In this life table analysis, 1 in 5 women in King County, Washington, had chlamydia by age 34 years. Black women had a higher risk than white and Hispanic women. One in 1667 women is estimated to develop chlamydia-associated tubal factor infertility.
Keywords: chlamydia, pelvic inflammatory disease, infertility, birth cohort, life table
Abstract
Background
Chlamydia trachomatis is the most common reportable infection in the United States and can cause pelvic inflammatory disease (PID) and tubal factor infertility (TFI).
Methods
We created life tables to estimate the “lifetime” risk of chlamydia diagnosis among women aged 15–34 years in King County, Washington, between 1992 and 2014. We estimated the lifetime risk of chlamydia-associated PID and TFI incorporating published estimates of the risk of sequelae.
Results
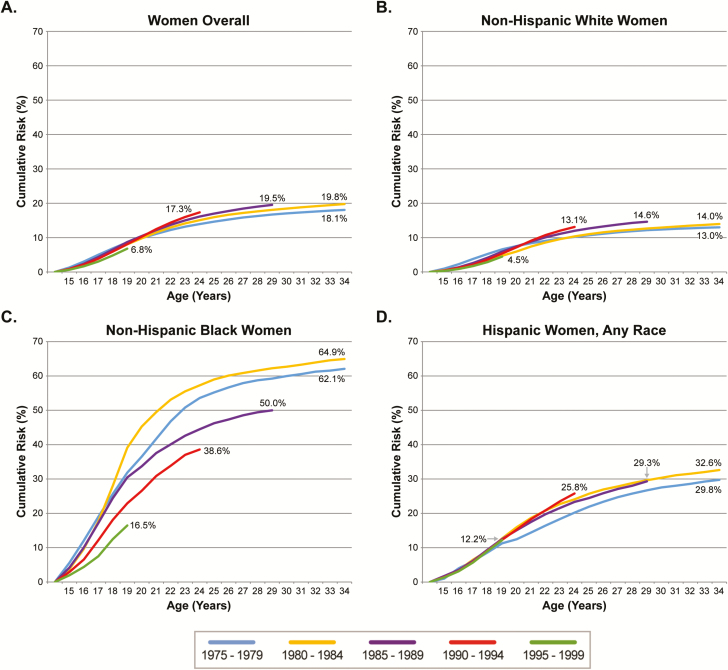
There were 51464 first chlamydia diagnoses in 1992—2014. For women born between 1980 and 1984, the lifetime risk of chlamydia diagnosis was 19.8% overall and 14.0% for non-Hispanic white, 64.9% for non-Hispanic black, and 32.6% for Hispanic women. The cumulative risk of chlamydia by age 24 increased overall from 13.9% to 17.3% among women born between 1975 and 1994 but declined among non-Hispanic black women, among whom risk by age 24 declined from 57.3% among women born between 1980 and 1984 to 38.6% among women born between 1990 and 1994. The lifetime risk of chlamydia-associated PID among women born between 1980 and 1984 ranged from 0.33% to 1.14%. Among non-Hispanic white, non-Hispanic black, and Hispanic women, the lifetime risk of chlamydia-associated TFI was 0.04%, 0.20%, and 0.10%, respectively.
Conclusions
Over 60% of non-Hispanic black women had at least 1 chlamydia diagnosis by age 34 in the birth cohorts most affected, a risk almost 5 times that in non-Hispanic whites. An estimated 1 in 500 non-Hispanic black women develops chlamydia-associated TFI. More effective control measures are needed.
Chlamydia trachomatis is the most commonly reported infection in the United States; more than 1.5 million cases were reported in 2015 [1]. Among women, chlamydial infection can cause pelvic inflammatory disease (PID), ectopic pregnancy, and tubal factor infertility (TFI) [2, 3]. Because of these serious complications and the high prevalence of asymptomatic chlamydial infection [4], the United States Preventive Services Task Force (USPSTF) recommends annual screening of all sexually active women aged ≤24 years and of women aged >24 years who are at increased risk of infection [5].
Although the Centers for Disease Control and Prevention (CDC) estimate the incidence of chlamydia diagnosis annually, such estimates include a mixture of first-time and repeat diagnoses and provide little insight into what percentage of women are diagnosed with chlamydia throughout their lifetime. To our knowledge, the cumulative risk of chlamydia diagnosis throughout a woman’s lifetime has only been estimated for one geographic setting in the United States (Florida [6]). The extent to which the risk is similar in other populations and how risk has varied over time are largely unknown. Furthermore, although chlamydia has been associated with sequelae [2, 3], the lifetime risk of a woman in the United States experiencing chlamydia-associated sequelae is unknown.
Washington State was among the first areas in the United States to institute a chlamydial screening program. Screening has been ongoing for almost 30 years [7], with relatively high levels of coverage [8]. Because of this long history, data from the area provide a unique opportunity to assess trends in women’s cumulative lifetime risk of chlamydia diagnosis and how trends in that risk may vary by race/ethnicity. We created life tables to assess women’s “lifetime” risk of chlamydia diagnosis, cumulative risk of chlamydia diagnosis by birth cohort, and lifetime risk of experiencing chlamydia-associated PID and TFI, overall and by race/ethnicity in King County, Washington.
METHODS
Study Design and Population
We grouped women aged 15–34 years in King County from 1992 through 2014 into the following five 5-year birth cohorts: 1975–1979, 1980–1984, 1985–1989, 1990–1994, and 1995–1999. Of note, women born after 1980 were not yet age 34 in 2014. For the younger birth cohorts, we examined risk for ages with data available.
Data Sources
We used King County surveillance data to define the number of women with first and repeat chlamydia diagnoses by year, age, and race/ethnicity. Washington laws require laboratories and medical providers to report chlamydia cases to local health departments, which report cases to state authorities. Each patient’s first and last names, diagnosis, age, and race/ethnicity are included in the reports. In routine surveillance activities, the Washington Department of Health (DOH) checks for duplicate cases and patients using Link Plus [9] and identifies repeat diagnoses, requiring a date of birth, last name, and first name match. Documentation of treatment for the previous diagnosis is not required to define a repeat diagnosis; however, treatment was reported for 98% of cases. We used census data to define the number of women at risk each year. Single-year population estimates for women in King County by age and race/ethnicity were obtained from the DOH Community Health Assessment Tool [10].
Statistical Analysis: Cumulative Risk of Chlamydia Diagnosis
We created life tables to estimate the cumulative risk of first chlamydia diagnosis between age 15 and 34 among women in King County between 1992 and 2014. This life table approach [11] estimates a woman’s cumulative risk of chlamydia diagnosis by age 34 (includes diagnoses at age 34) or the oldest age for which data were available for her birth cohort. We estimated the cumulative risk overall, by birth cohort and by race/ethnicity (ie, non-Hispanic white, non-Hispanic black, and Hispanic [any race]). Because 94% of chlamydia diagnoses occur in women aged ≤34 [1], we considered the cumulative risk of chlamydia diagnosis by age 34 for the 1980–1984 birth cohort to reflect current lifetime risk. We compared women across birth cohorts at age 24 because it was the oldest age at which 4 cohorts could be compared.
To create life tables for each birth cohort, we calculated an age- and year-specific risk of first chlamydia diagnosis by dividing the number of age- and year-specific first chlamydia diagnoses by the King County age- and year-specific population at risk of first chlamydia diagnosis (Supplementary Material, equation 1). To estimate the population at risk of first chlamydia diagnosis for each year and age, we subtracted the estimated number of women who had a chlamydia diagnosis prior to that age from the population. We assumed no women had a first chlamydia diagnosis before age 15. King County attracts a substantial number of young in-migrants, some of whom were diagnosed with chlamydia before moving to the area. To account for this, we subtracted the expected number of repeat chlamydia diagnoses among new population members from the number of first chlamydia diagnoses for each age and year. We estimated the number of new population members based on the change in the population size within a birth cohort for each age and estimated the number of repeat diagnoses in that population by applying the age- and year-specific incidence rate of repeat diagnosis to the number of new population members. For each birth cohort, we added the age- and year-specific risks to obtain the cumulative risk of first chlamydia diagnosis (Supplementary Material, equation 2). We repeated this process for each racial/ethnic group. Due to the large percentage of first chlamydia diagnoses with unknown race/ethnicity (36%), we distributed these cases between race/ethnicity categories based on the distribution of first chlamydia diagnoses with known race/ethnicity by year and age group (Supplementary Material).
Statistical Analysis: Lifetime Risk of Chlamydia-associated PID and TFI
We estimated the lifetime risk of chlamydia-associated PID and TFI overall and by race/ethnicity using our estimate of the lifetime risk of chlamydia diagnosis for the 1980–1984 birth cohort, because it most closely reflected current risk. We used estimates of the cumulative risk of each sequela from a Danish population-based study by Davies and colleagues [12] among women who ever tested positive (PID = 3.11%, TFI = 0.59%), only tested negative (PID = 2.48%, TFI = 0.51%), and were never tested (PID = 0.60%, TFI = 0.10%) for chlamydia. Based on the distribution of testing in Denmark, we calculated the risk of each sequela among women who were never diagnosed with chlamydia using a weighted average of the risk among women who only tested negative (44.2%) and women who were never tested (55.8%; Supplementary Material, equation 3). Subsequently, we estimated the cumulative risk of each sequela that was attributable to diagnosed chlamydia by subtracting the risk of the sequela that was expected in the absence of diagnosed chlamydia (Supplementary Material, equation 4). We present point estimates and ranges based on the bounds of the 95% confidence intervals for estimates in each testing group in Davies’ study. Davies’ estimate for PID incorporated only hospital episodes of PID. However, approximately 71% of diagnosed PID in the United States [13] and 76% of diagnosed PID in Denmark [14] are managed in the outpatient setting. Thus, we considered our analysis using Davies’ estimate to yield a “low” estimate of the lifetime risk of chlamydia-associated PID. To account for PID managed in the outpatient setting, we derived a “high” estimate of the lifetime risk of chlamydia-associated PID, assuming that Davies’ estimate did not capture 71% of diagnosed PID cases (Supplementary Material, equation 5).
We conducted a sensitivity analysis that incorporated data from a widely cited study of hospitalized cases of PID by Weström and colleagues in Sweden [15]. We estimated the risk of chlamydia-associated TFI among women estimated to have developed chlamydia-associated PID (using our high and low estimates), assuming that 10.8% of women with PID develop TFI (Supplementary Material, equation 6).
The University of Washington Human Subjects Division approved this study. We prepared the analysis dataset using Stata 13 (StataCorp, College Station, Texas) and created life tables in Excel 2010 (Microsoft Office, Bellevue, Washington).
RESULTS
From 1 January 1992 through 31 December 2014, there were 71352 chlamydia diagnoses reported among women aged 15–34 in King County, Washington, of whom 51464 were first diagnoses and 19888 were repeat diagnoses. Of the 51464 first diagnoses, 24567 were among non-Hispanic white women (48%), 10829 were among non-Hispanic black women (21%), 7544 were among Hispanic women (15%), and 8524 were among women of other or multiple racial groups (17%).
Cumulative Risk of Chlamydia Diagnosis
For women born between 1980 and 1984, the lifetime risk of chlamydia diagnosis was 19.8% overall and 14.0% for non-Hispanic white, 64.9% for non-Hispanic black, and 32.6% for Hispanic women. The cumulative risk of chlamydia diagnosis increased modestly over time among birth cohorts born between 1975 and 1994 (Figure 1A). For women born between 1975 and 1979, 1980 and 1984, 1985 and 1989, and 1990 and 1994, the risk of chlamydia diagnosis by age 24 was 13.9%, 15.1%, 16.1%, and 17.3%, respectively. The increase in risk across birth cohorts was primarily evident among women in their early 20s; risk during the teenage years was stable across cohorts. The trend toward higher risk of diagnosis was not evident in the youngest birth cohort (1995–1999), although they were not yet age 24.
Figure 1.
Cumulative risk of Chlamydia trachomatis diagnosis among women by age and birth cohort, overall (A) and by race/ethnicity (B-D) in King County, Washington, 1992–2014.
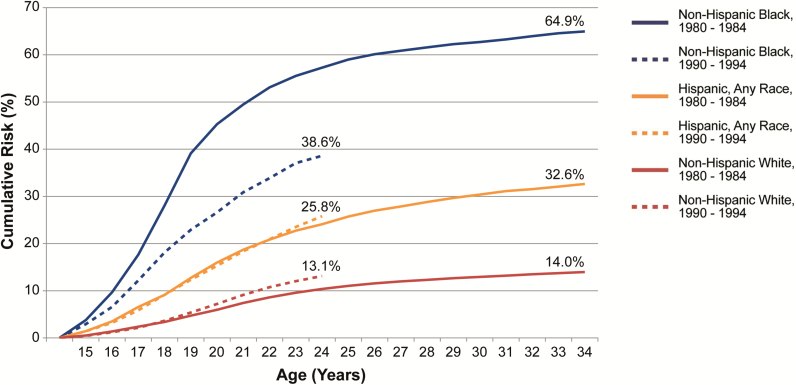
Secular trends also varied by race/ethnicity (Figures 1B–D and 2). Across all birth cohorts but the youngest, the cumulative risk of chlamydia by age 24 ranged from 10.2% to 13.1% among non-Hispanic white, 38.6% to 57.3% among non-Hispanic black, and 20.2% to 25.8% among Hispanic women. The cumulative risk increased somewhat for younger birth cohorts among non-Hispanic white and Hispanic women but declined substantially for non-Hispanic black women, among whom the cumulative risk by age 24 peaked at 57.3% in women born between 1980 and 1984 and declined to 38.6% in women born between 1990 and 1994. As a result, disparities between blacks and whites decreased for younger cohorts. Non-Hispanic black women born between 1980 and 1984 had approximately 5.5 times the risk of chlamydia by age 24 compared to non-Hispanic white women; this decreased by almost 50% to 2.9 times the risk among women born 10 years later in 1990–1994.
Figure 2.
Cumulative risk of Chlamydia trachomatis diagnosis among women born between 1980 and 1984 and between 1990 and 1994 by age and race/ethnicity in King County, Washington, 1992–2014.
Lifetime Risk of Chlamydia-associated PID and TFI
Based on Davies’ estimates of the risk of sequelae and our adjustment to account for outpatient PID, we estimated that 1.68% (low; range, 1.64–1.75) to 5.79% (high; range, 5.66–6.03) of King County women diagnosed with chlamydia develop chlamydia-associated PID and 0.31% develop chlamydia-associated TFI (range, 0.28–0.32). After applying these estimates to our estimate of the lifetime risk of chlamydia diagnosis for women born between 1980 and 1984, the low estimate of the lifetime risk of chlamydia-associated PID was 0.33% (range, 0.32–0.35) and the high estimate was 1.14% (range, 1.12–1.19; Table 1). Our primary estimate of the lifetime risk of chlamydia-associated TFI was 0.06% (range, 0.06–0.06). For non-Hispanic black women born between 1980 and 1984, the lifetime risk of chlamydia-associated TFI was 0.20% compared to 0.04% for non-Hispanic white and 0.10% for Hispanic women. Our primary estimates of the risk of TFI consistently fell within the ranges derived from our sensitivity analysis (Table 2).
Table 1.
Lifetime Risk of Chlamydia trachomatis–associated Pelvic Inflammatory Disease and Tubal Factor Infertility among Women Born between 1980 and 1984, Overall and by Race/Ethnicity, in King County, Washington
| Racial/Ethnic Group | Lifetime Risk of PID—Low,a % (Range)b | Lifetime Risk of PID—High,a % (Range)b | Lifetime Risk of Tubal Factor Infertility,a % (Range)b |
|---|---|---|---|
| Overall | 0.33 (0.32–0.35) | 1.14 (1.12–1.19) | 0.06 (0.06–0.06)c |
| Non-Hispanic White | 0.23 (0.23–0.24) | 0.81 (0.79–0.84) | 0.04 (0.04–0.04)c |
| Non-Hispanic Black | 1.09 (1.07–1.14) | 3.76 (3.68–3.92) | 0.20 (0.18–0.21) |
| Hispanic, Any Race | 0.55 (0.54–0.57) | 1.89 (1.85–1.97) | 0.10 (0.09–0.10) |
Incorporates estimates of the risk of PID and tubal factor infertility (TFI) among women in Denmark by chlamydia testing history (ever positive, always negative, never tested) [12].
Abbreviation: PID, pelvic inflammatory disease.
aLifetime risk was defined as the risk by age 34 years (includes age 34).
bThe range is based on the bounds of the 95% confidence intervals for estimates of the risk of PID and TFI among women who ever tested positive for chlamydia, always tested negative for chlamydia, and were never tested for chlamydia, respectively.
cNumbers differ in the thousandth decimal place.
Table 2.
Sensitivity Analysis of the Lifetime Risk of Chlamydia trachomatis–associated Tubal Factor Infertility among Women Born between 1980 and 1984, Overall and by Race/Ethnicity, in King County, Washington
| Racial/Ethnic Group | Lifetime Risk of TFI—Low,a % (Range)b | Lifetime Risk of TFI—High,a % (Range)b |
|---|---|---|
| Overall | 0.04 (0.04–0.04)c | 0.12 (0.12–0.13) |
| Non-Hispanic White | 0.03 (0.02–0.03) | 0.09 (0.09–0.09)c |
| Non-Hispanic Black | 0.12 (0.12–0.12)c | 0.41 (0.40–0.42) |
| Hispanic, Any Race | 0.06 (0.06–0.06)c | 0.20 (0.20–0.21) |
Incorporates historically used estimates of the proportion of women hospitalized with pelvic inflammatory disease who develop TFI [15].
Abbreviation: TFI, tubal factor infertility.
aLifetime risk was defined as the risk by age 34 years (includes age 34).
bThe ranges are based on the range of our low and high estimates of the lifetime risk of chlamydia-associated pelvic inflammatory disease.
cNumbers differ in the thousandth decimal place.
DISCUSSION
In King County, Washington, approximately 1 in 5 women are diagnosed with at least 1 chlamydial infection in their lifetime. There are substantial racial/ethnic disparities in the lifetime risk of chlamydia, with more than 60% of non-Hispanic black women diagnosed with at least 1 infection in the most affected birth cohorts, a risk that is almost 5-fold higher than that of non-Hispanic white women. Overall, between 1 in 88 and 1 in 300 women develops chlamydia-associated PID. Moreover, approximately 1 in 500 non-Hispanic black women experience chlamydia-associated TFI compared to 1 in 2500 non-Hispanic white women. Our study highlights racial/ethnic disparities in chlamydia risk and the need for innovative control measures to decrease the associated morbidity.
Our results are consistent with findings from a small number of studies on the cumulative risk of chlamydia and racial/ethnic disparities in the incidence of chlamydia in the United States. We build on these findings by showing how disparities in chlamydia risk have changed over time and may result in disparities in major morbidity. While our estimate of the overall lifetime risk of chlamydia diagnosis is almost identical to that from a study of women in Florida (20%), we identified a higher lifetime risk among black women in King County compared to black women in Florida (50% vs 36% for roughly comparable birth cohorts) [6]. Importantly, comparisons across geographic settings reflect both true differences in the risk of chlamydial infection and differences in testing patterns. For example, yearly screening coverage in Washington is roughly 50% [8, 16]. Had screening coverage overall or among black women been higher, more infections would have been detected, leading to a higher risk of diagnosis (assuming that screening does not affect the risk of infection at the population level). However, our estimates of the relative lifetime risk of chlamydia diagnosis among black and Hispanic women compared to white women in King County (4.6 and 2.3 times the risk, respectively) are generally consistent with a nationally representative study of chlamydia prevalence in young adults [17] and chlamydia incidence estimates from 2 randomized, controlled trials among young adults who attended sexually transmitted diseases clinics [18, 19], which were not subject to bias in testing patterns. Of note, a study of English women aged 30–34 (born between 1976 and 1982) found that 34% had antibody to C. trachomatis [20], highlighting that our estimate (20%), which is based on diagnoses rather than antibodies indicative of prior infection, underestimates true lifetime risk of infection. Although our estimates of the lifetime risk of chlamydia-associated PID and TFI are imprecise given the variety of assumptions required, they do provide a general estimate of the magnitude of these important conditions and demonstrate how racial/ethnic disparities in risk of chlamydia translate into disparities in major reproductive tract sequelae. Modeling studies from the United Kingdom estimated that by age 25–34, approximately 6.8% of women have had at least 1 episode of chlamydia-associated PID (includes undiagnosed PID) [21] and 0.1%–0.25% of women have experienced chlamydia-associated TFI [22]. Our estimates of the risk of chlamydia-associated PID and TFI are lower (PID, 0.32%–1.19%; TFI, 0.04%–0.13%), in part, because they do not include the risk that results from undiagnosed chlamydial infections, which likely carry the highest risk of sequelae [23]. Thus, our estimates are best considered lower-bound estimates of the risk of chlamydia-associated PID and TFI.
Although black women had the highest risk of chlamydia diagnosis in our population, the risk among black women declined dramatically over the study period, even as the risk increased in other racial/ethnic groups. A number of factors may help explain these trends. First, national chlamydial screening programs, including the CDC Infertility Prevention Project (IPP) and screening undertaken in response to CDC and USPSTF recommendations, may have disproportionately benefited the population with the highest prevalence of infection. IPP, in particular, focused on low-income and minority women [24], and Medicaid data suggest that black women are more likely to be tested for chlamydia than white women [25, 26]. Our observation is also consistent with findings from mathematical models that predict that screening programs would have their greatest impact on age groups with the highest prevalence of infection [27, 28]. Second, secular changes in the age of sexual debut may have contributed to high but decreasing risk of chlamydia diagnosis among black women. Although, on average, black women start having sex earlier than white and Hispanic women [29], from 1991 through 2011, the percentage of high school students who have ever had sex decreased more dramatically for blacks than whites (26% vs 11% decrease) [30], perhaps contributing to the disparate trends observed. Third, the observed trends could reflect changes in screening practices. We estimated the risk of chlamydia diagnosis not infection. If levels of screening declined among black women or disproportionately increased among non-blacks, it could result in the disparate trends observed.
Though the lifetime risk of chlamydia-associated TFI overall is relatively low, the number of women impacted and societal costs are not trivial. For example, if the risk of chlamydia-associated TFI were similar across the United States and remained stable in the coming decades, of approximately 76165033 women and girls who are currently aged <35 in the United States, about 45700 would experience chlamydia-associated TFI, with 41% of cases occurring among the 12% of women who are black. These racial/ethnic disparities in chlamydia-associated TFI are particularly worrisome given women’s limited access to assistive reproductive technology services, which are not typically covered by health insurance, and some evidence that suggests that minority women have worse outcomes even when such services are sought [31–33].
There are important limitations to our analysis. First, undiagnosed chlamydial infections were not included, leading to an underestimate of the true lifetime risk of chlamydial infection. The risk of sequelae is likely substantially higher for untreated infections, so our estimates represent a lower bound for the risk of chlamydia-associated PID and TFI. Second, to minimize bias due to missing race/ethnicity data, we distributed these cases between race/ethnicity categories based on the available data. However, our approach assumed that race/ethnicity was missing at random given the year and woman’s age group. The extent to which that assumption holds is unknown, but we did not find evidence that missing race/ethnicity was related to other characteristics. Third, for our estimates of the risk of chlamydia-associated PID and TFI, we used data from Denmark, and the data may not reflect risk in King County. However, the Denmark study was population based, incorporated similar years of data, included diagnosed infections, and reported the same risk of chlamydia diagnosis (20%). In addition, the Denmark study included only hospital episodes of PID. We aimed to account for this by deriving low- and high-end estimates. Nonetheless, substantial uncertainty remains; our estimates are imprecise. Fourth, we assumed the risks of chlamydia-associated PID and TFI were stable from 1992 through 2014; the risk of these sequelae may have changed with more widespread testing and treatment. Fifth, our estimates ignore the impact of repeat infections; women with multiple chlamydial infections are probably at higher risk of sequelae, meaning that our estimates likely underestimate the true risk of PID and TFI. Finally, the extent to which observed changes in chlamydia risk reflect true changes in incidence vs changes in testing patterns is uncertain. Despite these limitations, we used surveillance data that allowed us to examine population-based trends that spanned more than 20 years.
In conclusion, our analysis provides some evidence that while the lifetime risk of chlamydia diagnosis appears to be increasing in non-Hispanic whites, screening initiatives that prioritize black women in King County have been successful. Despite this positive trend, racial/ethnic disparities in the lifetime risk of chlamydia diagnosis persist and contribute to disparities in serious reproductive morbidity. New and innovative prevention tools are needed, including strategies to increase testing and rescreening.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: 2016 National STD Prevention Conference. Atlanta, GA, 20–23 September 2016. Abstract 6C1.
Author contributions. Authors contributed to the manuscript in the following manner. Study concept: C. M. K., M. R. G. Study design: L. C. C., C. M. K., M. R. G. Analysis and interpretation of data: L. C. C., C. M. K., D. A. K., J. C. D., L. E. M., M. R. G. Drafting of the manuscript: L. C. C. Critical revision of the manuscript: L. C. C., C. M. K., D. A. K., J. C. D., L. E. M., M. R. G.
Acknowledgments. The authors thank the Public Health–Seattle and King county disease intervention specialists. We also gratefully acknowledge Dr Katy Turner for her advice on estimates of the risk of chlamydia-associated sequelae and Dr Berit Andersen for information on trends in chlamydia testing in Denmark
Financial support. This work was funded by Public Health–Seattle and King County; the National Institutes of Health (NIH; grant TL1 TR002318 trainee support to L. C. C.; grant U19 AI113173, T32 AI007140 trainee support to C. M. K.); and the University of Washington Center for AIDS Research, an NIH-funded program (grant P30 AI027757), which is supported by the following NIH institutes and centers: National Institute of Allergy and Infectious Diseases; National Cancer Institute; National Institutes of Mental Health; National Institute on Drug Abuse; National Institute of Child Health and Human Development; National Heart, Lung, and Blood Institute; and National Institute on Aging.
Potential conflicts of interest. J. C. D. has conducted studies unrelated to this work funded by grants to the University of Washington from Hologic, Curatek, Quidel, ELITech, and Genentech. L. E. M. has received donations of test kits and reagents from Hologic. All other authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2015. Atlanta, GA: CDC, 2016. [Google Scholar]
- 2. Haggerty CL, Gottlieb SL, Taylor BD, Low N, Xu F, Ness RB. Risk of sequelae after Chlamydia trachomatis genital infection in women. J Infect Dis 2010; 201(Suppl 2):S134–55. [DOI] [PubMed] [Google Scholar]
- 3. Stamm WE. Chlamydia trachomatis infections in the adult. In: Holmes KK, Sparling PF, Stamm WE. et al. , eds. Sexually transmitted diseases. 4th ed. New York City, US: McGraw Hill Medical; 2008:575–94. [Google Scholar]
- 4. Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med 2003, 36:502–9. [DOI] [PubMed] [Google Scholar]
- 5. US Preventive Services Task Force. Final recommendation statement, gonorrhea and chlamydia: screening. Rockville, MD: USPSTF, 2014. [Google Scholar]
- 6. Peterman TA, Newman DR, Torrone E et al. Cumulative risk of chlamydial infection among young women in Florida, 2000–2011. J Adolesc Health 2014; 55:241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Britton TF, Delisle S, Fine D. STDs and family planning clinics: a regional program for chlamydia control that works. Am J Gynecol Health 1992; 6:80–7. [PubMed] [Google Scholar]
- 8. Broad JM, Manhart LE, Kerani RP, Scholes D, Hughes JP, Golden MR. Chlamydia screening coverage estimates derived using healthcare effectiveness data and information system procedures and indirect estimation vary substantially. Sex Transm Dis 2013; 40:292–7. [DOI] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention. Link Plus version 2.0 [probabilistic record linkage software]. Atlanta, GA: CDC, 2006. [Google Scholar]
- 10. Washington State Department of Health Community Health Assessment Tool. Washington State Office of Financial Management Forecasting Division: Single year intercensal estimates, revised 2001–2009, 2011–2016. Olympia, WA: CHAT, 2016. [Google Scholar]
- 11. Hosmer DW, Lemeshow S, May S. Descriptive methods for survival data. In: Balding DJ, Cressie NAC, Fitzmaurice GM. et al. , eds. Applied survival analysis: regression modeling of time-to-event data. 2nd ed. Hoboken, NJ: John Wiley & Sons, Inc; 2008. [Google Scholar]
- 12. Davies B, Turner KME, Frølund M et al. ; Danish Chlamydia Study Group Risk of reproductive complications following chlamydia testing: a population-based retrospective cohort study in Denmark. Lancet Infect Dis 2016; 16:1057–64. [DOI] [PubMed] [Google Scholar]
- 13. Moore MS, Golden MR, Scholes D et al. Assessing trends in chlamydia positivity and gonorrhea incidence and their associations with the incidence of pelvic inflammatory disease and ectopic pregnancy in Washington State, 1988–2010. Sex Transm Dis 2016; 43:2–8. [DOI] [PubMed] [Google Scholar]
- 14. Andersen B, van Valkengoed I, Sokolowski I, Møller JK, Østergaard L, Olesen F. Impact of intensified testing for urogenital Chlamydia trachomatis infections: a randomised study with 9-year follow-up. Sex Transm Infect 2011; 87:156–61. [DOI] [PubMed] [Google Scholar]
- 15. Weström L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis 1992; 19:185–92. [PubMed] [Google Scholar]
- 16. Khosropour CM, Broad JM, Scholes D, Saint-Johnson J, Manhart LE, Golden MR. Estimating chlamydia screening coverage: a comparison of self-report and health care effectiveness data and information set measures. Sex Transm Dis 2014; 41:665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miller WC, Ford CA, Morris M et al. Prevalence of chlamydial and gonococcal infections among young adults in the United States. JAMA 2004; 291:2229–36. [DOI] [PubMed] [Google Scholar]
- 18. Bolu OO, Lindsey C, Kamb ML et al. ; Project RESPECT Study Group Is HIV/sexually transmitted disease prevention counseling effective among vulnerable populations?: A subset analysis of data collected for a randomized, controlled trial evaluating counseling efficacy (Project RESPECT). Sex Transm Dis 2004; 31:469–74. [DOI] [PubMed] [Google Scholar]
- 19. Peterman TA, Tian LH, Metcalf CA et al. ; RESPECT-2 Study Group High incidence of new sexually transmitted infections in the year following a sexually transmitted infection: a case for rescreening. Ann Intern Med 2006; 145:564–72. [DOI] [PubMed] [Google Scholar]
- 20. Woodhall SC, Wills GS, Horner PJ et al. Chlamydia trachomatis Pgp3 antibody population seroprevalence before and during an era of widespread opportunistic chlamydia screening in England (1994–2012). PLoS One 2017; 12:e0152810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Price MJ, Ades AE, Soldan K et al. Chapter 8: Cumulative incidence of pelvic inflammatory disease: results from a Markov model. In: A natural history of Chlamydia trachomatis infection in women: a multi-parameter evidence synthesis. Health Technol Assess, 2016; 20:77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Price MJ, Ades AE, Soldan K et al. Chapter 10: Pelvic inflammatory disease and tubal factor infertility. In: A natural history of Chlamydia trachomatis infection in women: a multi-parameter evidence synthesis. Health Technol Assess, 2016; 20:111–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oakeshott P, Kerry S, Aghaizu A et al. Randomised controlled trial of screening for Chlamydia trachomatis to prevent pelvic inflammatory disease: the POPI (Prevention of Pelvic Infection) trial. BMJ 2010; 340:c1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Centers for Disease Control and Prevention. Projects & Initiatives Archive: Infertility Prevention Project Available at: http://www.cdc.gov/STD/infertility/ipp-archive.htm. Accessed 6 April 2016.
- 25. Christiansen-Lindquist L, Tao G, Hoover K et al. Chlamydia screening of young sexually active, Medicaid-insured women by race and ethnicity, 2002–2005. Sex Transm Dis 2009; 36:642–6. [DOI] [PubMed] [Google Scholar]
- 26. Patel CG, Chesson HW, Tao G. Racial differences in receipt of chlamydia testing among Medicaid-insured women in 2013. Sex Transm Dis 2016; 43:147–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Turner KM, Adams EJ, Lamontagne DS, Emmett L, Baster K, Edmunds WJ. Modelling the effectiveness of chlamydia screening in England. Sex Transm Infect 2006; 82:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Regan DG, Wilson DP, Hocking JS. Coverage is the key for effective screening of Chlamydia trachomatis in Australia. J Infect Dis 2008; 198:349–58. [DOI] [PubMed] [Google Scholar]
- 29. Cavazos-Rehg PA, Krauss MJ, Spitznagel EL et al. Age of sexual debut among US adolescents. Contraception 2009; 80:158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Centers for Disease Control and Prevention. Trends in HIV-related risk behaviors among high school students—United States, 1991–2011. Atlanta, GA: CDC, 2012. [Google Scholar]
- 31. Seifer DB, Zackula R, Grainger DA et al. Trends of racial disparities in assisted reproductive technology outcomes in black women compared with white women: Society for Assisted Reproductive Technology 1999 and 2000 vs. 2004–2006. Fertil Steril 2010; 93:626–35. [DOI] [PubMed] [Google Scholar]
- 32. Huddleston HG, Cedars MI, Sohn SH et al. Racial and ethnic disparities in reproductive endocrinology and infertility. Am J Obstet Gynecol 2010; 202:413–19. [DOI] [PubMed] [Google Scholar]
- 33. Armstrong A, Plowden TC. Ethnicity and assisted reproductive technologies. Clin Pract (Lond) 2012; 9:651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.