Abstract
Background
Premature cardiovascular disease in patients with chronic kidney disease (CKD) is not explained by traditional risk factors and oxidative stress may contribute via endothelial and vascular dysfunction. We investigated the effect of ascorbic acid on oxidative stress and vascular function in CKD patients compared with controls with hypertension (HTN).
Methods
A crossover study of intravenous saline and ascorbic acid was conducted. Biomarkers of oxidative stress were measured, while pulse wave analysis and brachial flow-mediated dilatation were performed to assess large artery and endothelial function.
Results
Twenty HTN and 30 CKD patients Stages 3–5 were recruited. Serum ascorbic acid was significantly lower in patients with CKD. In both groups, ascorbic acid significantly increased total antioxidant potential and superoxide. Asymmetric dimethylarginine (ADMA) was reduced significantly by ascorbic acid in the CKD group and on multivariate regression analysis, age and the presence of CKD were predictors of ADMA response to ascorbic acid. Although no effect on FMD was observed, central blood pressure and augmentation index were reduced significantly in both groups.
Conclusions
Ascorbic acid has pro- and antioxidant effects, reducing central blood pressure and augmentation index in HTN and CKD. Ascorbic acid reduces serum ADMA in CKD, which may have longer-term benefits.
Keywords: antioxidants, arterial stiffness, chronic kidney disease, endothelial dysfunction, oxidative stress
Introduction
The risk of cardiovascular disease (CVD) is significantly elevated in patients with chronic kidney disease (CKD) [1], with most patients dying of a cardiovascular cause prior to requiring renal replacement therapy. The prevalence of CVD can be explained partly by shared risk factors, but these do not fully account for the degree of observed CVD and its prevalence remains higher after adjustment for age and comorbidities [2].
Some suggest that non-conventional risk factors specific to CKD may explain this cardiovascular risk, including hyperphosphataemia, anaemia and inflammation. Oxidative stress is another risk factor characterized by an excess of reactive oxygen species (ROS) and other oxidants, which cause derangement in redox signalling, activating pathways that lead to deleterious changes to vascular biology [3].
In vivo, this is demonstrated by evidence of endothelial dysfunction and by an increase in oxidative modification of macromolecules. For example, advanced oxidative protein products (AOPPs), 8-hydroxydeoxyguanosine and F2-isoprostanes are all elevated in CKD, while restoration of renal function by kidney transplantation results in a significant reduction in these biomarkers [4, 5]. Furthermore, plasma concentration of the nitric oxide synthase (NOS) inhibitor asymmetric dimethylarginine (ADMA) are increased in CKD, which causes increased ROS production via uncoupling of endothelial NOS [6–8].
Previous studies have investigated the use of exogenous antioxidants as therapy or as manipulators of redox homeostasis to explore the mechanisms behind oxidative stress in CKD. For example, antioxidant supplementation has been shown to improve markers of oxidative stress and blood pressure (BP) in animal models of CKD and hypertension (HTN) [9, 10]. In humans, however, the data are conflicting, with some studies showing certain benefits of antioxidant therapy in patients with CKD [11, 12], and other studies finding no effect [13, 14].
In order to explore the mechanisms underlying vascular dysfunction in CKD and test the hypothesis that antioxidant administration ameliorates oxidative stress and endothelial dysfunction, we carried out a crossover study of intravenous ascorbic acid and normal saline in a population of patients with CKD Stages 3–5 [15] in comparison with a cohort with HTN and high cardiovascular risk.
Materials and methods
Subjects
Subjects with CKD were recruited from general nephrology and peritoneal dialysis (PD) clinics; subjects with HTN and normal renal function were recruited from an HTN clinic. Participants with diabetes, renovascular disease, liver disease or active infection were excluded, or if there was a history of vitamin C supplementation, renal stone disease or systemic oxalosis. Haemodialysis patients were excluded, given the need for forearm cannulation. The study was carried out in accordance with the Declaration of Helsinki, approved by the West of Scotland Research Ethics Committee, with informed consent received from all participants. The study is registered with a clinical trials registry (ISRCTN 31272864).
Study protocol
Participants attended in the morning, refraining from caffeine and nicotine and after an overnight fast. Control infusions (100 mL 0.9% saline) and ascorbic acid (2000 mg in 100 mL 0.9% saline) were given intravenously over 10 min and phlebotomy and vascular function studies performed as described below. Phlebotomy was carried out on a third occasion 1 h after ascorbic acid. Vascular function studies and analyses were performed by a single operator. Participant details were removed from both flow-mediated dilatation (FMD) and biomarker data to facilitate blinding.
Biochemical measurements
Baseline biochemistry was performed in an accredited biochemistry department. The estimated glomerular filtration rate (eGFR) was calculated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [16].
Biomarkers of oxidative stress
Blood samples were taken via an intravenous cannula contralateral to the infusion arm after the control infusion of saline, after ascorbic acid administration, and 1 h after ascorbic acid administration. Samples were analysed for a panel of biomarkers of oxidative stress as described below.
A colorimetric assay was used to measure 8-iso-prostaglandin F2α (F2-isoprostane) as a marker of lipid peroxidation (Direct 8-iso-Prostaglandin F2α Enzyme Immunoassay Kit, Assay Designs, Ann Arbor, MI, USA). The assay has an intra- and interassay coefficient of variation (CV) of 5–10%, with a cross-reactivity to other eicosanoids of <5%. The ratio of glutathione to glutathione disulphide (GSH/GSSG) was taken as a measure of reduced glutathione availability, and this was measured using a colorimetric assay (Bioxytech GSH/GSSG-412, OxisResearch, Portland, OR, USA). Intra- and interassay CVs for GSH were 0.96 and 3.11% and for GSSG were 6.45 and 7.61%, respectively. Total antioxidant potential (TAP) was measured using a quantitative colorimetric assay (Bioxytech AOP-450 Quantitative Assay for Total Antioxidant Potential, OxisResearch). The intra- and interassay CVs were 0.7 and 1.5%, respectively [17]. High-performance liquid chromatography (HPLC) was used to measure both ascorbic acid and ADMA in plasma. Intra- and interassay CVs were 1.9 and 2.5% for ADMA [18] and 1.0 and 3.7% for ascorbic acid. Whole blood samples were also taken and underwent electron paramagnetic resonance spectrometry (EPR) to measure the rate of superoxide (O2−) production (E-scan EPR system, Bruker, Billerica, MA, USA).
Assessment of endothelial function
Endothelial function was assessed by measuring the dilator response of the brachial artery to increased blood flow induced by reactive hyperaemia, termed FMD. Longitudinal recordings of the brachial artery were taken using a 7-MHz ultrasound transducer and a Siemens Accuson Sequoia ultrasound system (Siemens, Berlin, Germany). Recordings were made at baseline, after 5 min inflation of a BP cuff and after 25 µg sublingual glyceryl trinitrate (GTN). FMD was performed according to the 2002 guidelines [19] and recent update [20]. Image analysis was performed offline using bespoke software (Brachial Analyzer 5, Medical Imaging Applications, Coralville, IA, USA). Absolute FMD was calculated as the baseline diameter subtracted from the peak diameter following cuff inflation, while the percentage of FMD was calculated as the absolute FMD divided by the baseline diameter. The intra-operator CV of FMD was 14.68%.
Assessment of arterial function
Arterial properties were evaluated using the SphygmoCor Vx system (Atcor Medical, West Ryde, NSW, Australia) of applanation tonometry. The augmentation index (Aix) was measured at the radial artery over 15 s and corrected to a heart rate of 75 bpm to derive the adjusted Aix (adjAix). Central BP was derived from the central pulse wave by means of a Fourier transformation of the measured radial pulse wave. Pulse wave velocity (PWV) was measured by measuring the distance between the right carotid and femoral pulses (via the umbilicus) and then the time between the R wave of the electrocardiogram and the upstroke of the arterial waveform was measured at each pulse. The carotid to femoral PWV is equal to the distance between the two pulses divided by the transit time. The intra-operator CVs for adjAix and PWV were 5.25 and 4.83%, respectively.
Statistics
In a sample size calculation, based on a previous study where FMD was improved by ascorbic acid in renal transplant recipients [12], 26 patients with CKD would need to be examined to demonstrate a clinically relevant improvement in FMD from the saline infusion to the ascorbic acid infusion of >2% with a standard deviation of 3%, power of 90%, α = 0.05 and with a paired comparison.
Between-group data were compared using a two-tailed Student’s t-test or Mann–Whitney U-test. For related data, a paired samples Student’s t-test or Wilcoxon’s test was used. P < 0.05 was considered significant.
A comparison of response to ascorbic acid between the CKD and HTN groups was made using a difference in differences analysis (DID): a regression analysis was performed with the mean of the measured biomarker as the dependent variable and the treatment, time point and treatment × time point as predictor variables.
The relationship between the change in ADMA following ascorbic acid and the presence of CKD was examined via regression analysis initially with a univariate analysis (simple correlation) and then stepwise multiple linear regression analysis, with inclusion of variables at P < 0.05. Given the small sample size, covariates were entered into the multivariate model based on published literature on ADMA regulation [21–24]. All statistical testing was performed using SPSS version 22.0 (IBM, Armonk, NY, USA).
Results
Thirty subjects with CKD (22 CKD Stages 3–5; 8 PD) and 20 subjects with HTN were recruited, with eGFRs of 22.4 ± 12.6 mL/min/1.73 m2 and 94.4 ± 11.7 mL/min/1.73 m2, respectively (P < 0.001). The two groups were well matched for age, body mass index (BMI), smoking status and medication history, but BP was higher in the HTN group (Table 1).
Table 1.
Baseline parameters in both cohorts
| Parameter | HTN | CKD | P-value |
|---|---|---|---|
| Age (years) | 56 ± 10 | 59 ± 14 | 0.34 |
| BMI (kg/m2) | 30.9 ± 5.4 | 28.1 ± 6.7 | 0.13 |
| Peripheral BP (mmHg) | 150/95 ± 17/10 | 141/82 ± 15/10 | 0.04* |
| Mean arterial BP (mmHg) | 112 ± 12 | 101.6 ± 10.2 | 0.002* |
| Creatinine (µmol/L) | 71 ± 11 | 359 ± 274 | <0.001* |
| eGFR (mL/min/1.73m2) | 94.4 ± 11.7 | 22.4 ± 12.6 | <0.001* |
| Angiotensin-converting enzyme inhibitor, n (%) | 9 (45) | 15 (50) | 0.62 |
| Angiotensin receptor blocker, n (%) | 6 (30) | 7 (23) | 0.60 |
| Beta-blocker, n (%) | 3 (15) | 10 (33) | 0.15 |
| Calcium channel blocker, n (%) | 10 (50) | 15 (50) | 1.00 |
| Statin, n (%) | 6 (30) | 16 (53) | 0.10 |
| Allopurinol, n (%) | 2 (10) | 10 (33) | 0.06 |
| Spironolactone, n (%) | 2 (10) | 0 (0) | 0.07 |
| Current smoker, n (%) | 4 (20) | 6 (20) | 0.62 |
Indicate a significant difference.
Biomarkers
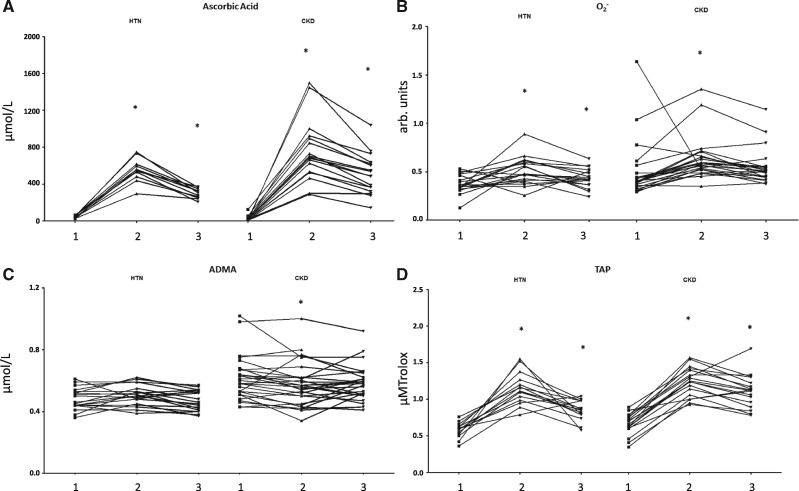
Table 2 and Figure 1 show the baseline and change in biomarkers of oxidative stress after ascorbic acid in each group.
Table 2.
Results of biomarker assays before and after administration of ascorbic acid
| Parameter | HTN |
CKD |
||||
|---|---|---|---|---|---|---|
| Before | After | P-value | Before | After | P-value | |
| Ascorbic acid (µmol/L) | 44.0 ± 14.1a | 552.9 ± 127.9b | <0.001 | 22.5 ± 27.5a | 705.7 ± 347.5b | <0.001 |
| ADMA (µmol/L) | 0.48 ± 0.07 | 0.51 ± 0.07 | 0.36 | 0.61 ± 0.14 | 0.58 ± 0.14 b | 0.039 |
| F2-isoprostanes (pg/mL) | 899.0 ± 643.0 | 834.9 ± 651.0 | 0.63 | 1036.9 ± 1048.6 | 1015.3 ± 994.3 | 0.93 |
| TAP (µM Trolox) | 0.58 ± 0.10a | 1.15 ± 0.22b | <0.001 | 0.66 ± 0.15a | 1.24 ± 0.20b | <0.001 |
| GSH:GSSG ratio | 140.0 ± 448.8 | 111.3 ± 390.4 | 0.84 | 91.1 ± 251.9 | 51.9 ± 92.6 | 0.30 |
| production (arbitrary units) | 0.37 ± 0.10 | 0.50 ± 0.14b | 0.005 | 0.50 ± 0.29 | 0.63 ± 0.22b | 0.038 |
Indicates parameters where there was a significant difference in baseline values between the two groups in an independent samples t-test.
This, along with P-values, refers to a paired samples t-test comparing values measured before and after ascorbic acid.
Fig. 1.
Change in (A) ascorbic acid, (B) production, (C) ADMA and (D) TAP in both groups after administration of ascorbic acid. Measurements of biomarkers of oxidative stress made in both groups are shown at (1) baseline, (2) after ascorbic acid and (3) at 1 h after ascorbic acid. *Indicates a significant difference (P < 0.05) in comparison with baseline.
Baseline ascorbic acid was lower in patients with CKD than in HTN (22.5 ± 27.5 versus 44.0 ± 14.1 µmol/L; P = 0.023), while ADMA (0.61 ± 0.14 versus 0.48 ± 0.07 µmol/L; P < 0.001) and TAP (0.66 ± 0.15 µM Trolox versus 0.58 ± 0.10 µM Trolox; P = 0.027) were higher. At baseline there were no between-group differences in F2-isoprostanes (1036.9 ± 1048.6 versus 899.0 ± 643.0 pg/mL; P = 0.61), GSH:GSSG (91.1 ± 251.9 versus 140.0 ± 448.8; P = 0.75) or production (0.50 ± 0.29 versus 0.37 ± 0.95 arbitrary units; P = 0.08).
Serum ascorbic acid levels rose immediately after its administration, before falling, albeit to higher than baseline, at the third time point (22.5, 705.7 and 483.2 versus 44.0, 552.9 and 297.5 µmol/L; P < 0.001). In CKD, TAP changed from 0.66 ± 0.15 to 1.24 ± 0.20 (P < 0.001) to 1.11 ± 0.22 µM Trolox (P < 0.001), while production changed from 0.50 ± 0.29 to 0.63 ± 0.22 (P = 0.038) to 0.55 ± 0.18 arbitrary units (P = 0.42). In HTN, TAP changed from 0.58 ± 0.10 to 1.15 ± 0.22 (P < 0.001) to 0.85 ± 0.14 µM Trolox (P < 0.001) and O2− changed from 0.37 ± 0.10 to 0.50 ± 0.14 (P = 0.005) to 0.43 ± 0.10 arbitrary units (P = 0.007).
There was no change in F2-isoprostanes observed in CKD (1036.9 ± 1048.6, 1015.3 ± 994.3, 855.9 ± 383.5 pg/mL; P = 0.93) or HTN (899.0 ± 643.0, 834.9 ± 651.0, 728.6 ± 463.8 pg/mL; P = 0.63), nor was there a change in GSH:GSSG in CKD (91.1 ± 251.9, 51.9 ± 92.6, 45.2 ± 82.0; P = 0.30) or HTN (140.0 ± 448.8, 111.3 ± 390.4, 41.6 ± 74.7; P = 0.84).
After administration of ascorbic acid there was a reduction in ADMA in the CKD group (0.61 ± 0.14 to 0.58 ± 0.14 µmol/L; P = 0.039), but not the HTN group (0.48 ± 0.07 to 0.51 ± 0.07 µmol/L; P = 0.36), which reached statistical significance in the second but not the third phlebotomy time point. The degree of change in ADMA was also significantly different between the two groups (–0.04 ± 0.09 versus 0.02 ± 0.06 µmol/L; P = 0.013).
In a DID analysis, there was no between-group differences in the response to ascorbic acid of ascorbic acid (174.3 µmol/L; P = 0.12), ADMA (0.06 µmol/L; P = 0.22), F2-isoprostanes (42.4 pg/mL; P = 0.91), TAP (0.01 µM Trolox; P = 0.91), GSH:GSSH ratio (26.2; P = 0.84) or O2− (0.00 arbitrary units; P = 0.99).
A regression analysis was carried out on the determinants of the response of ADMA to ascorbic acid with age, presence of CKD, BMI, BP and gender entered into the model (Table 3). Due to the small sample size, only a limited number of variables were entered. These variables were included because of published data suggesting their involvement in ADMA regulation [21–24] and first entered into a simple regression analysis. In a multiple regression analysis, age was positively associated with a change in ADMA in response to ascorbic acid and the presence of CKD was negatively associated, while BMI, BP and gender were not significant predictors (R2 = 0.21, P = 0.008).
Table 3.
Simple and multiple linear regression analysis of the predictors of the change in ADMA in nanomoles in response to ascorbic acid administration.
| Predictor | Increment | Simple regression |
Multiple regression |
||||
|---|---|---|---|---|---|---|---|
| B | 95% CI | P-value | B | 95% CI | P-value | ||
| CKD | −60.81 | −108.08 to −13.54 | 0.01 | −70.89 | −119.91 to −21.86 | 0.006 | |
| Age (years) | 10 | 14.70 | −4.94–34.35 | 0.14 | 19.96 | 0.72–39.19 | 0.042 |
| Central systolic BP (mmHg) | 10 | −0.69 | −16.45–15.07 | 0.93 | |||
| BMI (kg/m2) | 1 | 2.05 | −1.80–5.90 | 0.29 | |||
| Male | 1.51 | −49.64–52.67 | 0.95 | ||||
CI, confidence interval.
Arterial stiffness
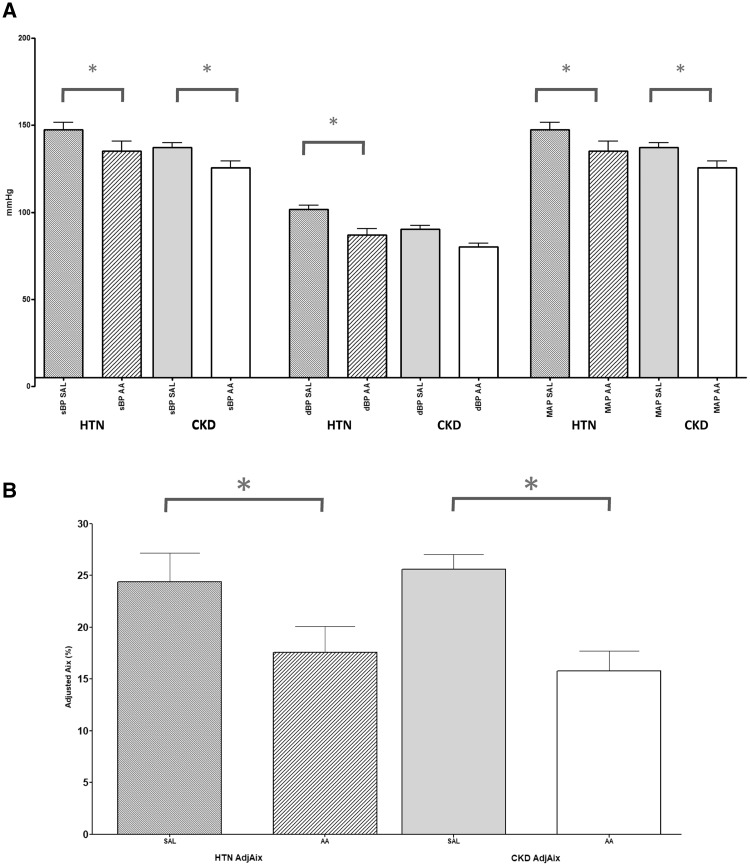
Figure 2 and Table 4 demonstrate measurements of vascular function studies before and after ascorbic acid.
Fig. 2.
Change in (A) central BP and (B) Aix in both HTN and CKD. *Indicate a significant difference (P < 0.05) occurring after ascorbic acid. SAL, normal saline; AA, ascorbic acid; NS, not significant.
Table 4.
Results of vascular function tests before and after administration of ascorbic acid
| Parameter | HTN |
CKD |
||||
|---|---|---|---|---|---|---|
| Before | After | P-value | Before | After | P-value | |
| Central BP (mmHg) | 142/97 ± 18/10a | 130/81 ± 25/15b | 0.002 | 132/85 ± 15/12a | 120/75 ± 21/11b | 0.005 |
| adjAix (%) | 24.4 ± 12.3a | 17.6 ± 11.3b | <0.001 | 25.6 ± 8.0a | 15.8 ± 10.4b | <0.001 |
| PWV (m/s) | 8.6 ± 1.7a | 8.4 ± 1.5 | 0.28 | 10.2 ± 2.5a | 9.7 ± 3.0 | 0.11 |
| Brachial artery diameter (mm) | 4.71 ± 0.81 | 5.15 ± 0.84b | <0.001 | 4.40 ± 0.67 | 4.76 ± 0.87b | <0.001 |
| FMD (mm) | 0.19 ± 0.07 | 0.25 ± 0.14 | 0.12 | 0.20 ± 0.11 | 0.27 ± 0.15 | 0.10 |
| FMD (%) | 4.13 ± 1.99 | 5.08 ± 3.00 | 0.31 | 4.89 ± 3.16 | 5.77 ± 3.62 | 0.35 |
| GTN-mediated dilatation (mm) | 0.55 ± 0.25 | 0.32 ± 0.23b | <0.001 | 0.61 ± 0.16 | 0.33 ± 0.22 b | <0.001 |
| GTN-mediated dilatation (%) | 12.30 ± 5.91 | 6.63 ± 4.91b | <0.001 | 14.17 ± 4.66 | 7.60 ± 5.39 b | <0.001 |
Indicates parameters where there was a significant difference in baseline values between the two groups in an independent samples t-test.
This, along with P-values, refers to a paired samples t-test comparing values measured before and after ascorbic acid.
Baseline central BP was higher in the HTN group (132/85 ± 15/11 versus 142/97 ± 18/10 mmHg; P = 0.042) but there was no difference in adjAix (25.6 ± 8.0 versus 24.4 ± 12.3%; P = 0.70). PWV was higher in the CKD group (10.2 ± 2.5 versus 8.6 ± 1.7 m/s; P = 0.022).
Following administration of ascorbic acid, central BP fell in both CKD (132/85 ± 15/11 to 120/75 ± 21/11 mmHg; P = 0.005) and HTN (142/97 ± 18/10 to 130/81 ± 25/15 mmHg; P = 0.002). A reduction in adjAix was seen in CKD (25.6 ± 8.0 to 15.8 ± 10.4%; P < 0.001) and HTN (24.4 ± 12.3 to 17.6 ± 11.3%; P < 0.001), but no change in PWV was observed in either group.
In a DID analysis, there was no between-group difference in response to ascorbic acid of central systolic BP (0.62 mmHg P = 0.94), adjAix (3.0%, P = 0.49) or PWV (0.29 m/s, P = 0.77).
Endothelial dysfunction
At baseline, no significant difference was observed in the diameter of the brachial artery between the HTN and CKD groups (4.71 ± 0.81 versus 4.40 ± 0.67 mm; P = 0.18) (Table 4). Neither was there a between-group difference in FMD in either absolute (0.19 ± 0.07 versus 0.19 ± 0.12 mm; P = 0.27) or proportional (4.13 ± 1.99 versus 4.89 ± 3.16%; P = 0.51) terms.
In the HTN group, after administration of ascorbic acid, FMD changed from 0.19 ± 0.07 to 0.25 ± 0.14 (P = 0.12) and the percentage FMD changed from 4.13 ± 1.99 to 5.08 ± 3.00 (P = 0.31). In the CKD group FMD changed from 0.20 ± 0.11 to 0.27 ± 0.15 mm (P = 0.10) and proportional FMD changed from 4.89 ± 3.16 to 5.77 ± 3.62 (P = 0.35) after ascorbic acid. There was a significant reduction in endothelial independent dilatation after ascorbic acid administration, in both the HTN group (12.30 ± 5.91 versus 6.63 ± 4.91%; P < 0.001) and the CKD group (14.17 ± 4.66 versus 7.60 ± 5.39%; P < 0.001).
In a DID analysis there was no between-group difference in the change in FMD after ascorbic acid (0.43 mm, P = 0.75)
Discussion
Oxidative stress is a state of disturbed redox signalling due to an excess of ROS and derivatives and a consequent depletion of cardioprotective signalling molecules such as nitric oxide (NO). Understanding the mechanisms underlying these processes may lead to the development of novel therapeutics that will reduce the burden of CVD in patients with CKD.
In both cohorts there was a numerical increase in FMD following administration of ascorbic acid, which did not reach statistical significance. Results of previous studies into the vascular effects of ascorbic acid are conflicting: Cross et al. [13] found no improvement in FMD after parenteral administration of ascorbic acid, but Williams et al. [12] found improvement in endothelium-dependent vasodilatation after ascorbic acid supplementation in a cohort of transplant patients with mild impairment of kidney function. In an earlier study by Taddei et al. [25], ascorbic acid augmented the increase in forearm blood flow caused by acetylcholine but not sodium nitroprusside, and this effect was reversed by NOS inhibition, suggesting that ascorbic acid increases endothelium-dependent vasodilatation in an NO-dependent manner. That no effect on FMD was observed in this study could be attributed to the method of administration, given as an intravenous pulse rather than an intra-arterial infusion or oral supplement. It is also possible that the studied population was of inherently high cardiovascular risk compared with other studies, as demonstrated by the lower FMD and high PWV at baseline, which may result in more resistant vascular dysfunction.
The presence of CKD was associated with a decrease in ADMA after administration of ascorbic acid, echoing the results of earlier studies that have shown a reduction in ADMA levels in patients with CKD supplemented with vitamin E [26]. Given that ADMA is a significant predictor of cardiovascular outcome [27], this may represent an important mechanism by which antioxidants exert a beneficial cardiovascular effect, which should be explored in a larger randomized placebo-controlled study.
Conversely, ascorbic acid reduced central BP in both the HTN and CKD groups by 12/15 and 12/10 mmHg, respectively, and this was associated with a reduction in Aix. This was also reflected in an increase in the diameter of the brachial artery at the baseline stage of FMD, suggesting that this might have occurred due to systemic vasodilatation. Juraschek et al. [28] previously conducted a meta-analysis of the effect of longitudinal ascorbic acid supplementation on BP, finding that systolic and diastolic BPs were reduced by 4.85 and 1.67 mmHg, respectively.
It is interesting that the vascular effects of ascorbic acid are little different in HTN compared with CKD and that there was little difference in either biomarkers of oxidative stress or endothelial dysfunction at baseline. While baseline FMD in the CKD group was similar to other studies in this cohort [29–31], in the HTN group, FMD was 4.13%, which is lower than in many other studies in similar populations. Having been recruited from a tertiary referral BP clinic, it is possible that this represents a population with higher cardiovascular risk than in previously studied populations with HTN and normal renal function [32]. Similarly, although the two study groups were well matched for age, BMI, smoking status and medication history, BP was higher in the HTN group. In the absence of any confounding factors, such as diabetes or pre-existing coronary artery disease, it is possible that such higher BP might offset the differences in vascular disease occurring due to renal impairment.
There are limitations to this study that should be acknowledged. Patients with diabetes were excluded to examine the specific effect of uraemia on vascular function and oxidative stress, but it could be argued that this limits the applicability of these data to those with diabetes. Bias may also have been introduced by the need for participants to undergo a prolonged study visit that may select out healthier patients. Vascular function studies and biomarkers are evaluated on a single day and repeated arterial occlusion and exposure to GTN may confound the interpretation of brachial artery ultrasound measurements. Nevertheless, performing the study on a single day removes the confounding effect of day-to-day variability from the results. Serum levels of ascorbic acid rose to very high levels during the study, higher than would be predicted in healthy subjects [33] or during oral supplementation [34], such that the relevance of the effects of such levels of antioxidant could be questioned, although it has previously been shown that supraphysiological doses of ascorbic acid are required to manipulate vascular function [35]. Furthermore, the sample size calculation was based on between-group differences in FMD, so differences in other measured parameters could be due to chance rather than true between-group differences. Indeed, the study is limited in large part by its small sample size and should be viewed as a proof-of-concept study. Nevertheless, our findings demonstrate that more robust studies with well-characterized cohorts are required to more precisely measure the oxidative stress associated specifically with uraemia, separate from other risk factors.
In summary, in comparison with matched hypertensive controls, CKD patients have ascorbic acid deficiency but otherwise similar levels of oxidative stress and endothelial dysfunction. In both CKD and in a high-risk hypertensive population, parenteral ascorbic acid reduces central BP and Aix in a manner independent of endothelial function. Further studies are required to assess the effects of chronic ascorbic acid on vascular function in these populations.
Funding
This study was funded by the Glasgow Renal and Transplant Endowment fund.
Conflict of interest statement
None declared.
References
- 1. Culleton BF, Larson MG, Wilson PW. et al. Cardiovascular disease and mortality in a community-based cohort with mild renal insufficiency. Kidney Int 1999; 56: 2214–2219 [DOI] [PubMed] [Google Scholar]
- 2. Go AS, Chertow GM, Fan D. et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305 [DOI] [PubMed] [Google Scholar]
- 3. Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol 2008; 295;C849–C868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simmons EM, Langone A, Sezer MT. et al. Effect of renal transplantation on biomarkers of inflammation and oxidative stress in end-stage renal disease patients. Transplantation 2005; 79: 914–919 [DOI] [PubMed] [Google Scholar]
- 5. Yilmaz MI, Saglam M, Caglar K. et al. Endothelial functions improve with decrease in asymmetric dimethylarginine (ADMA) levels after renal transplantation. Transplantation 2005; 80: 1660–1666 [DOI] [PubMed] [Google Scholar]
- 6. Fujiwara N, Nakamura T, Sato E. et al. Renovascular protective effects of erythropoietin in patients with chronic kidney disease. Intern Med 2011; 50: 1929–1934 [DOI] [PubMed] [Google Scholar]
- 7. Valli A, Suliman ME, Meert N. et al. Overestimation of advanced oxidation protein products in uremic plasma due to presence of triglycerides and other endogenous factors. Clin Chim Acta 2007; 379: 87–94 [DOI] [PubMed] [Google Scholar]
- 8. Oberg BP, McMenamin E, Lucas FLEE. et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int 2004; 65: 1009–1016 [DOI] [PubMed] [Google Scholar]
- 9. Tian N, Rose RA, Jordan S. et al. N-Acetylcysteine improves renal dysfunction, ameliorates kidney damage and decreases blood pressure in salt-sensitive hypertension. J Hypertens 2006; 24: 2263–2270 [DOI] [PubMed] [Google Scholar]
- 10. Shing CM, Fassett RG, Peake JM. et al. Effect of tocopherol on atherosclerosis, vascular function, and inflammation in apolipoprotein E knockout mice with subtotal nephrectomy. Cardiovasc Ther 2014; 32: 270–275 [DOI] [PubMed] [Google Scholar]
- 11. Boaz M, Smetana S, Weinstein T. et al. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): randomised placebo-controlled trial. Lancet 2000; 356: 1213–1218 [DOI] [PubMed] [Google Scholar]
- 12. Williams MJA, Sutherland WHF, McCormick MP. et al. Vitamin C improves endothelial dysfunction in renal allograft recipients. Nephrol Dial Transplant 2001; 16: 1251–1255 [DOI] [PubMed] [Google Scholar]
- 13. Cross JM, Donald AE, Nuttall SL. et al. Vitamin C improves resistance but not conduit artery endothelial function in patients with chronic renal failure. Kidney Int 2003; 63: 1433–1442 [DOI] [PubMed] [Google Scholar]
- 14. Ramos LF, Kane J, McMonagle E. et al. Effects of combination tocopherols and alpha lipoic acid therapy on oxidative stress and inflammatory biomarkers in chronic kidney disease. J Ren Nutr 2011; 21: 211–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapter 2: Definition, identification, and prediction of CKD progression. Kidney Int Suppl2013; 3: 63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Apak R, Güçlü K, Özyürek M. et al. Total antioxidant capacity assay of human serum using copper(II)-neocuproine as chromogenic oxidant: the CUPRAC method. Free Radic Res 2005; 39: 949–961 [DOI] [PubMed] [Google Scholar]
- 18. Blackwell S, O’Reilly DS, Talwar DK. et al. HPLC analysis of asymmetric dimethylarginine (ADMA) and related arginine metabolites in human plasma using a novel non-endogenous internal standard. Clin Chim Acta 2009; 401: 14–19 [DOI] [PubMed] [Google Scholar]
- 19. Corretti MC, Anderson TJ, Benjamin EJ. et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002; 39: 257–265 [DOI] [PubMed] [Google Scholar]
- 20. Thijssen DHJ, Black MA, Pyke KE. et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 2011; 300: H2–H12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sibal L, Agarwal SC, Home PD. et al. The role of asymmetric dimethylarginine (ADMA) in endothelial dysfunction and cardiovascular disease. Curr Cardiol Rev 2010; 6: 82–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eid HM, Arnesen H, Hjerkinn EM. et al. Relationship between obesity, smoking, and the endogenous nitric oxide synthase inhibitor, asymmetric dimethylarginine. Metabolism 2004; 53: 1574–1579 [DOI] [PubMed] [Google Scholar]
- 23. Panza JA, Quyyumi AA, Brush JE Jr. et al. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med 1990; 323: 22–27 [DOI] [PubMed] [Google Scholar]
- 24. Juonala M, Viikari JSA, Alfthan G. et al. Brachial artery flow-mediated dilation and asymmetrical dimethylarginine in the cardiovascular risk in young Finns study. Circulation 2007; 116: 1367–1373 [DOI] [PubMed] [Google Scholar]
- 25. Taddei S, Virdis A, Ghiadoni L. et al. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation 1998; 97: 2222–2229 [DOI] [PubMed] [Google Scholar]
- 26. Saran R, Novak JE, Desai A.. Impact of vitamin E on plasma asymmetric dimethylarginine (ADMA) in chronic kidney disease (CKD): a pilot study. Nephrol Dial Transplant 2003; 18: 2415–2420 [DOI] [PubMed] [Google Scholar]
- 27. Zoccali C, Bode-Böger SM, Mallamaci F. et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet 2001; 358: 2113–2117 [DOI] [PubMed] [Google Scholar]
- 28. Juraschek SP, Guallar E, Appel LJ. et al. Effects of vitamin C supplementation on blood pressure: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2012; 95: 1079–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Recio-Mayoral A, Banerjee D, Streather C, et al. Endothelial dysfunction, inflammation and atherosclerosis in chronic kidney disease–a cross-sectional study of predialysis, dialysis and kidney-transplantation patients. Atherosclerosis 2011; 216: 446–451 [DOI] [PubMed] [Google Scholar]
- 30. Takahashi N, Morimoto S, Okigaki M. et al. Decreased plasma level of vitamin C in chronic kidney disease: comparison between diabetic and non-diabetic patients. Nephrol Dial Transplant 1252; 26: 1252–1257 [DOI] [PubMed] [Google Scholar]
- 31. Yilmaz MI, Saglam M, Caglar K. et al. The determinants of endothelial dysfunction in CKD: oxidative stress and asymmetric dimethylarginine. Am J Kidney Dis 2006; 47: 42–50 [DOI] [PubMed] [Google Scholar]
- 32. Shimbo D, Muntner P, Mann D. et al. Endothelial dysfunction and the risk of hypertension: the multi-ethnic study of atherosclerosis. Hypertension 2010; 55: 1210–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khaw K-T, Bingham S, Welch A. et al. Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study. Lancet 2001; 357: 657–663 [DOI] [PubMed] [Google Scholar]
- 34. Heart Protection Study Collaborative G. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360: 23–33 [DOI] [PubMed] [Google Scholar]
- 35. Sherman DL, Keaney JF Jr, Biegelsen ES. et al. Pharmacological concentrations of ascorbic acid are required for the beneficial effect on endothelial vasomotor function in hypertension. Hypertension 2000; 35: 936–941 [DOI] [PubMed] [Google Scholar]