medical management.
On histologic examination, which is only available for those who have undergone surgical valve replacement, CAV is characterized by a thickened valve due to fibrous proliferation, with the absence of inflammatory cells, thrombus, and calcification [8]. The mechanism of valvular pathology as a cabergoline-induced adverse event is biologically plausible via stimulation of serotonin (5-hydroxytryptamine) receptor subtype 5-hydroxytryptamine(2B), which is expressed in heart valves and is known to mediate mitogenesis and proliferation of fibroblasts [9].
In a cohort of patients with Parkinson disease at risk of CAV, the mean cumulative dose was 4015 ± 3208 mg), giving one standard deviation less at ~720 mg [10]. Only 10% of patients with prolactinoma will require high doses of cabergoline (>3 mg/wk) [11]. Those taking doses of 3 mg/wk will reach cumulative doses of 720 mg after 5 years of treatment. Most patients with prolactinoma will receive doses ≤1 mg/wk.
In the reported data, two cases of CAV in patients with prolactinoma have been confirmed. One cases of CAV occurred with a cumulative dose of only 252 mg [8]. That patient had fulminant heart failure, with echocardiographic findings of severe mitral regurgitation and a thickened and restricted valve (in the absence of stenosis), confirming CAV. The histologic findings were available because the patient had required valve replacement, which supported the echocardiographic diagnosis. In the second case of CAV, moderate mitral regurgitation occurred at an extremely high dose of 5252 mg, similar to our case [12]. That patient also had all three features of CAV found on the echocardiogram; however, valve replacement was not required. One case of moderate tricuspid regurgitation has been reported; however, no morphological features of CAV were found on the echocardiogram, and the patient had only had a cumulative cabergoline dose of 48 mg. These findings together would make CAV less likely [13].
Our patient had been taking a high dose of cabergoline for many years, reaching doses comparable to those used for Parkinson disease. She had also received several years of bromocriptine, another ergot dopamine agonist. Despite normal echocardiographic findings when the cumulative dose was ~3000 mg, she subsequently developed CAV, which was initially detected clinically by the presence of a new murmur although she was asymptomatic.
A recent community study from the United Kingdom demonstrated that very few patients with hyperprolactinemia had undergone baseline echocardiography (2 of 45), and only 5 of the 45 patients had undergone serial echocardiograms 2 years apart [3]. Even for those with Parkinson disease, a study showed that very few patients had had serial echocardiograms [14]. Three studies have followed up a total of 185 patients with prolactinoma with serial echocardiograms after 2 to 5 years and did not find an increased incidence of CAV [2–4]. Thus, echocardiography is seldom used; however, more importantly, routine interval scanning has not been shown to be useful. In July 2011, the FDA recommended “regular echocardiograms every 6 to 12 months” in all users of cabergoline “or as clinically indicated with the presence of signs and symptoms such as dyspnea, edema, new cardiac murmur, or congestive heart failure” [7]. We would argue that the frequency of echocardiograms recommended by the FDA has no scientific basis. We have previously shown the limitations of echocardiography as a screening tool, in particular the risk of overreporting findings, failure to recognize the cardinal features of CAV (compared with age-related changes or myxomatous disease), and the associated financial and psychological costs [1].
In conclusion, it is reassuring to endocrinologists (and patients) that the prevalence of CAV in patients with prolactinoma is extremely low. Given this low prevalence, routine screening with echocardiography is not indicated. The recommended screening procedure should be an annual cardiovascular examination, with echocardiography reserved for patients with a murmur or those with high cumulative cabergoline doses.
Acknowledgments
Financial Support: C.C. was supported by an Australian Government Research Training Program Scholarship.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- CAV
cabergoline-associated valvulopathy
- FDA
Food and Drug Administration
References and Notes
- 1. Caputo C, Prior D, Inder WJ. The need for annual echocardiography to detect cabergoline-associated valvulopathy in patients with prolactinoma: a systematic review and additional clinical data. Lancet Diabetes Endocrinol. 2015;3(11):906–913. [DOI] [PubMed] [Google Scholar]
- 2. Vroonen L, Lancellotti P, Garcia MT, Dulgheru R, Rubio-Almanza M, Maiga I, Magne J, Petrossians P, Auriemma R, Daly AF, Beckers A. Prospective, long-term study of the effect of cabergoline on valvular status in patients with prolactinoma and idiopathic hyperprolactinemia. Endocrine. 2017;55(1):239–245. [DOI] [PubMed] [Google Scholar]
- 3. Gamble D, Fairley R, Harvey R, Farman C, Cantley N, Leslie SJ. Screening for valve disease in patients with hyperprolactinaemia disorders prescribed cabergoline: a service evaluation and literature review. Ther Adv Drug Saf. 2017;8(7):215–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khare S, Lila AR, Patil R, Phadke M, Kerkar P, Bandgar T, Shah NS. Long-term cardiac (valvulopathy) safety of cabergoline in prolactinoma. Indian J Endocrinol Metab. 2017;21(1):154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Delgado V, Biermasz NR, van Thiel SW, Ewe SH, Marsan NA, Holman ER, Feelders RA, Smit JW, Bax JJ, Pereira AM. Changes in heart valve structure and function in patients treated with dopamine agonists for prolactinomas, a 2-year follow-up study. Clin Endocrinol (Oxf). 2012;77(1):99–105. [DOI] [PubMed] [Google Scholar]
- 6. Auriemma RS, Pivonello R, Perone Y, Grasso LF, Ferreri L, Simeoli C, Iacuaniello D, Gasperi M, Colao A. Safety of long-term treatment with cabergoline on cardiac valve disease in patients with prolactinomas. Eur J Endocrinol. 2013;169(3):359–366. [DOI] [PubMed] [Google Scholar]
- 7. Food and Drug Administration, Department of Health and Human Services. Supplement approval revision. Available at: www.accessdata.fda.gov/drugsatfda_docs/appletter/2011/020664s011ltr.pdf. 2011. Accessed 12 May 2018.
- 8. Cawood TJ, Bridgman P, Hunter L, Cole D. Low-dose cabergoline causing valvular heart disease in a patient treated for prolactinoma. Intern Med J. 2009;39(4):266–267. [DOI] [PubMed] [Google Scholar]
- 9. Van Camp G, Flamez A, Cosyns B, Weytjens C, Muyldermans L, Van Zandijcke M, De Sutter J, Santens P, Decoodt P, Moerman C, Schoors D. Treatment of Parkinson’s disease with pergolide and relation to restrictive valvular heart disease. Lancet. 2004;363(9416):1179–1183. [DOI] [PubMed] [Google Scholar]
- 10. Zanettini R, Antonini A, Gatto G, Gentile R, Tesei S, Pezzoli G. Valvular heart disease and the use of dopamine agonists for Parkinson’s disease. N Engl J Med. 2007;356(1):39–46. [DOI] [PubMed] [Google Scholar]
- 11. Colao A, Galderisi M, Di Sarno A, Pardo M, Gaccione M, D’Andrea M, Guerra E, Pivonello R, Lerro G, Lombardi G. Increased prevalence of tricuspid regurgitation in patients with prolactinomas chronically treated with cabergoline. J Clin Endocrinol Metab. 2008;93(10):3777–3784. [DOI] [PubMed] [Google Scholar]
- 12. Gu H, Luck S, Carroll PV, Powrie J, Chambers J. Cardiac valve disease and low-dose dopamine agonist therapy: an artefact of reporting bias? Clin Endocrinol (Oxf). 2011;74(5):608–610. [DOI] [PubMed] [Google Scholar]
- 13. Bhat MH, Mushtaq S, Saba S, Saif R, Ali G. Cabergoline-induced tricuspid regurgitation: case report and review of literature. Indian J Endocrinol Metab. 2011;15(2):137–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Italiano D, Bianchini E, Ilardi M, Cilia R, Pezzoli G, Zanettini R, Vacca L, Stocchi F, Bramanti P, Ciurleo R, Di Lorenzo G, Polimeni G, de Luise C, Ross D, Rijnbeek P, Sturkenboom M, Trifirò G. Effectiveness of risk minimization measures for cabergoline-induced cardiac valve fibrosis in clinical practice in Italy. J Neural Transm (Vienna). 2015;122(6):799–808. [DOI] [PubMed] [Google Scholar]