Abstract
Calciphylaxis has high mortality. Vitamin K deficiency is common in haemodialysis patients and may be a trigger for calciphylaxis due to its role in activating matrix Gla protein (a tissue inhibitor of calcification). We report the case of a 43-year-old female haemodialysis patient who developed calciphylaxis. Two months prior to the diagnosis she was found to have an undetectable plasma vitamin K concentration. The calciphylaxis completely resolved with vitamin K supplementation and an increase in haemodialysis frequency. She did not receive sodium thiosulphate or bisphosphonates. Supplementation of vitamin K in deficient patients may improve the outcome of this condition.
Keywords: calciphylaxis, malabsorption, matrix Gla protein, renal dialysis, vitamin K
Background
Calciphylaxis is a disease of painful, ulcerating, violaceous skin nodules that progress to subcutaneous tissue necrosis. It usually occurs in maintenance dialysis patients, is associated with small vessel calcification and carries a high mortality [1].
Vitamin K is needed to gamma-carboxylate the family of proteins called gamma-carboxyglutamic acid (Gla) proteins. Matrix Gla protein (MGP) is one such protein and, when carboxylated to cMGP, it acts to inhibit tissue and vascular calcification [2]. Approximately half of patients with calciphylaxis are known to be prescribed warfarin (a vitamin K antagonist) at disease onset [1] and such patients are also known to have a lower fraction of cMGP [3].
We report the successful treatment of calciphylaxis with high-dose vitamin K. The patient has provided informed consent for the publication of this case report.
Case report
A 43-year-old white female with a body mass index (BMI) of 51 kg/m2, received three cycles of fluorouracil, epirubicin and cyclophosphamide followed by docetaxel for breast cancer. After docetaxel administration, she developed ischaemic gut and septic shock, ischaemic hepatitis and became dialysis dependent. She underwent total colectomy and a 2.3 m small bowel resection with jejuno-rectal anastomosis and received 2 months of total parenteral nutrition before recommencing adequate oral intake.
One year after the presentation with septic shock, she presented with an ulcerating, painful nodular rash over her calf and lateral upper thigh. At presentation she was normocalcaemic, had mild hyperphosphataemia (1.62 mmol/L), her parathyroid hormone was 28 pmol/L (reference range <7.5 pmol/L) and she received thrice weekly haemodialysis (260 min/session) using a 1.25 mmol/L calcium dialysate. She took sevelamer as a phosphate binder and did not take vitamin D analogues or warfarin.
X-ray of the soft tissue showed diffuse subcutaneous calcification. Histology of the rash demonstrated mural vascular calcification with intraluminal thrombi and fat necrosis. Immunofluorescence was negative. Serum vitamin K1 measured using C18 solid-phase extraction and zinc reduction followed by high-performance liquid chromatography was <0.3 nmol/L (reference range 0.3–2.6) and her vitamin A level was 0.8 μmol/L (reference range 1.6–2.3).
After diagnosis of calciphylaxis, her dialysate calcium was reduced to 1.0 mmol/L and dialysis frequency increased to four sessions/week. Intravenous therapy was begun with 10 mg vitamin K and a multivitamin given at every dialysis session. The multivitamin contained retinol 3500 IU, cholecalciferol 5.5 μg, vitamin E 11.2 IU, vitamin C 125 mg, thiamine 3.51 mg, riboflavine 4.14 mg, pyridoxine 4.53 mg, cyanocobalamin 6 μg, folic acid 414 μg, dexpanthenoic acid 17.25 mg, d-biotin 69 μg, niacin 46 mg, glycocholic acid 140 mg and lecithin 112.5 mg. No bisphosphonate or sodium thiosulphate was administered. Plasma vitamin K levels rose to 20 nmol/L (reference range 0.3–2.6). She experienced one episode of bacteraemia prior to complete resolution of all skin lesions 12 months after commencing vitamin K. The time course of plasma calcium, phosphate and intact parathyroid hormone (iPTH) changes are shown in Figure 1. (iPTH was measured on an Abbott ci16200 analyser with an Architect iPTH second-generation assay.)
Fig. 1.
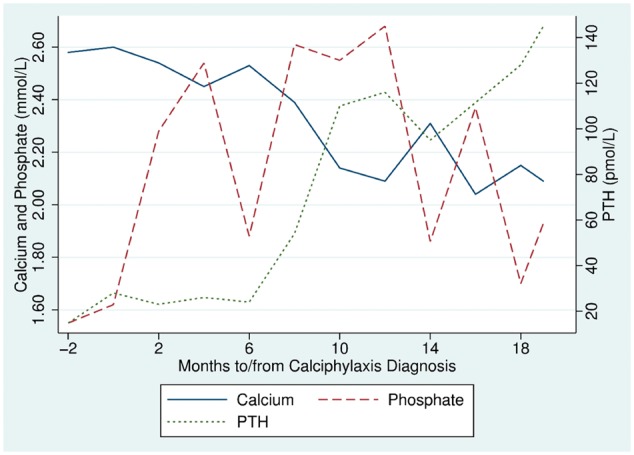
Time course of plasma calcium, phosphate and PTH.
Discussion
There are two types of natural vitamin K: vitamin K1, which is found in green vegetables, and K2, which is found in cheese, natto and similar fermented foods. Both K1 and K2 activate vitamin K–dependant proteins, including MGP. Dialysis patients are often advised to restrict their intake of sodium- and potassium-containing foods (such as cheese and green vegetables). Probably for this reason, vitamin K deficiency is common in the dialysis population [4]. In our patient, this predisposition to deficiency was likely exacerbated by poor absorption secondary to extensive past small bowel resection.
The two pathological processes in calciphylaxis are cutaneous arteriolar stenosis and vascular occlusion. Vascular stenosis occurs insidiously from medial arteriolar calcification and is an active process of vascular smooth muscle cells that is inhibited by cMGP [2]. Vascular thrombosis tends to occur acutely and leads to painful ulceration. Our patient had some classic risk factors for calciphylaxis, such as obesity and female sex. Other traditional risk factors, including PTH and plasma phosphate [5], actually deteriorated during her recovery, probably secondary to stimulation of the parathyroid by the low-calcium dialysate. For this reason we believe the trigger for developing calciphylaxis was most likely her severe vitamin K deficiency.
All calciphylaxis lesions healed with vitamin K supplementation along with the co-interventions of lowered dialysate calcium and increased dialysis frequency. It seems likely that, of the co-interventions, vitamin K supplementation had the greatest impact on the patient’s recovery. Recovery did not rely on improvements in plasma phosphate or PTH but may have been assisted by the increased frequency of dialysis.
Conflict of interest statement
None declared.
References
- 1. Brandenburg VM, Kramann R, Rothe H. et al. Calcific uraemic arteriolopathy (calciphylaxis): data from a large nationwide registry. Nephrol Dial Transplant 2017; 32: 126–132 [DOI] [PubMed] [Google Scholar]
- 2. Tyson KL, Reynolds JL, McNair R. et al. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol 2003; 23: 489–494 [DOI] [PubMed] [Google Scholar]
- 3. Nigwekar SU, Bloch DB, Nazarian RM. et al. Vitamin K-dependent carboxylation of matrix Gla protein influences the risk of calciphylaxis. J Am Soc Nephrol 2017; 28: 1717–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cranenburg ECM, Schurgers LJ, Uiterwijk HH. et al. Vitamin K intake and status are low in hemodialysis patients. Kidney Int 2012; 82: 605–610 [DOI] [PubMed] [Google Scholar]
- 5. Fine A, Zacharias J.. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int 2002; 61: 2210–2217 [DOI] [PubMed] [Google Scholar]


