Abstract
Background
Linear relationships are commonly observed between shoot magnesium ([Mg]shoot) and shoot calcium ([Ca]shoot) concentrations among angiosperm species growing in the same environment.
Scope and Conclusions
This article argues that, in plants that do not exhibit ‘luxury’ accumulation of Mg or Ca, (1) distinct stoichiometric relationships between [Mg]shoot and [Ca]shoot are exhibited by at least three groups of angiosperm species, namely commelinid monocots, eudicots excluding Caryophyllales, and Caryophyllales species; (2) these relationships are determined by cell wall chemistry and the Mg/Ca mass quotients in their cell walls; (3) differences between species in [Mg]shoot and [Ca]shoot within each group are associated with differences in the cation exchange capacity (CEC) of the cell walls of different species; and (4) Caryophyllales constitutively accumulate more Mg in their vacuoles than other angiosperm species when grown without a supra-sufficient Mg supply.
Keywords: Angiosperm, calcium (Ca), Caryophyllales, cation exchange capacity (CEC), cell wall, commelinid monocot, magnesium (Mg), Poales, shoot, stoichiometry, vacuole
INTRODUCTION
Calcium (Ca) and magnesium (Mg) are both plant nutrients (Hawkesford et al., 2012). Calcium is essential for cell wall and membrane integrity and for cytosolic signalling. Magnesium is required for protein synthesis, energy metabolism, and photosynthesis as a constituent of chlorophyll. Although each of these elements has unique biological functions, linear relationships between shoot Ca concentration ([Ca]shoot) and shoot Mg concentration ([Mg]shoot) are commonly observed among angiosperm species growing in the same environment, with the exception of species of the Caryophyllales order (White et al., 2015). These relationships have been observed in both field (Garten, 1976; Thompson et al., 1997; Watanabe et al., 2007; Fyllas et al., 2009; White et al., 2012) and glasshouse studies (Broadley et al., 2004; White et al., 2015). This article suggests an anatomical basis for such relationships.
SHOOT CALCIUM AND MAGNESIUM CONCENTRATIONS CORRELATE WITH CELL WALL CATION EXCHANGE CAPACITY
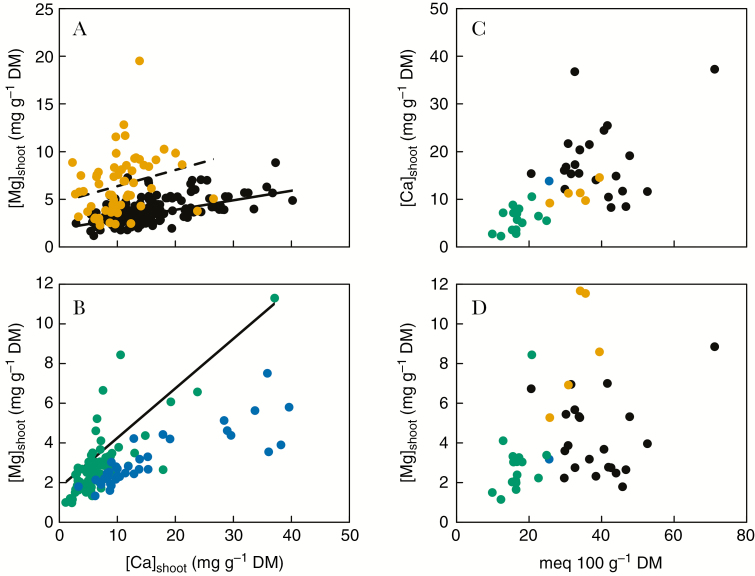
The [Ca]shoot of Ca-replete angiosperms generally lies between 1 and 50 mg Ca g–1 dry matter (DM) and the [Mg]shoot of Mg-replete angiosperms between 1 and 10 mg Mg g–1 DM, depending on plant species and growth conditions (Hawkesford et al., 2012). Eudicot species, and species of the non-commelinid monocots, generally have greater [Ca]shoot and [Mg]shoot than species of the commelinid monocots (Fig. 1A, B; Thompson et al., 1997; Broadley et al., 2003, 2004; Watanabe et al., 2007; White et al., 2012, 2015). There are often strong correlations in both relative [Ca]shoot and relative [Mg]shoot of angiosperm species between studies, indicating that the ranking of angiosperm species for both [Ca]shoot and [Mg]shoot is largely independent of environment (Broadley et al., 2003; White et al., 2012).
Fig. 1.
(A) The relationship between shoot Mg concentration ([Mg]shoot) and shoot Ca concentration ([Ca]shoot) among 212 eudicot species (black and orange circles), of which 61 were members of the Caryophyllales order (orange circles). The lines are predictions for the relationships between [Mg]shoot and [Ca]shoot for non-Caryophyllales eudicots (solid line) and Caryophyllales species (broken line) from the model based on leaf anatomy described in the text and the data presented in Table 1. (B) The relationship between [Mg]shoot and [Ca]shoot among 76 commelinid (green circles) and 35 non-commelinid monocot species (blue circles). The original data set contained three non-commelinid species with [Ca]shoot >50 mg g–1 DM that are not plotted. The line is a prediction for the relationship between [Mg]shoot and [Ca]shoot for commelinid monocots from the model based on leaf anatomy described in the text and the data presented in Table 1. (C) The relationship between [Ca]shoot and the cation exchange capacity (CEC) of root cell walls of 44 angiosperm species, comprising 16 commelinid monocots (green circles), one non-commelinid monocot (blue circle), five Caryophyllales (orange circles) and 22 other eudicots (black circles). (D) The relationship between [Mg]shoot and the CEC of root cell walls of the same 44 angiosperm species. The [Ca]shoot and [Mg]shoot for angiosperm species are mean values obtained in the six hydroponic experiments described by White et al. (2017) and collated by Neugebauer et al. (2018). Values for the CEC of root cell walls were obtained from the literature survey of White and Broadley (2003), and are estimated means from a REML analysis of the data.
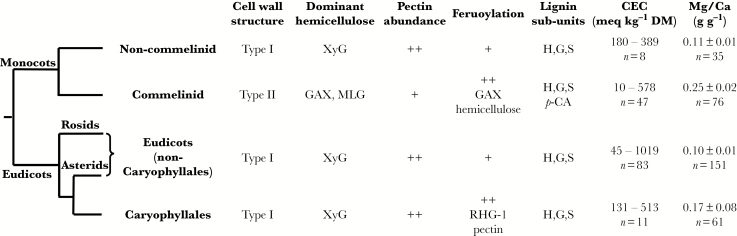
Asher and Ozanne (1961) observed that [Ca]shoot was directly related to the cation exchange capacity (CEC) of root cell walls among angiosperm species, and this has been confirmed in other studies (White and Broadley, 2003; Ray and George, 2011). It is observed that the gradient of the relationship between [Ca]shoot and root CEC is similar for all angiosperm species (Fig. 1C). The CEC of cell walls in the shoot is generally similar to, or greater than, that of root cell walls (Knight et al., 1973). When the same tissue is assayed in plants grown under similar conditions, cell wall CEC is generally greater in eudicots and non-commelinid monocots than in commelinid monocots (Fig. 1C, D; White and Broadley, 2003). Estimates of the CEC of root cell walls range from 45 to 1019 meq kg–1 DM in eudicots, from 180 to 389 meq kg–1 DM in non-commelinid monocots and from 10 to 578 meq kg–1 DM in commelinid monocots (White and Broadley, 2003). An equivalent is the number of moles of an ion multiplied by the valence of that ion (e.g. 1 meq = 0.5 mmol for Ca2+ and Mg2+). Cell wall CEC is not constant for a plant species but can vary with development, growth conditions and the tissue sampled (Heintz, 1961). The CEC is dominated by the free carboxyl groups of polygalacturonic acids (pectins) in the middle lamella of cell walls (White and Broadley, 2003; Taiz et al., 2015). Although there are many different pectin structures (Ridley et al., 2001; Sénéchal et al., 2014; Daher and Braybrook, 2015; Park and Cosgrove, 2015; Anderson, 2016; Bidhendi and Geitmann, 2016) and pectin content can differ between and within tissues, change with development and respond to both abiotic and biotic challenges (Popper et al., 2011; Sénéchal et al., 2014; Daher and Braybrook, 2015; LeGall et al., 2015; Park and Cosgrove, 2015; Anderson, 2016), the cell walls of eudicots and non-commelinid monocots generally have similar pectin contents, and both have more pectin than cell walls of commelinid monocots (Fig. 2; Jarvis et al., 1988; Harris et al., 1997; Smith and Harris, 1999; Popper et al., 2011; Banasiak, 2015). The cell walls of eudicots excluding Caryophyllales, Caryophyllales, non-commelinid monocots and commelinid monocots also differ in other cell wall properties (Fig. 2; Harris and Tretheway, 2010; Popper et al., 2011; Banasiak, 2015). In particular, non-commelind monocots generally contain greater amounts of xyloglucans, mixed linkage glucans and ester-related p-coumaric acids than eudicots and non-commelinid monocots (Banasiak, 2015; Hatfield et al., 2017), and the hemicelluloses of non-commelinid monocots and the rhamnogalacturonan-I pectins of Caryophyllales can be covalently cross-linked by feruoylation (Ridley et al., 2001; Harris and Tretheway, 2010; Hatfield et al., 2017). It has been speculated that the CEC of cell walls might influence free Ca2+ concentrations in the apoplast and, thereby, cell signalling (Hepler and Winship, 2010), but there is, as yet, no direct evidence that cell wall CEC affects Ca2+ signalling across the plasma membrane of mature plant cells.
Fig. 2.
Phylogenetic relationships between non-commelinid monocots, commelinid monocots, eudicots excluding Caryophyllales (rosids and asterids) and Caryophyllales according to the Angiosperm Phylogeny Group IV (2016). The presence of Type I [cellulose microfibrils surrounded by xyloglucan (XyG) with large amounts of pectin; lignin containing H (p-hydroxyphenyl), G (guaiacyl) and S (syringyl) subunits] or Type II [cellulose microfibrils surrounded by glucuronoarabinoxylan (GAX) and some mixed linkage glucans (MLG), with little pectin; lignins containing H, G, S and ester-related p-coumaric acid (p-CA) sub-units] cell walls, dominant hemicelluloses, pectin abundance (++ = large amounts, + = small amounts), feruoylation (++ = large amounts, + = small amounts) and lignin sub-units are indicated. Data for cell wall composition are summarized from Harris and Trethewey (2010), Popper et al. (2011), Banasiak (2015) and Hatfield et al. (2017). Root cell wall cation exchange capacities (CECs, expressed as the range for n species) are taken from White and Broadley (2003), and gradients of the relationships between [Mg]shoot and [Ca]shoot are derived from the data shown in Fig. 1A and B.
Cell walls also bind substantial amounts of Mg2+ (Hawkesford et al., 2012), and [Mg]shoot, like [Ca]shoot, is correlated with cell wall CEC among angiosperm species (Fig. 1D). However, the gradient of the relationship between [Mg]shoot and root CEC is greater among commelinid monocots than among eudicots, with the exception of Caryophyllales species (Fig. 1D). Since the relationship between [Ca]shoot and root CEC does not differ between angiosperm species, these data indicate that cell walls of commelinid monocots have a lower Ca/Mg selectivity than cell walls of most eudicots.
ESTIMATES OF CALCIUM AND MAGNESIUM CONCENTRATIONS IN CELL WALLS AND INTRACELLULAR COMPARTMENTS OF SHOOTS
Estimates of the Ca concentration in cell walls of eudicot shoots range from 0.47 to 38.9 mg Ca g–1 DM (Nakajima et al., 1981; Goldberg et al., 1987; Mühling and Sattelmacher, 1995; Miklós et al., 2000, Carr et al., 2003; Liu et al., 2015), which convert to values of 11.7–970 mm if it is assumed that water makes up about two-thirds of cell wall mass in growing tissues (Cosgrove, 1997), and Ca concentration in cell walls of commelinid monocot shoots range from 0.26 to 3.0 mg Ca g–1 DM, which equates to 6.5–74.8 mm (Turan et al., 2009; Zeng et al., 2010). Estimates of the Mg concentration in cell walls of eudicot shoots range from 0.024 to 0.99 mg Mg g–1 DM (Nakajima et al., 1981; Mühling and Sattelmacher, 1995; Carr et al., 2003) and the Mg concentration in rice shoots has been estimated to be 0.072 mg Mg g–1 DM (Zeng et al., 2010), which are equivalent to 0.99–40.7 mm and 2.96 mm, respectively. Estimates of Ca/Mg mass quotients in shoot cell walls of eudicots range from 5.63 to 17.60 (Nakajima et al., 1981; Mühling and Sattelmacher, 1995; Carr et al., 2003) and that of rice shoots has been estimated to be 3.56 (Zeng et al., 2010). Cell walls of eudicot shoots generally contain between 70 and 99 % of the total tissue Ca, although values as low as 17 % have been reported (Mühling and Sattelmacher, 1995), but only 1–11 % of the total tissue Mg (Nakajima et al., 1981; Mühling and Sattelmacher, 1995; Miklós et al., 2000; Liu et al., 2015), although 80 % of the Mg in the first trifoliate leaf of a sub-terranean clover plant was found to be associated with a fibre fraction by Scott and Robson (1990). Greater concentrations of Ca and Mg, and greater Ca/Mg mass quotients, have also been observed in root cell walls of eudicots than in those of commelinid monocots (e.g. Mehlich, 1953), and the Ca/Mg selectivity of root cell walls has been found to increase with increasing CEC among Poales species (Waquant, 1977).
In plants that contain no precipitated Ca salts, the Ca concentration in shoot vacuoles generally lies between 2 and 20 mm, but can reach 80 mm in some cells, and that in chloroplasts is between 7 and 12 mm (Carr et al., 2003; Stael et al., 2012). The open cytoplasm contains between 0.1 and 1 mm Ca, and the endoplasmic reticulum (ER), mitochondria and nuclei contain about 2 mm Ca (White and Broadley, 2003; Stael et al., 2012). Chloroplasts contain 5–10 % of total leaf Mg in Mg-replete plants, but up to 20–35 % of leaf Mg in Mg-deficient plants (Scott and Robson, 1990; Hawkesford et al., 2012). The Mg is present in chlorophyll, at a concentration of about 100 mm, and in solution, at a concentration of 5–20 mm (Shaul, 2002). Between 60 and 90 % of the total Mg in leaves of Mg-replete plants is in a water-soluble form (Hawkesford et al., 2012). In Mg-replete plants, vacuolar Mg concentrations generally lie between 3 and 20 mm, but Mg concentrations of up to 120 mm have been reported in some cells (Shaul, 2002; Carr et al., 2003; Hawkesford et al., 2012). It is thought that the open cytosol contains 2–10 mm Mg, mitochondria contain 7–11 mm Mg and the ER and nuclei contain 10–20 mm Mg (Hawkesford et al., 2012; Gout et al., 2014). These values are similar to the Mg concentrations found in these organelles in animal cells (Romani, 2011). Cameron et al. (1984) estimated that the open cytoplasm of onion root cells contained 16–32 mmol Ca kg–1 DM and 67–156 mmol Mg kg–1 DM (Ca/Mg mass quotient = 0.34–0.39), and nuclei contained 9–36 mmol Ca kg–1 DM and 61–139 mmol Mg kg–1 DM (Ca/Mg mass quotient = 0.24–0.43).
THE CONTRIBUTIONS OF CELL WALLS AND INTRACELLULAR COMPARTMENTS TO SHOOT CALCIUM AND MAGNESIUM CONCENTRATIONS
Although it is acknowledged that both leaves and cell types within leaves differ in their Ca and Mg concentrations (Conn and Gilliham, 2010; Hawkesford et al., 2012), this article refers to a composite shoot that integrates these differences. This composite shoot is considered to have several features with different Ca and Mg concentrations and distinct Ca/Mg mass quotients: the cell wall, the cytoplasm, comprising the open cytosol, ER, nucleus, mitochondria and chloroplasts, and the vacuole. Their approximate contributions to the volume of a mature leaf are assumed to be: cell wall 6 %, open cytoplasm 5 %, chloroplasts 5 %, nuclei 0.5 %, mitochondria 1 %, ER 0.5 % and vacuole 82 % (Table 1; Heldt and Piechulla, 2010; Hawkesford et al., 2012). However, it must be stressed that these contributions can vary greatly between plant species, in leaves of different ages and in plants grown under contrasting environmental conditions.
Table 1.
Data used to predict shoot calcium concentrations ([Ca]shoot) and magnesium concentrations ([Mg]shoot) of angiosperm species.
| Cell compartment | |||||||
|---|---|---|---|---|---|---|---|
| Cell wall | Cytosol | ER | Nucleus | Mitochondria | Chloroplasts | Vacuoles | |
| Volume (% leaf)* | |||||||
| Angiosperms | 6.0 | 5.0 | 0.5 | 0.5 | 1.0 | 5.0 | 82 |
| Calcium concentration (mM)† | |||||||
| Angiosperms | 0.1 | 2 | 2 | 2 | 7 | 2 | |
| Magnesium concentration (mM)‡ | |||||||
| Angiosperms except Caryophyllales | 2 | 10 | 10 | 7 | 105 | 3 | |
| Caryophyllales | 2 | 10 | 10 | 7 | 105 | 14 | |
| Ca/Mg quotient in material above the minimal cell wall (g g–1)§ | |||||||
| Commelinids | 4.00 | ||||||
| Eudicots except Caryophyllales | 10.0 | ||||||
| Caryophyllales | 5.88 | ||||||
Broadley et al. (2004) suggested that there was a linear relationship between [Mg]shoot and [Ca]shoot among all angiosperm species, with the exception of Caryophyllales that had greater [Mg]shoot at any given [Ca]shoot than other angiosperm species. They also suggested that the commelinid monocots had smaller [Mg]shoot and [Ca]shoot than other angiosperm species. In the data set assembled here, which includes more species than the study of Broadley et al. (2004), it appears that the data can be separated into at least three groups: (1) commelinid monocots (Arecales, Commelinales, Poales and Zingiberales); (2) eudicots excluding Caryophyllales; and (3) Caryophylalles species (Fig. 1A, B). The non-commelinid monocots might form a fourth group. In all groups there appears to be a linear relationship between [Mg]shoot and [Ca]shoot, but the gradient of this relationship and [Mg]shoot at zero [Ca]shoot differ between groups (Fig. 1A, B). The equations for linear regressions of the relationships between [Mg]shoot and [Ca]shoot, both expressed as mg g–1 DM, are [Mg]shoot = 1.09 ± 0.20 + (0.25 ± 0.02 × [Ca]shoot) for commelinid monocots, [Mg]shoot = 2.17 ± 0.21 + (0.10 ± 0.01 × [Ca]shoot) for eudicots excluding Caryophyllales, and [Mg]shoot = 4.68 ± 0.92 + (0.17 ± 0.08 × [Ca]shoot) for Caryophyllales species. The commelinid monocots have a greater gradient and smaller [Mg]shoot at zero [Ca]shoot than the eudicots, whilst the Caryophyllales have an intermediate gradient but a considerably greater [Mg]shoot at zero [Ca]shoot than other angiosperm species. In agreement with Broadley et al. (2004), the magnitudes of [Mg]shoot and [Ca]shoot are generally less in the commelinid monocots than in other angiosperm species.
The data set presented in Fig. 1 comprises data from six experiments undertaken in hydroponics using the same nutrient solution (White et al., 2017; Neugebauer et al., 2018). Although experiments in hydroponics might underestimate the consequences of vagaries in the phytoavailability of nutrients and toxic elements in soil and the intimate interactions between the root and the soil on the shoot ionome (Brown et al., 2017; Neugebauer et al., 2018), it is noteworthy that the gradients of the relationships between [Mg]shoot and [Ca]shoot among angiosperm species obtained in the hydroponic system described by White et al. (2017) are similar to those obtained in more natural environments (Broadley et al., 2004; White et al., 2012).
If the relationship between [Mg]shoot and [Ca]shoot can be attributed to variation in a single, common anatomical feature within each group, then the reciprocal of the gradient of this relationship is the Ca/Mg mass quotient of that anatomical feature. In the data set compiled here, this is 4.00 for commelinid monocots, 10.0 for eudicots excluding the Caryophyllales, and 5.88 for Caryophyllales species. For comparison, the equivalent values for all angiosperms excluding Caryophyllales in the hydroponic study of Broadley et al. (2003) and the field studies of Garten et al. (1976) and Thompson et al. (1997) were all 7.7, for the field study of White et al. (2012) it was 8.9 and for the hydroponic study of White et al. (2015) it was 11.1. These values correspond to the Ca/Mg mass quotients reported for cell walls of rice shoots (3.56; Zeng et al., 2010) and eudicots (5.63–17.60; Nakajima et al., 1981; Mühling and Sattelmacher 1995; Carr et al., 2003). It is therefore possible that the stoichiometric relationships between [Mg]shoot and [Ca]shoot of groups of angiosperm species reflects the relative binding of these cations in their cell walls, which differs between the three groups. The absolute binding capacity of cell walls of individual species within these groups is likely to be related to their characteristic cell wall CEC.
If the plants growing hydroponically in the data set reported here do not exhibit ‘luxury’ accumulation of either Ca or Mg, the minimal intracellular requirement for Ca and Mg for cellular functions might be estimated from the minimal concentrations of these elements in cellular compartments reported in plants growing with adequate nutrition (Table 1). Expressed on a leaf volume basis, the minimal intracellular concentrations are 2.04 mm Ca and 7.98 mm Mg, which equate to 816 mg Ca kg–1 leaf DM and 1939 mg Mg kg–1 leaf DM assuming a leaf DM/fresh weight quotient of 0.10 (Broadley et al., 2003). For comparison, simple linear regressions of the data for [Mg]shoot and [Ca]shoot suggest an [Mg]shoot of 1294 and 2252 mg kg–1 leaf DM for commelinid monocots and eudicots excluding Caryophyllales at a [Ca]shoot of 816 mg Ca kg–1 leaf DM, respectively. Assuming minimal leaf concentrations of 1 mg Ca g–1 DM and 2 mg Mg g–1 DM for a commelinid monocot, cell wall concentrations of 0.37 mg Ca g–1 cell wall DM and 0.12 mg Mg g–1 cell wall DM can be calculated based on a the cell wall contributing 50 % of the total leaf DM (Sugiyama and Shimazaki, 2007). Both these values are similar to estimates of the Ca concentration (0.26–3.0 mg Ca g–1 DM; Turan et al., 2009; Zeng et al., 2010) and Mg concentration (0.072 mg Mg g–1 DM; Zeng et al., 2010) in cell walls of monocot leaves, and the calculated cell wall Ca/Mg mass quotient of 3.02 is comparable with that in shoots of rice (3.56 mg Mg g–1 DM; Zeng et al., 2010). These values suggest that at least 18 % of leaf Ca and 3 % of leaf Mg by mass will be present in the cell walls of monocots. Given the lack of precision in the estimates of the volumes of cellular compartments within leaves, total Ca and Mg concentrations in cellular compartments, leaf DM/fresh weight quotient and the contribution of the cell wall to leaf biomass, the prediction is remarkably concordant with measured values. From these observations, the relationship between [Mg]shoot and [Ca]shoot among commelinid monocots (Fig. 1A) can then be predicted assuming a cell wall Ca/Mg mass quotient of 4.00 using the equation [Mg]shoot = 1.75 + (0.25 × [Ca]shoot). Assuming similar intracellular Ca and Mg concentrations in all angiosperms, the relationship between [Mg]shoot and [Ca]shoot among eudicots excluding Caryophyllales species (Fig. 1B) can be predicted assuming a cell wall Ca/Mg mass quotient of 10.0 using the equation [Mg]shoot = 1.90 + (0.10 × [Ca]shoot). These predictions fit the data reasonably well, although the predictions of [Mg]shoot for monocots are generally greater than the observed [Mg]shoot at a given [Ca]shoot, suggesting that intracellular Mg might be overestimated in monocots, and that intracellular Mg might be less in commelinid monocots than in eudicots (Fig. 1A, B).
White et al. (2015) suggested that Caryophyllales species have larger [Mg]shoot and smaller shoot Ca/Mg quotients than other angiosperms because of greater accumulation of Mg in vacuoles of shoot cells. The accumulation of Mg, but the same amount of Ca, in a vacuole can give rise to both phenomena if all other factors remain equal and produce the relationship between [Ca]shoot and [Mg]shoot among Caryophyllales species observed in the data set analysed here (Fig. 1B) as well as in previous studies (Thompson et al., 1997; Broadley et al., 2004; White et al., 2012, 2015). Assuming that the Mg concentration in other cellular compartments remains equal, and that the Mg concentration in the cell wall of a plant with a shoot Ca concentration of 1 mg kg–1 is 0.12 mg Mg g–1 cell wall DM, then the vacuolar Mg concentration in this plant can be calculated to be 14.19 mM (Table 1). The relationship between [Mg]shoot and [Ca]shoot in Caryophyllales can be predicted assuming a cell wall Ca/Mg mass quotient of 5.88, using the equation [Mg]shoot = 4.68 + (0.17 × [Ca]shoot).
CONCLUSIONS AND PERSPECTIVE
This article presents a novel, quantitative and universal explanation of the differences in shoot Ca/Mg quotients and absolute Ca and Mg concentrations in the shoots of angiosperm species. The arguments and analysis presented lead to several hypotheses, i.e. that in plants that do not exhibit ‘luxury’ accumulation of Ca or Mg, (1) distinct linear relationships between [Mg]shoot and [Ca]shoot are exhibited by at least three groups of angiosperm species, namely commelinid monocots, eudicots excluding Caryophyllales, and Caryophylalles species; (2) these relationships are determined by cell wall chemistry and the Mg/Ca mass quotients in their cell walls; (3) differences between species in [Ca]shoot and [Mg]shoot within groups are associated with their cell wall CEC; and (4) Caryophyllales constitutively accumulate more Mg in their vacuoles than other angiosperm species.
These hypotheses might be tested through further experimentation. The hypothesis that different groups of angiosperm species exhibit distinct linear relationships between [Mg]shoot and [Ca]shoot might be tested by surveying the shoot ionomes of more species within each group. Similarly, the hypothesis that the relative concentrations of Ca and Mg in shoot cell walls differ between groups of angiosperm species and correlate with the gradient of their [Mg]shoot and [Ca]shoot relationships might be tested by determining the cationic composition of shoot cell walls of more plant species from each group. The hypothesis that the absolute Ca and Mg concentrations in shoot cell walls of species within each group are determined by their CEC might be tested by assaying the cell wall Ca and Mg concentrations and CEC of shoots of more plant species from each group. The role of particular cell wall compounds in determining CEC and the absolute and relative concentrations of Ca and Mg in the cell wall might be tested using mutants with less or more of these compounds. The greater accumulation of Mg in the vacuole of Caryophyllales might be tested by comparing Mg localization at sub-cellular resolution in shoots of species from different angiosperm orders grown under identical conditions.
ACKNOWLEDGEMENTS
This work was supported by the Rural and Environment Science and Analytical Services Division (RESAS) of the Scottish Government (P.J.W. and T.S.G), the Distinguished Scientist Fellowship Program of King Saud University (P.J.W. and H.A.E-S.) and a University of Nottingham/James Hutton Institute Postgraduate Studentship (K.N.).
LITERATURE CITED
- Anderson CT. 2016. We be jammin’: an update on pectin biosynthesis, trafficking and dynamics. Journal of Experimental Botany 67: 495–502. [DOI] [PubMed] [Google Scholar]
- Angiosperm Phylogeny Group IV 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181: 1–20. [Google Scholar]
- Asher CJ, Ozanne PG. 1961. The cation exchange capacity of plant roots and its relationship to the uptake of insoluble nutrients. Australian Journal of Agricultural Research 12: 755–766. [Google Scholar]
- Banasiak A. 2015. Evolution of the cell wall components during terrestrialization. Acta Societatis Botanicorum Poloniae 83: 349–362. [Google Scholar]
- Bidhendi AJ, Geitmann A. 2016. Relating the mechanics of the primary plant cell wall to morphogenesis. Journal of Experimental Botany 67: 449–461. [DOI] [PubMed] [Google Scholar]
- Broadley MR, Bowen HC, Cotterill HL, et al. 2003. Variation in the shoot calcium content of angiosperms. Journal of Experimental Botany 54: 1431–1446. [DOI] [PubMed] [Google Scholar]
- Broadley MR, Bowen HC, Cotterill HL, et al. 2004. Phylogenetic variation in the shoot mineral concentration of angiosperms. Journal of Experimental Botany 55: 321–336. [DOI] [PubMed] [Google Scholar]
- Brown LK, George TS, Neugebauer K, White PJ. 2017. The rhizosheath – a potential trait for future agricultural sustainability occurs in orders throughout the angiosperms. Plant and Soil 418: 115–128. [Google Scholar]
- Cameron IL, Hunter KE, Smith NKR. 1984. The subcellular concentration of ions and elements in thin cryosections of onion root meristem cells. An electron-probe EDS study. Journal of Cell Science 72: 295–230. [DOI] [PubMed] [Google Scholar]
- Carr HP, Lombi E, Kupper H, McGrath SP, Wong MH. 2003. Accumulation and distribution of aluminium and other elements in tea (Camellia sinensis) leaves. Agronomie 23: 705–710. [Google Scholar]
- Conn S, Gilliham M. 2010. Comparative physiology of elemental distributions in plants. Annals of Botany 105: 1081–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. 1997. Assembly and enlargement of the primary cell wall in plants. Annual Review of Cell and Developmental Biology 13: 171–201. [DOI] [PubMed] [Google Scholar]
- Daher FB, Braybrook SA. 2015. How to let go: pectin and plant cell adhesion. Frontiers in Plant Science 6: 523. doi: 10.3389/fpls.2015.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyllas NM, Patino S, Baker TR, et al. 2009. Basin-wide variations in foliar properties of Amazonian forest: phylogeny, soils and climate. Biogeosciences 6: 2677–2708. [Google Scholar]
- Garten CT. 1976. Correlations between concentrations of elements in plants. Nature 261: 686–688. [Google Scholar]
- Goldberg R, Liberman M, Mathieu C, Pierron M, Catesson AM. 1987. Development of epidermal cell wall peroxidases along the mung bean hypocotyl: possible involvement in the cell wall stiffening process. Journal of Experimental Botany 38: 1378–1390. [Google Scholar]
- Gout E, Rébeillé F, Douce R, Bligny R. 2014. Interplay of Mg2+, ADP, and ATP in the cytosol and mitochondria: unravelling the role of Mg2+ in cell respiration. Proceedings of the National Academy of Sciences, USA 111: E4560–E4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PJ, Trethewey JAK. 2010. The distribution of ester-linked ferulic acid in the cell walls of angiosperms. Phytochemistry Reviews 9: 19–33. [Google Scholar]
- Harris PJ, Kelderman MR, Kendon MF, Mckenzie RJ. 1997. Monosaccharide compositions of unlignified cell walls of monocotyledons in relation to the occurrence of wall-bound ferulic acid. Biochemical Systematics and Ecology 25: 167–179. [Google Scholar]
- Hatfield RD, Rancour DM, Marita JM. 2017. Grass cell walls: a story of cross-linking. Frontiers in Plant Science 7: 2056. doi: 10.3389/fpls.2016.02056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkesford M, Horst W, Kichey T, et al. 2012. Functions of macronutrients. In: Marschner P, ed. Marschner’s mineral nutrition of higher plants, 3rd edn London: Academic Press, 135–189. [Google Scholar]
- Heintz SG. 1961. Studies on cation-exchange capacities of roots. Plant and Soil 13: 365–383. [Google Scholar]
- Heldt H-W, Piechulla B. 2010. Plant biochemistry, 4th edn Amsterdam: Academic Press. [Google Scholar]
- Hepler PK, Winship LJ. 2010. Calcium at the cell wall–cytoplast interface. Journal of Integrative Plant Biology 52: 147–160. [DOI] [PubMed] [Google Scholar]
- Jarvis MC, Forsyth W, Duncan HJ. 1988. A survey of the pectic content of non-lignified monocot cell walls. Plant Physiology 88: 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight AH, Crooke WM, Burridge JC. 1973. Cation exchange capacity, chemical composition and balance of carboxylic acids in the floral parts of various plant species. Annals of Botany 37: 159–166. [Google Scholar]
- LeGall H, Philippe F, Domon J-M, Gillet F, Pelloux J, Rayon C. 2015. Cell wall metabolism in response to abiotic stress. Plants 4: 112–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GD, Wang RD, Wu LS, Peng SA, Wang YH, Jiang CC. 2015. Differential changes in cell-wall content and boron and calcium concentration in Newhall navel orange grafted on two rootstocks differing in boron-deficiency responses. Communications in Soil Science and Plant Analysis 46: 439–453. [Google Scholar]
- Mehlich A. 1953. Factors affecting adsorption of cations by plant roots. Soil Science Society of America Journal 17: 231–234. [Google Scholar]
- Miklós E, Szõke L, Kozma P, Erdei L. 2000. Differences in cell wall binding capacity of grapevine cultivars. Acta Horticulturae 526: 183–186. [Google Scholar]
- Mühling KH, Sattelmacher B. 1995. Apoplastic ion concentration of intact leaves of field bean (Vicia faba) as influenced by ammonium and nitrate nutrition. Journal of Plant Physiology 147: 81–86. [Google Scholar]
- Nakajima N, Morikawa H, Igarashi S, Senda M. 1981. Differential effect of calcium and magnesium on mechanical properties of pea stem cell walls. Plant and Cell Physiology 22: 1305–1315. [Google Scholar]
- Neugebauer K, Broadley MR, El-Serehy HA, et al. 2018. Variation in the angiosperm ionome. Physiologia Plantarum (in press). [DOI] [PubMed] [Google Scholar]
- Park YB, Cosgrove DJ. 2015. Xyloglucan and its interactions with other components of the growing cell wall. Plant and Cell Physiology 56: 180–194. [DOI] [PubMed] [Google Scholar]
- Popper ZA, Michel G, Hervé C, et al. 2011. Evolution and diversity of plant cell walls: from algae to flowering plants. Annual Review of Plant Biology 62: 567–590. [DOI] [PubMed] [Google Scholar]
- Ray JG, George KJ. 2011. Cation exchange capacity of roots of wild grasses and their ecological implications. Ecology and Noospherology Journal 22: 58–72. [Google Scholar]
- Ridley BL, O’Neill MA, Mohnen D. 2001. Pectins: structure, biosynthesis, and oligogalacturonide-related signalling. Phytochemistry 57: 929–967. [DOI] [PubMed] [Google Scholar]
- Romani AMP. 2011. Cellular magnesium homeostasis. Archives of Biochemistry and Biophysics 512: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BJ, Robson AD. 1990. Changes in the content and form of magnesium in the first trifoliate leaf of subterranean clover under altered or constant root supply. Australian Journal of Agricultural Research 41: 511–519. [Google Scholar]
- Sénéchal F, Wattier C, Rustérucci C, Pelloux J. 2014. Homogalacturonan-modifying enzymes: structure, expression, and roles in plants. Journal of Experimental Botany 65: 5125–5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul O. 2002. Magnesium transport and function in plants: the tip of the iceberg. Biometals 15: 309–323. [DOI] [PubMed] [Google Scholar]
- Sugiyama S, Shimazaki T. 2007. Increased cell-wall mass and resistance to freezing and snow mold during cold acclimation of winter wheat under field conditions. Plant Production Science 10: 383–390. [Google Scholar]
- Smith BG, Harris PJ. 1999. The polysaccharide composition of Poales cell walls: Poaceae cell walls are not unique. Biochemical Systematics and Ecology 27: 33–53. [Google Scholar]
- Stael S, Wurzinger B, Mair A, Mehlmer N, Vothknecht UC, Teige M. 2012. Plant organellar calcium signalling: an emerging field. Journal of Experimental Botany 63: 1525–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan MA, Taban N, Taban S. 2009. Effect of calcium on the alleviation of boron toxicity and localization of boron and calcium in cell wall of wheat. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 37: 99–103. [Google Scholar]
- Taiz L, Zeiger E, Møller IM, Murphy A. 2015. Plant physiology and development, 6th edn Sunderland, MA: Sinauer Associates. [Google Scholar]
- Thompson K, Parkinson JA, Band SR, Spencer RE. 1997. A comparative study of leaf nutrient concentrations in a regional herbaceous flora. New Phytologist 136: 679–689. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Broadley MR, Jansen S, et al. 2007. Evolutionary control of leaf element composition in plants. New Phytologist 174: 516–523. [DOI] [PubMed] [Google Scholar]
- Waquant J-P. 1977. Physicochemical selectivity for cations and CEC of grass roots. Plant and Soil 47: 257–262. [Google Scholar]
- White PJ, Broadley MR. 2003. Calcium in plants. Annals of Botany 92: 487–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, Broadley MR, Thompson JA, et al. 2012. Testing the distinctness of shoot ionomes of angiosperm families using the Rothamsted Park Grass Continuous Hay Experiment. New Phytologist 196: 101–109. [DOI] [PubMed] [Google Scholar]
- White PJ, Bowen HC, Farley E, et al. 2015. Phylogenetic effects on shoot magnesium concentration. Crop and Pasture Science 66: 1241–1248. [Google Scholar]
- White PJ, Bowen HC, Broadley MR, et al. 2017. Evolutionary origins of abnormally large shoot sodium accumulation in non-saline environments within the Caryophyllales. New Phytologist 214: 284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F, Ali S, Qiu B, Wu F, Zhang G. 2010. Effects of chromium stress on the subcellular distribution and chemical form of Ca, Mg, Fe, and Zn in two rice genotypes. Journal of Plant Nutrition and Soil Science 173: 135–148. [Google Scholar]