Abstract
Background
Current antidepressants in clinical use always take weeks or even months to exert full therapeutic effects, and sometimes have serious side effects. Thus, it is very necessary to develop novel antidepressants with better efficacy and fewer adverse effects. The present study focused on investigating the antidepressant potential of matrine and its possible mechanisms of action.
Methods
The forced swim test, tail suspension test, and chronic unpredictable mild stress model of depression were used to reveal the antidepressant-like effects of matrine on mice. Western blotting, immunohistochemistry, and lentivirus were further used together to explore the antidepressant mechanism of matrine.
Results
It was found that matrine exhibited significant antidepressant actions in the forced swim test and tail suspension test without affecting the locomotor activity of mice. Chronic matrine administration fully reversed the chronic unpredictable mild stress-induced depressive-like symptoms in forced swim test, tail suspension test, and sucrose preference test. After that, western blotting analysis revealed that chronic matrine treatment restored the decreasing effects of chronic unpredictable mild stress on the PI3K/Akt/mammalian target of rapamycin signaling in hippocampus, but not prefrontal cortex. Furthermore, pharmacological and genetic blockade of the PI3K/Akt/mammalian target of rapamycin signaling in hippocampus abolished the antidepressant actions of matrine on mice.
Conclusions
Taken together, matrine produces antidepressant-like effects on mice via promoting the hippocampal PI3K/Akt/ mammalian target of rapamycin signaling.
Keywords: chronic unpredictable mild stress, depression, hippocampus, matrine, mTOR
Significance Statement
This study shows that matrine possess antidepressant-like effects that are mediated by activating the PI3K/Akt/mTOR signaling in hippocampus. Its significance is as follows: (1) Extending the knowledge of matrine’s pharmacological effects; (2) providing a potential antidepressant with better efficacy or fewer side effects; (3) further proving that the central mTOR signaling is a good target for developing novel antidepressants.
Introduction
Depression is one of the leading public health problems in the world, and antidepressants are among the most commonly prescribed medications (Bowden, 2005; Shelton, 2007; Krishnan et al., 2008). Currently, most antidepressants used in clinical practice elevate the level of monoaminergic neurotransmitters in brain, particularly serotonin and norepinephrine (Berton et al., 2006; Hirschfeld, 2012). However, it always takes weeks or even months for these antidepressant drugs to exert their full therapeutic effects, and many patients are resistant to them (Berton et al., 2006; Hirschfeld, 2012). Therefore, it is necessary to develop novel antidepressants based on different pharmacological targets.
Mammalian target of rapamycin (mTOR) is a large serine/threonine kinase that regulates initiation of protein translation (Abe et al., 2010). Activation of mTOR by phosphatidylinositol-3 kinase (PI3K)/protein kinase B (AKT) results in its phosphorylation at serine 2448, thereby promoting its downstream molecules, p70 ribosomal protein S6 kinase (p70S6K) and eukaryotic initiation factor 4E-binding protein 1 (4E-BP-1). Then, p70S6K and 4E-BP-1 promote initiation of protein translation for synaptic protein synthesis (Hay et al., 2004; Dann et al., 2007; Hashimoto, 2011). Recent studies support the hypothesis that major depressive disorder may be a consequence of deficiency in the mTOR signaling (Hashimoto, 2011; Ludka et al., 2016). It has also been demonstrated that upregulation of the mTOR signaling in prefrontal cortex (PFC) and hippocampus leads to rapid and consistent antidepressant-like effects (Li et al., 2010; Hashimoto, 2011; Zhou et al., 2014).
Matrine, a quinolizidine alkaloid compound extracted from the root of Sophorae flavescens, is known for its various pharmacological activities. Matrine has been shown to have antiinflammatory, antiallergic, antivirus, antitumor, and cardiovascular protective effects (Li et al., 2010; Fu et al., 2014; Liu et al., 2014; Kan et al., 2015; Wu et al., 2017). Recently, more and more matrine-induced pharmacologic effects on the central nervous system have been reported. For example, Gong et al. reported that matrine had neuroprotective effects on a mouse model of vincristine-induced neuropathic pain (Gong et al., 2016). Zhang et al. showed that matrine improved cognitive impairments in a rat model of Alzheimer’s disease (Zhang et al., 2015). Meng et al. showed that matrine also had neuroprotective effects against MPTP-induced Parkinson’s disease (Meng et al., 2017). Here, we noticed that in 2017, matrine had been demonstrated to have promoting effects on the PI3K/Akt/mTOR signaling in corpus callosum (Liu et al., 2017). Thus, we assume that matrine may possess antidepressant-like effects via activating mTOR. In this study, we investigated this assumption using various methods, including the forced swimming test (FST), tail suspension test (TST), and chronic unpredictable mild stress (CUMS) model of depression.
Materials and Methods
Animals
Adult male C57BL/6J mice (8 weeks old) were obtained from the Experimental Animal Center of Medical College, Nantong University. Before use, the mice were housed under standard conditions (12-hour-light/-dark cycle; lights on from 7:00 am to 7:00 pm; 2°C ±1°C ambient temperature; 55%±10% relative humidity) for 1 week with free access to food and water. Behavioral experiments were carried out during the light phase. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (8th edition, Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences, Washington DC).
Materials
Matrine (purity >98%) and fluoxetine were bought from Sigma and dissolved in 1% DMSO in 0.9% saline. LY294002 was purchased from Tocris, rapamycin was purchased from Target Mol, and they were dissolved in 1% DMSO in ACSF. The doses of matrine (15, 30, 60, and 120 mg/kg), fluoxetine (20 mg/kg), LY294002 (10 nmol/site), and rapamycin (0.2 nmol/site) were chosen based on previous reports (Jiang et al., 2015; Gong et al., 2016; Ludka et al., 2016; Jiang et al., 2017). Matrine and fluoxetine were i.p. injected in a volume of 10 mL/kg. LY294002 and rapamycin were intracerebroventricularly (i.c.v.) infused.
FST
FST was carried out as previously described (Jiang et al., 2015; Jiang et al., 2017). Briefly, each mouse was individually placed in a glass cylinder (15 cm diameter, 25 cm height) filled with 10 cm of 25°C±1°C water and forced to swim for 6 minutes. The immobility duration of the last 4 minutes was recorded by an investigator blind to the study. Water was exchanged between 2 trails. The time during which each mouse was floating in water without struggling and only making minor movements to keep its nose above the water was regarded as the immobility time.
TST
TST was carried out as previously described (Jiang et al., 2015, 2017). Briefly, each mouse was suspended on the shelf, which was 70 cm above the ground, for 6 minutes. A tape was placed at about 1.5 cm away from the tip of tail. The immobility time during which each mouse was completely motionless was recorded for the last 4 minutes. The observer was unaware of mice grouping.
Locomotor Activity Test
As reported (Jiang et al., 2015, 2017), briefly, each mouse was individually placed in the middle of a wooden box (50×50×40 cm) that consisted of 25 equal squares on the floor (10×10 cm). The number of squares each mouse crossed was recorded for 5 minutes by an observer unaware of mice grouping. The apparatus was cleaned after each test.
CUMS
In this procedure, mice were subjected to a random sequence of unpredictable and mild stressors for 8 weeks. The stressors are listed as follows: (1) food or water deprivation for 24 hours; (2) damp sawdust for 12 hours; (3) restraint for 2 hours; (4) cage shaking for 15 minutes; (5) inversion of light/dark cycle; (6) 45°C cage tilting in empty cage for 12 hours; (7) tail pinch for 5 minutes. Control mice were left undisturbed in home cages in a separate room. Repeated administration of vehicle/fluoxetine/matrine/inhibitors was given daily during the last 2 weeks. After CUMS and drug treatments, FST, TST, and the sucrose preference test were performed.
Sucrose Preference Test
For this test, mice were first adapted to 1% sucrose solution for 48 hours, then deprived of water and food for 24 hours, and finally given 2 preweighed bottles containing 1% sucrose solution and tap water, respectively. All the mice were housed individually during this test, and the index of sucrose preference was calculated as a percentage of the volume of sucrose consumed/total volume of liquid consumed.
Intracerebroventricular (i.c.v.) Injections
As reported (Jiang et al., 2016, 2017), each mouse was deeply anesthetized with 0.5% pentobarbital sodium (50 mg/kg, i.p.) and placed in a stereotaxic device (Stoelting). Scalp skin was shaved with clippers and disinfected using iodine. After opening the scalp skin and exposing the skull, infusion cannulas were implanted into the left lateral brain ventricle (AP=−0.2 mm, ML=+1.0 mm, DV=+2.3 mm) and cemented. The mice were allowed to recover for 3 d. Osmotic minipumps were used to deliver 0.3 μL/min of LY294002 or rapamycin or 1% DMSO in ACSF (final volume, 3 μL/mouse).
Lentivirus Microinfusion
This was done according to previous reports (Jiang et al., 2014). Briefly, each mouse was deeply anesthetized with 0.5% pentobarbital sodium (50 mg/kg, i.p.) and placed in a stereotaxic device. After exposing the skull, 5 µL microsyringes (Hamilton) were inserted into hippocampus bilaterally to deliver lentivirus. The location is as follows: AP=-2.3 mm, ML=±1.6 mm, DV=+1.9 mm. Infusion of lentivirus was performed bilaterally at a rate of 0.3 µL/min (1.5 µL/side). The mice were allowed to recover for 3 d.
LV-mTOR-shRNA-EGFP and LV-Scrambled-shRNA-EGFP used in this study were produced and provided by Genechem Co., Ltd. The titers were adjusted to 6×109 TU/mL. The sequences for mTOR-shRNA and Scrambled-shRNA were 5’-GGCCTATGGTCGAGATTTA-3’ and 5’-TTCTCCGAACGTGT CACGT-3’, respectively.
Western Blotting
The hippocampus and PFC tissues were dissected and immediately homogenized in NP-40 lysis buffer containing protease inhibitor cocktail for 30 minutes. Then the homogenates were centrifuged at 12000×g for 30 minutes at 4°C. The supernatants were collected on ice, and protein concentrations were assayed using the BCA method. Then 30 µg of protein samples was separated by SDS-PAGE gels. After that, proteins were transferred to PVDF membranes. The transferred membranes were blocked in 5% bovine serum albumin and then incubated with different primary antibodies overnight at 4°C. Primary antibodies against mTOR (1:500; Abcam), p-mTOR (Ser2448, 1:500; Abcam), 4E-BP-1 (1:500; Cell Signaling), p-4E-BP-1 (Thr37/46, 1:500; Cell Signaling), p70S6K (1:1000; Cell Signaling), p-p70S6K (Thr389, 1:1000; Cell Signaling), AKT(1:500; Cell Signaling), p-AKT (Ser473, 1:500; Cell Signaling), and β-actin (1:5000; Immunoway) were used. After incubated with goat anti-rabbit or goat anti-mouse HRP-conjugated secondary antibody for 2 hours at room temperature, the blots were detected by enhanced chemiluminescence.
Immunohistochemistry
To analyze hippocampal neurogenesis, mice were anesthetized using 0.5% pentobarbital sodium (50 mg/kg, i.p.) and then transcardially perfused with normal saline followed by 4% paraformaldehyde in 0.01 M phosphate buffer saline (PBS). The brains were postfixed in 4% paraformaldehyde overnight and dehydrated in 30% sucrose solution for 2 days. Serial sections of 25 μm were cut throughout hippocampi using a freezing microtome (Leica) and preserved in 0.01 M PBS. After that, the sections were permeabilized with 0.3% Triton X-100 in PBS (30 minutes, first) and 3% bovine serum albumin in PBS (30 minutes, second) at room temperature. Then the sections were incubated with rat polyclonal anti-doublecortin (DCX, 1:100; Cell Signaling) primary antibody under 4°C overnight. The sections were further incubated in fluorescein isothiocyanate-labeled horse anti-rabbit IgG (1:50; Thermo Fisher) for 2 hours at room temperature. After washing, the sections were coverslipped and observed using a confocal fluorescence microscope (Leica). Examination of the DCX-positive (DCX+) cells was confined to dentate gyrus (DG), especially in the granule cell layer (GCL), including the subgranular zone of hippocampus that was defined as a 2-cell body-wide zone along the border between GCL and hilus. Quantifications of the DCX+ cells were respectively conducted from 1-in-6 series of hippocampal sections spaced at 150 μm and spanning the rostrocaudal extent of DG bilaterally. Every DCX+ cell within the GCL and subgranular zone was counted.
Statistical Analysis
All data were analyzed using SPSS 13.0 software (SPSS Inc.). Multiple group comparisons were performed using 1-way or 2-way ANOVA, as appropriate. P<.05 was considered statistically significant.
RESULTS
Effects of Matrine on the Immobility of Mice in FST and TST
As a first step of this study, FST and TST were used. Figure 1A and B showed the effects of matrine on the immobility duration of mice in FST and TST, respectively. It was found that both matrine and fluoxetine significantly reduced the immobility of mice in FST and TST compared with the control mice (n=10). For the FST data, ANOVA indicated a significant main effect of drug treatment [F(5, 54)=28.592, P<.01]. For the TST data, ANOVA also revealed a significant main effect of drug treatment [F(5, 54)=33.017, P<.01]. Detailed analysis showed that compared with the control mice, 15 mg/kg of matrine treatment moderately decreased the immobility, 30 mg/kg and 60 mg/kg of matrine treatment significantly decreased the immobility, while 120 mg/kg matrine produced a similar effect to 60 mg/kg matrine. Therefore, 30 mg/kg and 60 mg/kg were selected as the doses for matrine in the following studies. Besides, Figure 1C showed that matrine treatment did not affect the locomotor activity of mice (n=10), and ANOVA showed no significant effects of drug treatment [F(3, 36)=0.715, P=.386]. Together, matrine may have antidepressant-like effects on mice.
Figure 1.
Antidepressant-like effects of matrine in forced swim test (FST) and tail suspension test (TST). Naive mice were i.p. injected with a single dose of vehicle, fluoxetine (20 mg/kg), or matrine (15, 30, 60, or 120 mg/kg). The behavioral tests were conducted 30 minutes after the injection. Different groups of mice were used for these tests. (A) The matrine-treated mice had significantly less immobility than the vehicle-treated mice in FST. (B) The matrine-treated mice also showed significantly less immobility than the vehicle-treated mice in TST. (C) All groups of mice displayed similar locomotor activity in the locomotor activity test. Data are expressed as means±SEM (n=10); *P<.05, **P<.01; n.s., no significance. Comparisons were made by 1-way ANOVA followed by posthoc LSD test.
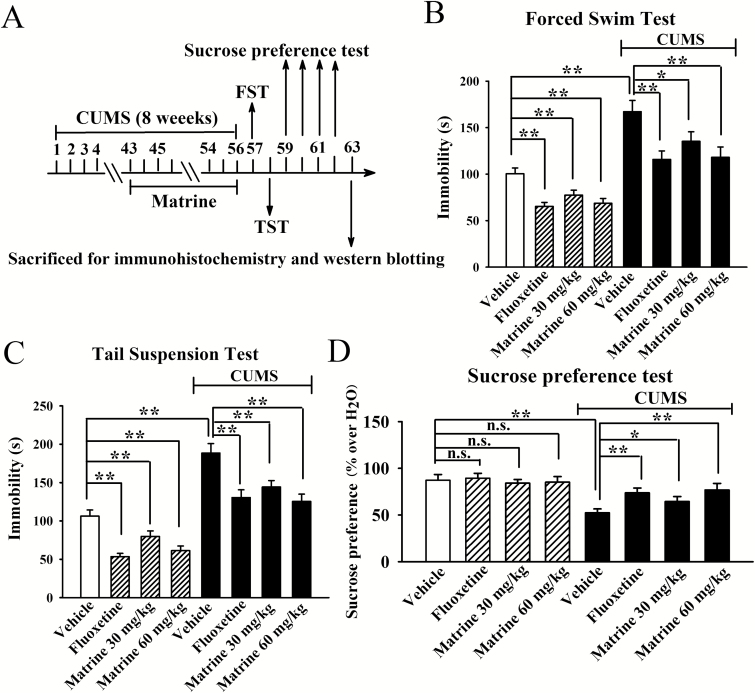
Effects of Matrine on the Depressive-Like Behaviors of Mice in the CUMS Model of Depression
Next, the CUMS model of depression was used to assay the antidepressant effects of matrine, with FST, TST, and the sucrose preference test used to evaluate the CUMS-induced depressive-like behaviors (Reid et al., 1997; Shields, 2006). Figure 2B illustrates the FST data. It was found that CUMS significantly enhanced the immobility of mice, while matrine administration fully restored this change (n=10). ANOVA revealed a significant interaction [F(3, 72)=33.097, P<.01] with significant effects of CUMS [F(1, 72)=43.767, P<.01] and drug treatment [F(3, 72)=26.143, P<.01]. Figure 2C illustrates the TST data, and similarly, matrine treatment significantly reversed the promoting effects of CUMS on the immobility of mice in TST (n=10). ANOVA revealed a significant interaction [F(3, 72)=42.045, P<.01] with significant effects of CUMS [F(1, 72)=54.134, P<.01] and drug treatment [F(3, 72)=38.578, P<.01]. Figure 2D illustrates the sucrose preference data. Also, we found that chronic injection of matrine protected against the CUMS-induced anhedonia behavior (n=10). ANOVA revealed a significant interaction [F(3, 72)=39.606, P<.01] with significant effects of CUMS [F(1, 72)=51.552, P<.01] and drug treatment [F(3, 72)=29.506, P<.01]. Thus, matrine indeed possessed antidepressant-like effects on mice.
Figure 2.
Antidepressant-like effects of matrine on the chronic unpredictable mild stress (CUMS) model of depression. Mice were exposed to 8 weeks of CUMS and received daily injection of vehicle, fluoxetine (20 mg/kg), or matrine (30 or 60 mg/kg) during the last 2 weeks. The behavioral tests were then conducted. (A) Schematic timeline of experimental procedures. (B) CUMS + matrine mice spent significantly less immobility than that of CUMS + vehicle mice in forced swim test (FST). (C) CUMS + matrine mice spent significantly less immobility than that of CUMS + vehicle mice in tail suspension test (TST). (D) CUMS + matrine mice displayed significantly higher sucrose preference than that of CUMS + vehicle mice. Data are expressed as means±SEM (n=10); *P<.05, **P<.01; n.s., no significance. Comparisons were made by 2-way ANOVA followed by posthoc Bonferroni’s test.
Depression is also accompanied with decreased hippocampal neurogenesis (Santarelli et al., 2003). Here, we examined whether matrine prevented the CUMS-induced effects on neurogenesis using DCX immuohistochemistry. It is known that DCX is a microtubule-associated protein that serves as a marker of neurogenesis because of its transient expression in newborn neurons (Brown et al., 2003). As shown in Figure 3, chronic matrine administration fully restored the decreasing effects of CUMS on the number of DCX+ cells in DG (n=5). ANOVA revealed a significant interaction [F(3, 17)=55.397, P<.01] with significant effects of CUMS [F(1, 17)=74.356, P<.01] and drug treatment [F(3, 17)=41.479, P<.01].
Figure 3.
Antidepressant-like effects of matrine on hippocampal neurogenesis in chronic unpredictable mild stress (CUMS)-stressed mice. (A) Representative confocal microscopic images showed the localization of doublecortin (DCX) in the dentate gyrus (DG) region. Scale bar is 150 μm for representative images and 75 μm for enlarged images, respectively. (B) Statistical analysis revealed the enhancing effects of matrine on the amount of DCX-positive cells in DG of CUMS-stressed mice. Data are expressed as means±SEM (n=5), **P<.01. Comparisons were made by 2-way ANOVA followed by posthoc Bonferroni’s test.
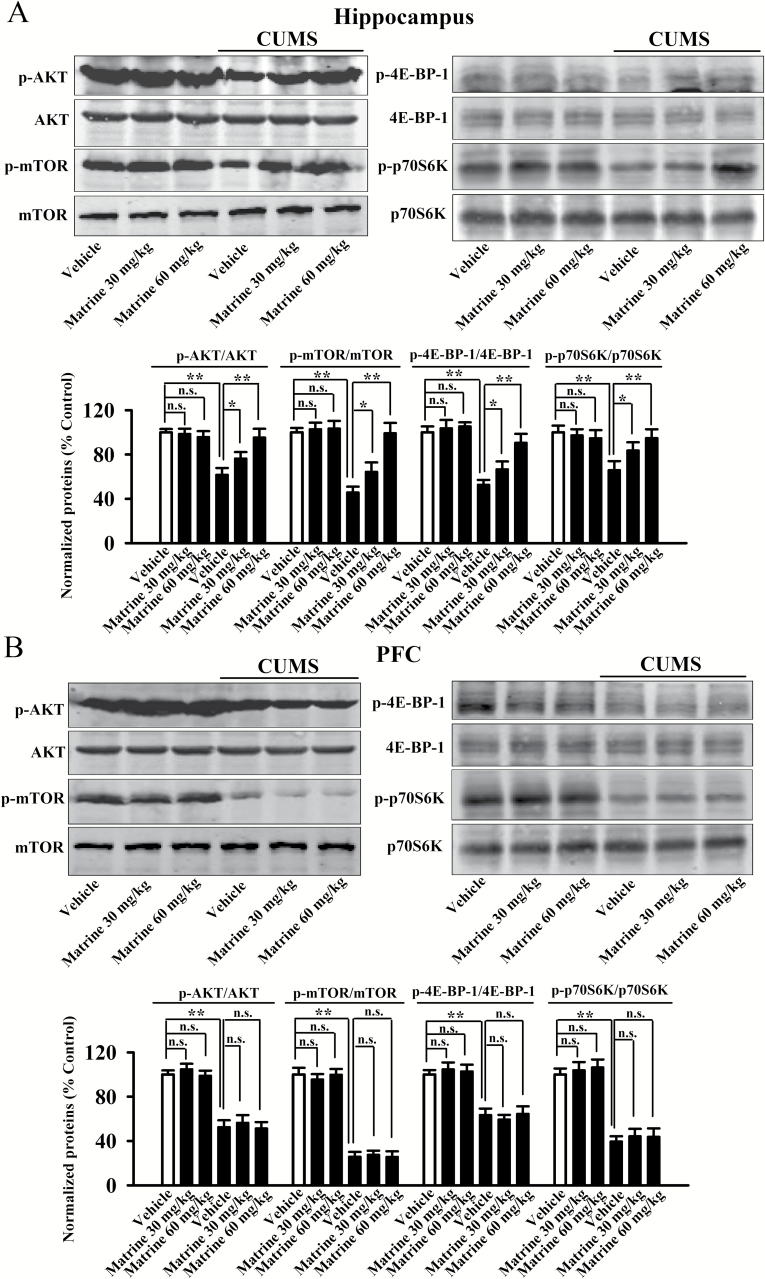
Effects of Matrine on the Central PI3K/AKT/mTOR Signaling in CUMS-Stressed Mice
After the behavioral tests, western blotting experiments were performed to detect the effects of matrine on levels of p-AKT (Ser473), AKT, p-mTOR (Ser2448), mTOR, p-4E-BP-1 (Thr37/46), 4E-BP-1, p-p70S6K (Thr389), p70S6K, and β-actin in both hippocampus and PFC. Figure 4B illustrates the PFC data. Although CUMS significantly decreased the expression of p-AKT, p-mTOR, p-4E-BP-1, and p-p70S6K in PFC, chronic matrine administration did not restore these changes (n=5). Figure 4A illustrates the hippocampus data, and we can see that chronic matrine administration fully reversed the decreased p-AKT, p-mTOR, p-4E-BP-1, and p-p70S6K expression in the hippocampus of CUMS-stressed mice (n=5). For the p-AKT data, ANOVA indicated a significant interaction [F(2, 24)=35.329, P<.01] with significant effects of CUMS [F(1, 24)=47.856, P<.01] and drug treatment [F(2, 24)=23.084, P<.01]. For the p-mTOR data, ANOVA indicated a significant interaction [F(2, 24)=32.592, P<.01] with significant effects of CUMS [F(1, 24)=44.648, P<.01] and drug treatment [F(2, 24)=23.628, P<.01]. For the p-4E-BP-1 data, ANOVA indicated a significant interaction [F(2, 24)=40.042, P<.01] with significant effects of CUMS [F(1, 24)=56.278, P<.01] and drug treatment [F(2, 24)=33.712, P<.01]. For the p-p70S6K data, ANOVA indicated a significant interaction [F(2, 24)=48.135, P<.01] with significant effects of CUMS [F(1, 24)=62.592, P<.01] and drug treatment [F(2, 24)=37.388, P<.01]. Matrine administration did not affect the level of the central PI3K/AKT/mTOR signaling in naive mice (n=5). In contrast, the levels of total AKT, mTOR, 4E-BP-1, and p70S6K were unchanged among all groups (n=5). Therefore, the antidepressant-like effects of matrine on mice may involve the PI3K/AKT/mTOR signaling in hippocampus.
Figure 4.
Chronic matrine treatment reversed chronic unpredictable mild stress (CUMS)-induced decrease in the phosphatidylinositol-3 kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signaling in hippocampus, but not prefrontal cortex (PFC). (A) Matrine treatment fully restored CUMS-induced decrease of p-AKT, p-mTOR, p-4E-BP-1, and p-p70S6K expressions in hippocampus, with the total AKT, mTOR, 4E-BP-1, p70S6K, and β-actin levels unchanged. (B) Matrine produced no effects on the levels of p-AKT, p-mTOR, p-4E-BP-1, and p-p70S6K in PFC of CUMS-stressed mice. Data are expressed as means±SEM (n=5), *P<.05, **P<.01; n.s., no significance. Comparisons were made by 2-way ANOVA followed by posthoc Bonferroni’s test.
Pharmacological Inhibition of the PI3K/AKT/mTOR Signaling Prevented the Antidepressant-Like Effects of Matrine on Mice
To test whether the PI3K/AKT/mTOR signaling is really necessary for the effects of matrine, selective inhibitors of AKT (LY294002) and mTOR (rapamycin) were used. In this study, these inhibitors were i.c.v. infused. As a first step, naive mice were first infused with LY294002 or rapamycin and then treated with matrine (60 mg/kg) followed by FST or TST. For LY294002, although its infusion alone produced no effects in FST or TST (n=10), its pretreatment significantly blocked the antidepressant-like effects of matrine in FST [ANOVA: matrine, F(1, 36)=28.598, P<.01; LY294002, F(1, 36)=17.269, P<.01; interaction, F(1, 36)=22.146, P<.01; Figure 5B] and TST [ANOVA: matrine, F(1, 36)=34.487, P<.01; LY294002, F(1, 36)=20.479, P<.01; interaction, F(1, 36)=25.524, P<.01; Figure 5C]. Similarly, for rapamycin, although its treatment alone induced no effects (n=10), it fully prevented the effects of matrine in FST [ANOVA: matrine, F(1, 36)=38.207, P<.01; LY294002, F(1, 36)=21.443, P<.01; interaction, F(1, 36)=27.288, P<.01; Figure 5B] and TST [ANOVA: matrine, F(1, 36)=36.087, P<.01; LY294002, F(1, 36)=28.123, P<.01; interaction, F(1, 36)=19.115, P<.01; Figure 5C].
Figure 5.
Pharmacological inhibition of the phosphatidylinositol-3 kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signaling prevented the antidepressant effects of matrine on mouse behaviors. (A) Schematic timeline of experimental procedures. (B) LY294002/rapamycin and matrine (60 mg/kg) were given 60 and 30 minutes before the forced swim test (FST), respectively. Both LY294002 and rapamycin prevented the reducing effects of matrine on the immobility of naive mice in FST (n=10). (C) LY294002/rapamycin and matrine (60 mg/kg) were given 60 and 30 minutes before the tail suspension test (TST), respectively. Both LY294002 and rapamycin prevented the decreasing effects of matrine on the immobility of naive mice in TST (n=10). (D) Both LY294002 and rapamycin blocked the reducing effects of matrine (60 mg/kg) on the immobility of CUMS-stressed mice in FST (n=10). (E) Both LY294002 and rapamycin blocked the decreasing effects of matrine (60 mg/kg) on the immobility of CUMS-stressed mice in TST (n=10). (F) Both LY294002 and rapamycin abolished the reversing effects of matrine (60 mg/kg) on the sucrose preference of CUMS-stressed mice (n=10). Data are expressed as means±SEM (n=10); *P<.05, **P<.01; n.s., no significance. Comparisons were made by 2-way ANOVA followed by posthoc Bonferroni’s test.
As a second step, CUMS-stressed mice were cotreated with matrine and LY294002/rapamycin followed by the behavioral tests. Figure 5D showed that both LY294002 and rapamycin prevented the reducing effects of matrine on the immobility of the stressed mice in FST (n=10). Figure 5E showed that LY294002 and rapamycin all blocked the decreasing effects of matrine on the immobility of the stressed mice in TST (n=10). Figure 5F showed that matrine could not restore the decreased sucrose preference of the stressed mice under existence of LY294002 or rapamycin (n=10). Moreover, western blotting and immunohistochemistry experiments were done. In parallel with the behavioral data, matrine could not reverse the decreased p-AKT, p-mTOR, p-4E-BP-1, p-p70S6K expressions, and neurogenesis in hippocampus of the stressed mice under existence of LY294002 or rapamycin (n=5, Figures 6 and 7).
Figure 6.
Pharmacological inhibition of the phosphatidylinositol-3 kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signaling prevented the reversing effects of matrine on hippocampal neurogenesis in the stressed mice. (A) Representative confocal microscopic images showed the localization of doublecortin (DCX) in dentate gyrus (DG) region. Scale bar is 150 μm for representative images and 75 μm for enlarged images, respectively. (B) Statistical analysis showed that the promoting effects of matrine (60 mg/kg) on the amount of DCX-positive cells in chronic unpredictable mild stress (CUMS)-stressed mice were fully blocked by infusion of LY294002 and rapamycin. Data are expressed as means±SEM (n=5); *P<.05, **P<.01; n.s., no significance. Comparisons were made by 2-way ANOVA followed by posthoc Bonferroni’s test.
Figure 7.
Usage of LY294002 and rapamycin prevented the reversing effects of matrine on the hippocampal phosphatidylinositol-3 kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signaling. (A) Representative images of the western blotting results. (B) Quantitative analysis revealed that matrine (60 mg/kg) could not restore the decreased p-AKT, p-mTOR, p-4E-BP-1, and p-p70S6K expressions in the hippocampus of chronic unpredictable mild stress (CUMS)-stressed mice under existence of LY294002 or rapamycin (n=5). Data are expressed as means±SEM; *P<.05, **P<.01; n.s., no significance. Comparisons were made by 2-way ANOVA followed by posthoc Bonferroni’s test.
Genetic Knockdown of mTOR in Hippocampus Abolished the Antidepressant-Like Effects of Matrine on Mice
Furthermore, we generated an EGFP-containing lentiviral vector that selectively expressed short hairpin RNA against mTOR (LV-mTOR-shRNA-EGFP). LV-mTOR-shRNA-EGFP or LV-Scrambled-shRNA-EGFP was infused into bilateral hippocampus of mice by stereotaxic injection, and after 14 d, numerous EGFP positive cells and decreased mTOR expression were observed in hippocampus (n=4; Figure 8A). It was found that mTOR knockdown in hippocampus fully abolished the antidepressant-like effects of matrine in FST [ANOVA: matrine, F(1, 36)=33.395, P<.01; mTOR-shRNA, F(1, 36)=26.493, P<.01; interaction, F(1, 36)=31.109, P<.01; n=10, Figure 8C] and TST [ANOVA: matrine, F(1, 36)=42.029, P<.01; mTOR-shRNA, F(1, 36)=37.432, P<.01; interaction, F(1, 36)=34.288, P<.01; n=10, Figure 8D]. Moreover, the mTOR-knockdown mice were subjected to CUMS and matrine administration followed by the behavioral tests. Figure 8E and F revealed that mTOR knockdown in hippocampus completely abolished the decreasing effects of matrine on the immobility of the stressed mice in FST (n=10) and TST (n=10), respectively. Figure 8G revealed that matrine could not restore the decreased sucrose preference of the stressed mice under existence of mTOR-shRNA (n=10). Collectively, the PI3K/AKT/mTOR signaling in hippocampus is necessary for the antidepressant actions of matrine.
Figure 8.
Genetic knockdown of mammalian target of rapamycin (mTOR) in hippocampus by mTOR-shRNA abolishes the antidepressant effects of matrine on mice. (A) Fluorescence of a fixed brain section which expressed LV-mTOR-shRNA-EGFP in the hippocampus 2 weeks after its stereotactic injection. Scale bar is 400 μm. Western blotting analysis showed the efficacy of mTOR-shRNA (n=4). (B) Schematic timeline of experimental procedures. (C) mTOR-shRNA fully abolished the reducing effects of matrine (60 mg/kg) on the immobility of naive mice in the forced swim test (FST) (n=10). (D) mTOR-shRNA also blocked the decreasing effects of matrine (60 mg/kg) on the immobility of naive mice in the tail suspension test (TST) (n=10). (E) mTOR-shRNA completely abolished the reducing effects of matine (60 mg/kg) on the immobility of chronic unpredictable mild stress (CUMS)-stressed mice in FST (n=10). (F) mTOR-shRNA also blocked the decreasing effects of matrine (60 mg/kg) on the immobility of CUMS-stressed mice in TST (n=10). (G) mTOR-shRNA completely antagonized the restoring effects of matrine (60 mg/kg) on the sucrose preference of CUMS-stressed mice (n=10). Data are expressed as means±SEM; **P<.01; n.s., no significance. Comparisons were made by 2-way ANOVA followed by posthoc Bonferroni’s test.
Discussion
The major findings of this study are as follows. First, matrine has antidepressant-like effects in multiple animal models screening for antidepressant activities, including the CUMS paradigm, FST, and TST. Second, the antidepressant-like effects of matrine require the PI3K/Akt/mTOR signaling in hippocampus. Together, these data indicate that matrine could be a potential and novel antidepressant.
Matrine was considered in our study because of knowledge that it had promoting effects on the PI3K/Akt/mTOR signaling in brain (Liu et al., 2017), while mTOR is closely involved in the pathophysiology of depression (Abelaira et al., 2014). Besides, previous studies have demonstrated that matrine has protecting and improving effects on neuropathic pain, Alzheimer’s disease, and Parkinson’s disease (Zhang et al., 2015; Gong et al., 2016; Meng et al., 2017). These reports may also imply the antidepressant-like actions of matrine, as these neurological disorders are always accompanied with depression (Li et al., 2014; Goto et al., 2018; Laumet et al., 2017; Sampath et al., 2017). In this study, we first detected the effects of matrine using FST and TST, as the 2 tests have high predictive validity for detecting potential antidepressant activities and are widely used (Porsolt et al., 1977; Steru et al., 1985). We found that a single injection of matrine produced a significant reduction of immobility in both FST and TST. Moreover, matrine treatment had no effects on the locomotor activity of mice, indicating that the matrine-induced reduction of immobility was not due to locomotor abnormality. We further used the CUMS model to validate the effects of matrine, as the CUMS model has excellent validity modulating the pathogenesis of depression (Papp et al., 1996; Reid et al., 1997). As a result, chronic administration of matrine fully ameliorated the behavioral deficits of CUMS-stressed mice.
mTOR is a serine/threonine protein kinase that modulates a lot of physiological processes including cell proliferation, neuronal survival, and synaptic plasticity (Hay et al., 2004; Tramutola et al., 2017). It has also been demonstrated that mTOR is involved in many diseases, such as diabetes, tremor, and Alzheimer’s disease (Dann et al., 2007). Since Li et al. reported in 2010 that ketamine produced rapid antidepressant effects via activating the mTOR pathway in PFC (Li et al., 2010), mTOR has been a popular target in depression research. Until now, numerous studies have explored the role of mTOR in depression and also found that many antidepressants used in clinical practice (fluoxetine, escitalopram, paroxetine, etc.) have promoting effects on mTOR activity in hippocampus (Park et al., 2014; Liu et al., 2015). Here, our results showed that matrine could reverse the CUMS-induced effects on the PI3K/Akt/mTOR signaling in hippocampus, but not PFC. It is interesting that the effects of matrine on the mTOR system are region selective, while currently there are no persuasive explanations for this finding, and more profound studies are required in the future. More importantly, both pharmacological and genetic inhibition of the mTOR system abolished the antidepressant actions of matrine, further proving our assumption. However, as the neurobiology of depression is very complex, involving a lot of factors like brain-derived neurotrophic factor, cAMP-response element binding protein, serotonin, and mTOR (Shelton, 2007; Krishnan et al., 2008; Albert et al., 2012), so it can not conclude that some other targets are also involved in the antidepressant actions of matrine. For this, we will perform further research using more selective inhibitors and shRNAs. It should be also noticed that matrine had inhibiting effects on the PI3K/Akt/mTOR signaling in lung cancer cells and acute myeloid leukaemia cells (Niu et al., 2014; Wu et al., 2017), while here we found a positive effect of matrine on this signaling in neurons, implying that the pharmacological effects of matrine in body were complex.
In addition, this study is also the first evidence showing the effects of matrine on hippocampal neurogenesis, which is interesting and may imply a proneurogenic compound. Matrine shall modulate hippocampal neurogenesis through the PI3K/Akt/mTOR pathway, as the correlation between mTOR and neurogenesis has been welldemonstrated (Lee, 2015; Tee et al., 2016), and more importantly, inhibition of the PI3K/Akt/mTOR system fully antagonized its effects on hippocampal neurogenesis.
Due to a prevalent belief that “natural shall be better,” currently a lot of public interests have focused on developing and identifying novel antidepressant medications derived from plant materials or natural products. As an important component in Sophorae flavescens, matrine has wide-ranging biological effects and many reveal positive therapeutic indices. Our study is the first evidence showing that matrine has antidepressant-like efficacy via promoting the hippocampal PI3K/Akt/mTOR signaling. Meanwhile, it should be recognized that matrine is found to be neurotoxic (Wang et al., 2010), which can be a major limiting feature for its CNS use. Anyhow, this study extends the knowledge of matrine’s pharmacological effects and sheds light on the development of novel antidepressants with better efficacy and fewer side effects.
Statement of Interest
None.
Acknowledgments
This study was supported by Innovation and Demonstration Projects of Nantong Social Science and Technology (HS2011024).
References
- Abe N, Borson SH, Gambello MJ, Wang F, Cavalli V(2010)Mammalian target of rapamycin (mtor) activation increases axonal growth capacity of injured peripheral nerves. J Biol Chem 285:28034–28043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abelaira HM, Réus GZ, Neotti MV, Quevedo J(2014)The role of mtor in depression and antidepressant responses. Life Sci 101:10–14. [DOI] [PubMed] [Google Scholar]
- Albert PR, Benkelfat C, Descarries L(2012)The neurobiology of depression–revisiting the serotonin hypothesis. I. Cellular and molecular mechanisms. Philos Trans R Soc Lond B Biol Sci 367:2378–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, Nestler EJ(2006)New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci 7:137–151. [DOI] [PubMed] [Google Scholar]
- Bowden CL.(2005)Treatment options for bipolar depression. J Clin Psychiatry 66:3–6. [PubMed] [Google Scholar]
- Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG(2003)Transient expression of doublecortin during adult neurogenesis. J Comp Neurol 467:1–10. [DOI] [PubMed] [Google Scholar]
- Dann SG, Selvaraj A, Thomas G(2007)Mtor complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med 13:252–259. [DOI] [PubMed] [Google Scholar]
- Fu Q, Wang J, Ma Z, Ma S(2014)Anti-asthmatic effects of matrine in a mouse model of allergic asthma. Fitoterapia 94:183–189. [DOI] [PubMed] [Google Scholar]
- Gong SS, Li YX, Zhang MT, Du J, Ma PS, Yao WX, Zhou R, Niu Y, Sun T, Yu JQ(2016)Neuroprotective effect of matrine in mouse model of vincristine-induced neuropathic pain. Neurochem Res 41:3147–3159. [DOI] [PubMed] [Google Scholar]
- Goto M, Kamagata K, Hatano T, Hattori N, Abe O, Aoki S, Hori M, Gomi T(2018)Depressive symptoms in Parkinson’s disease are related to decreased left hippocampal volume: correlation with the 15-item shortened version of the Geriatric Depression Scale. Acta Radiol 59:341–345. [DOI] [PubMed] [Google Scholar]
- Hashimoto K.(2011)Role of the mtor signaling pathway in the rapid antidepressant action of ketamine. Expert Rev Neurother 11:33–36. [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N(2004)Upstream and downstream of mtor. Genes Dev 18:1926–1945. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RM.(2012)The epidemiology of depression and the evolution of treatment. J Clin Psychiatry 73:5–9. [DOI] [PubMed] [Google Scholar]
- Jiang B, Wang F, Yang S, Fang P, Deng ZF, Xiao JL, Hu ZL, Chen JG(2014)SKF83959 produces antidepressant effects in a chronic social defeat stress model of depression through BDNF-TrkB pathway. Int J Neuropsychopharmacol 18:pii pyu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Huang C, Zhu Q, Tong LJ, Zhang W(2015)WY14643 produces anti-depressant-like effects in mice via the BDNF signaling pathway. Psychopharmacology (Berl) 232:1629–1642. [DOI] [PubMed] [Google Scholar]
- Jiang B, Song L, Wang CN, Zhang W, Huang C, Tong LJ(2016)Antidepressant-like effects of GM1 ganglioside involving the BDNF signaling cascade in mice. Int J Neuropsychopharmacol 19:piipyw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Wang YJ, Wang H, Song L, Huang C, Zhu Q, Wu F, Zhang W(2017)Antidepressant-like effects of fenofibrate in mice via the hippocampal brain-derived neurotrophic factor signalling pathway. Br J Pharmacol 174:177–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan QC, Lv P, Zhang XJ, Xu YM, Zhang GX, Zhu L(2015)Matrine protects neuro-axon from CNS inflammation-induced injury. Exp Mol Pathol 98:124–130. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ(2008)The molecular neurobiology of depression. Nature 455:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumet G, Zhou W, Dantzer R, Edralin JD, Huo X, Budac DP, O’Connor JC, Lee AW, Heijnen CJ, Kavelaars A(2017)Upregulation of neuronal kynurenine 3-monooxygenase mediates depression-like behavior in a mouse model of neuropathic pain. Brain Behav Immun 66:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY.(2015)Roles of mtor signaling in brain development. Exp Neurobiol 24:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS(2010)Mtor-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Yue N, Liu SB, Wang ZF, Mi WL, Jiang JW, Wu GC, Yu J, Wang YQ(2014)Effects of chronic electroacupuncture on depression- and anxiety-like behaviors in rats with chronic neuropathic pain. Evid Based Complement Alternat Med 2014:158987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhou R, Zheng P, Yan L, Wu Y, Xiao X, Dai G(2010)Cardioprotective effect of matrine on isoproterenol-induced cardiotoxicity in rats. J Pharm Pharmacol 62:514–520. [DOI] [PubMed] [Google Scholar]
- Linglu D, Yuxiang L, Yaqiong X, Ru Z, Lin M, Shaoju J, Juan D, Tao S, Jianqiang Y(2014)Antinociceptive effect of matrine on vincristine-induced neuropathic pain model in mice. Neurol Sci 35:815–821. [DOI] [PubMed] [Google Scholar]
- Liu SQ, Zhang ML, Zhang HJ, Liu FZ, Chu RJ, Zhang GX, Zhu L(2017)Matrine promotes oligodendrocyte development in CNS autoimmunity through the PI3K/akt signaling pathway. Life Sci 180:36–41. [DOI] [PubMed] [Google Scholar]
- Liu XL, Luo L, Mu RH, Liu BB, Geng D, Liu Q, Yi LT(2015)Fluoxetine regulates mtor signalling in a region-dependent manner in depression-like mice. Sci Rep 5:16024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Xu Y, Ji W, Li X, Sun B, Gao Q, Su C(2014)Anti-tumor activities of matrine and oxymatrine: literature review. Tumour Biol 35:5111–5119. [DOI] [PubMed] [Google Scholar]
- Ludka FK, Constantino LC, Dal-Cim T, Binder LB, Zomkowski A, Rodrigues AL, Tasca CI(2016)Involvement of PI3K/akt/GSK-3β and mtor in the antidepressant-like effect of atorvastatin in mice. J Psychiatr Res 82:50–57. [DOI] [PubMed] [Google Scholar]
- Meng F, Wang J, Ding F, Xie Y, Zhang Y, Zhu J(2017)Neuroprotective effect of matrine on MPTP-induced Parkinson’s disease and on nrf2 expression. Oncol Lett 13:296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu H, Zhang Y, Wu B, Zhang Y, Jiang H, He P(2014)Matrine induces the apoptosis of lung cancer cells through downregulation of inhibitor of apoptosis proteins and the akt signaling pathway. Oncol Rep 32:1087–1093. [DOI] [PubMed] [Google Scholar]
- Papp M, Moryl E, Willner P(1996)Pharmacological validation of the chronic mild stress model of depression. Eur J Pharmacol 296:129–136. [DOI] [PubMed] [Google Scholar]
- Park SW, Lee JG, Seo MK, Lee CH, Cho HY, Lee BJ, Seol W, Kim YH(2014)Differential effects of antidepressant drugs on mtor signalling in rat hippocampal neurons. Int J Neuropsychopharmacol 17:1831–1846. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M(1977)Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229:327–336. [PubMed] [Google Scholar]
- Reid I, Forbes N, Stewart C, Matthews K(1997)Chronic mild stress and depressive disorder: a useful new model?Psychopharmacology (Berl) 134:365–377. [DOI] [PubMed] [Google Scholar]
- Sampath D, Sathyanesan M, Newton SS(2017)Cognitive dysfunction in major depression and Alzheimer’s disease is associated with hippocampal-prefrontal cortex dysconnectivity. Neuropsychiatr Dis Treat 13:1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R(2003)Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301: 805–809. [DOI] [PubMed] [Google Scholar]
- Shelton RC.(2007)The molecular neurobiology of depression. Psychiatr Clin North Am 30:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields M.(2006)Stress and depression in the employed population. Health Rep 17:11–29. [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P(1985)The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 85:367–370. [DOI] [PubMed] [Google Scholar]
- Tee AR, Sampson JR, Pal DK, Bateman JM(2016)The role of mtor signalling in neurogenesis, insights from tuberous sclerosis complex. Semin Cell Dev Biol 52:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramutola A, Lanzillotta C, Di Domenico F(2017)Targeting mtor to reduce Alzheimer-related cognitive decline: from current hits to future therapies. Expert Rev Neurother 17:33–45. [DOI] [PubMed] [Google Scholar]
- Wang XY, Liang L, Chang JL, Yang MH, Li ZG(2010)Toxicity of matrine in kunming mice. Nan Fang Yi Ke Da Xue Xue Bao 30:2154–2155. [PubMed] [Google Scholar]
- Wu G, Zhou W, Zhao J, Pan X, Sun Y, Xu H, Shi P, Geng C, Gao L, Tianx (2017)Matrine alleviates lipopolysaccharide-induced intestinal inflammation and oxidative stress via CCR7 signal. Oncotarget 8:11621–11628. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wu J, Hu G, Dong Y, Ma R, Yu Z, Jiang S, Han Y, Yu K, Zhang S(2017)Matrine induces akt/mtor signalling inhibition-mediated autophagy and apoptosis in acute myeloid leukaemia cells. J Cell Mol Med 21:1171–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu M, Sun H, Yin K(2015)Matrine improves cognitive impairment and modulates the balance of th17/treg cytokines in a rat model of aβ1-42-induced Alzheimer’s disease. Cent Eur J Immunol 40:411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Wang N, Yang C, Li XM, Zhou ZQ, Yang JJ(2014)Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mtor and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry 29:419–423. [DOI] [PubMed] [Google Scholar]