Abstract
Background
In the UK, primary care records are electronic and require doctors to ascribe disease codes to direct care plans and facilitate safe prescribing. We investigated factors associated with coding of chronic kidney disease (CKD) in patients with reduced kidney function and the impact this has on patient management.
Methods
We identified patients meeting biochemical criteria for CKD (two estimated glomerular filtration rates <60 mL/min/1.73 m2 taken >90 days apart) from 1039 general practitioner (GP) practices in a UK audit. Clustered logistic regression was used to identify factors associated with coding for CKD and improvement in coding as a result of the audit process. We investigated the relationship between coding and five interventions recommended for CKD: achieving blood pressure targets, proteinuria testing, statin prescription and flu and pneumococcal vaccination.
Results
Of 256 000 patients with biochemical CKD, 30% did not have a GP CKD code. Males, older patients, those with more severe CKD, diabetes or hypertension or those prescribed statins were more likely to have a CKD code. Among those with continued biochemical CKD following audit, these same characteristics increased the odds of improved coding. Patients without any kidney diagnosis were less likely to receive optimal care than those coded for CKD [e.g. odds ratio for meeting blood pressure target 0.78 (95% confidence interval 0.76–0.79)].
Conclusion
Older age, male sex, diabetes and hypertension are associated with coding for those with biochemical CKD. CKD coding is associated with receiving key primary care interventions recommended for CKD. Increased efforts to incentivize CKD coding may improve outcomes for CKD patients.
Keywords: audit, chronic kidney disease, coding, management, primary care
INTRODUCTION
Chronic kidney disease (CKD) has an estimated prevalence in the UK of ∼5–7% [1, 2], based on creatinine measurements for Stages 3–5 disease. The majority of CKD patients are diagnosed and managed by primary care physicians rather than kidney specialists in secondary care settings. Early identification of people with CKD in primary care, particularly among populations with risk factors such as diabetes and hypertension, enables early management of high blood pressure and correction of adverse lifestyle factors. Progression of CKD can be delayed by such interventions [3] and the implementation of these interventions can be improved by use of quality improvement (QI) tools in primary care [4].
In the UK, primary care health records are computerized, with each condition given a diagnostic Read code to enable more systematic patient management and appropriate prescribing. The UK Quality and Outcomes Framework (QOF) [5] is an ongoing pay-for-performance system that incentivizes aspects of primary care delivery. Coding for CKD based on two estimated glomerular filtration rate (eGFR) measurements <60 mL/min/1.73 m2 within 90 days has been incentivized in the QOF; however, there is evidence to suggest that this system does not capture all CKD cases meeting diagnostic criteria. It has been reported that only 55–70% of patients with biochemical evidence of CKD (Stages 3–5) have an appropriate Read code in general practitioner (GP) practice databases [6–8]. Practice-level prevalence of coded CKD (as captured in the QOF) is positively associated with practice prevalence of diabetes and cardiovascular disease (CVD) and negatively associated with social deprivation [9]. The extent of this lack of coding varies widely by GP practice, even after accounting for practice-level differences in risk factors such as diabetes [7]. The QOF registers are also subject to error relating to cases coded as CKD in the absence of biochemical evidence; recent data suggests that 11% of cases on QOF registers do not fulfil biochemical testing criteria, rising to 36% among those with black ethnicity [1]. It has further been shown that appropriate coding of CKD in the primary care electronic record may be associated with improved blood pressure management and urinary albuminuria testing compared with those with uncoded CKD [6].
The National Chronic Kidney Disease Audit (NCKDA) [7] was set up to audit the testing, identification and management of CKD in primary care in the UK. The audit capitalized on the existence of computerized practice records and used an automated extraction tool that directly extracted data from the electronic health record with automatic encrypted upload to a central data safe haven. The first round of data collection (round 1) provided an initial snapshot of the above outcomes for the practices enrolled in the audit. Practices were encouraged to make use of the electronic QI tools for CKD, which were developed by the NCKDA team in collaboration with Informatica Systems as an integral component of the audit. The QI tools provided practice lists of people with risk factors who may need testing for CKD, people who may require CKD coding or coding removal and prompts to support the management of those with coded CKD. In addition, consultation prompts alerting clinicians to people with uncoded CKD could be activated [1]. A second extraction of data was made (round 2) at least 90 days after round 1 to ascertain the impact of the QI aspect of the audit process.
We used individual patient data from the NCKDA to investigate the associations between individual patient characteristics and coding for CKD among those with biochemical evidence of CKD based on creatinine measurements. We further sought to identify the characteristics associated with improvements in coding status at round 2. Among patients with biochemical evidence of CKD, we then investigated the relationship between coding and five key markers of primary care management of CKD [10]: (i) meeting blood pressure targets, (ii) being offered statins for CVD prevention, (iii) receipt of urinary albumin:creatinine ratio (ACR) or protein:creatinine ratio (PCR) testing, (iv) receipt of flu vaccine and (v) receipt of pneumococcal vaccine (for those with CKD Stages 4–5).
This work will help identify whether there are population subgroups for whom coding for CKD requires improvement and whether these same characteristics are associated with a lack of coding improvement or receipt of primary care interventions aimed at improving patient outcomes.
MATERIALS AND METHODS
Data source and study population
All practices in England and Wales who were current users of the Informatica Audit Plus software were invited to participate in NCKDA between March 2015 and July 2016. NCKDA round 1 data were collected from all GPs in 1039 GP practices representing an underlying population >18 years of age of 8.24 million in England and Wales. Coverage in England and Wales differed substantially as a result of technical difficulties and differential use of the software used to extract data for the NCKDA [1]; final coverage was ∼76% of practices in Wales and 9% of practices in England. All Welsh practices had Audit Plus installed (funded by the National Health Service in Wales) while in England, practices actively purchased Informatica Audit Plus software to support better disease management [1]. Data on CKD coding, eGFR test results and relating to CKD management were extracted for all patients with risk factor coding for CKD at least 1 year prior to data extraction. A full list of risk factor codes and full details regarding the study population are available elsewhere [1]. Practices received e-mail feedback about the prevalence of biochemical, coded and uncoded CKD suggesting that they might use the QI software to improve coding.
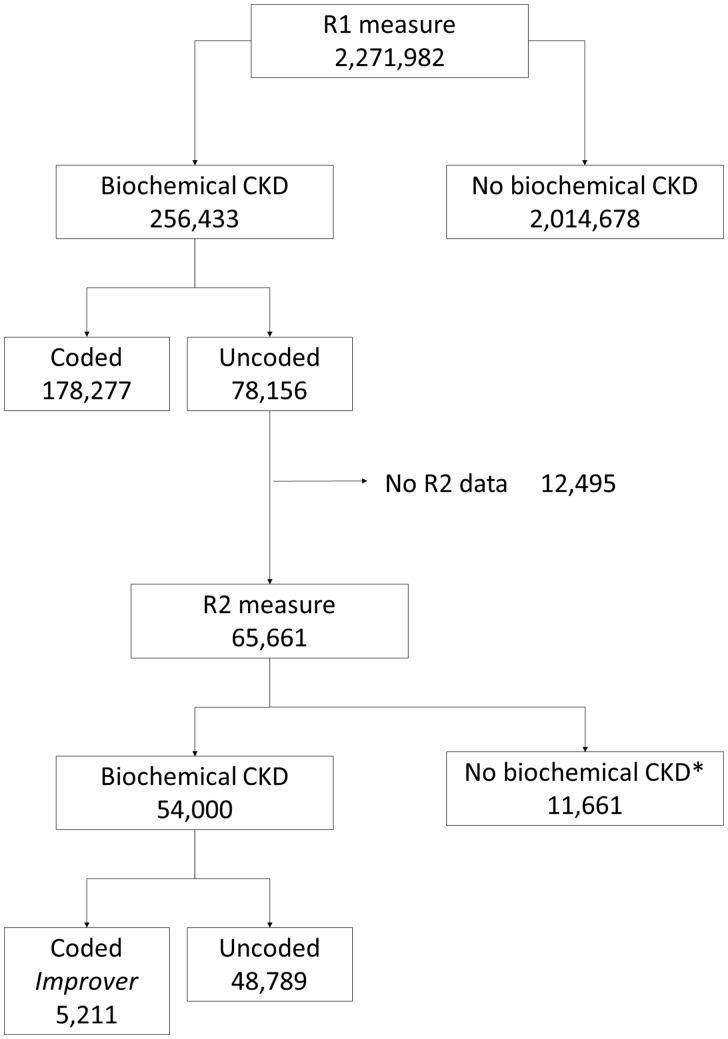
Round 2 data were collected from 948 of these practices, with a median of 8 months from round 1 (range 3–20 months). Figure 1 shows patient progress from round 1 to round 2 by coding status. A total of 65 661 patients with uncoded CKD at round 1 (i.e. no code for Stages 3–5 CKD, but with biochemical evidence for CKD) for whom round 2 data confirming biochemical CKD were available were included in an analysis of coding improvement.
FIGURE 1.
Flow chart showing progress through round 1 (R1) and round 2 (R2).
Information about referrals to secondary care was available through extraction of outpatient referral codes collected at round 1 from the GP record and linkage to outpatient records from Hospital Episode Statistics (HES) for England (data collected for the period 1 April 2012–30 June 2016) and NHS Wales Informatics Statistics (NWIS) (1 January 2012–30 June 2016).
Outcomes
Coding
Coding status for CKD (defined by the presence of a code for Stages 3–5 CKD) was analysed for 256 433 patients with biochemical evidence of CKD [two Modification of diet in renal disease-isotope dilute mass spectrometry (MDRD-IDMS) eGFR measurements <60 mL/min/1.73 m2 at least 90 days apart]. MDRD IDMS measurements incorporating the ethnicity adjustment were derived from creatinine measurements and used for this analysis, as the majority of laboratories in the UK do not report the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) eGFR.
Coding improvement
Among patients with uncoded CKD at round 1 and for whom round 2 data confirm biochemical CKD, coding improvers were defined as those who had a code for Stages 3–5 CKD at round 2.
Referrals to secondary care
Referral to secondary care is defined as any nephrologist referral code collected at round 1 from the GP records or any nephrologist outpatient clinic code held in the HES database (see Supplementary Appendix).
Primary care management of CKD
Blood pressure management
Patients were considered to have met blood pressure targets if they had blood pressure measurements taken in the previous year and had either (i) systolic blood pressure <130 mmHg and diastolic blood pressure <80 mmHg (for those with diabetes or proteinuria defined as last ACR ≥70 mg/mmol or last PCR ≥100 mg/mmol) or (ii) systolic blood pressure <140 mmHg and diastolic blood pressure <90 mmHg (for everyone else). Those with blood pressure measurements taken >1 year earlier were not included as meeting targets, regardless of the measurement. Only the single most recent blood pressure measurement was available from the GP record.
Statins
As part of a CVD prevention strategy, statin therapy is recommended for all individuals with CKD Stages 3–5 [11]. We report here on individuals for whom there was any previous recording of statin prescription collected at round 1 from the GP record.
Proteinuria testing
It is recommended that testing for proteinuria be carried out at least once a year for all individuals with CKD Stages 3–5 (increasing to four times a year for those at Stage 5) [10, 11]. Proteinuria testing was considered as having been undertaken if patients had an ACR or PCR test collected at round 1 that was from the previous year or the previous 2 years, to provide some insight into the extent of deviation from testing guidelines.
Flu vaccination
Guidelines state that all individuals with CKD Stages 3–5 should be offered an annual flu vaccination unless contraindicated [10]. We report the percentage of patients receiving this vaccination in the previous year.
Pneumococcal vaccination
For individuals with CKD Stages 4–5, it is additionally recommended that pneumococcal vaccination be administered unless contraindicated and that individuals should be offered re-vaccination within 5 years [10]. We report the percentage of patients with Stages 4–5 disease receiving this vaccination in the previous 5 years.
Among those with uncoded CKD, results are presented separately for those with and without a urological or renal diagnostic disorder code (a full list of corresponding read codes is available elsewhere [1]).
Predictors of coding and coding improvement
The following characteristics were considered as potentially being associated with coding and/or coding improvement:
Age: categorized in 10-year age bands (plus <50- and ≥90-year groups).
Index of multiple deprivation (IMD): categorized in approximate quintiles of the distribution for the study population, plus an additional category for those with missing IMD (these are all from Welsh practices; 93% of Welsh practices did not have any IMD data available).
Last known CKD stage: defined by categorizing the last known eGFR measurement using standard definitions [10]: Stage 3a (eGFR 46–59 mL/min/1.73 m2), Stage 3b (eGFR 31–45 mL/min/1.73 m2), Stage 4 (eGFR 16–30 mL/min/1.73 m2) and Stage 5 (eGFR <15 mL/min/1.73 m2).
Diabetes: defined as any previously recorded diagnosis for diabetes (incentivized by QOF).
Hypertension: defined as any previously recorded date for hypertension diagnosis (incentivized by QOF).
Statin prescription: defined as any previously recorded date on which statins were prescribed.
Country: indicator for Wales or England.
Statistical methods
Population-averaged logistic generalized estimating equations (GEE) models were fitted for having coded CKD (among those with biochemical CKD at round 1) and for coding improvement (among those with uncoded biochemical CKD at round 1 and biochemical CKD at round 2), allowing for clustering of patients within practices.
The use of five interventions for CKD was summarized among those with biochemical CKD according to CKD and renal disorder coding status. Odds ratios (ORs) comparing the coding groups for each of these management outcomes were estimated using population-averaged clustered logistic GEE models adjusted for IMD group, sex, age group, country, last known CKD stage, diabetes, hypertension, CVD and statins (except for statins outcome).
RESULTS
Predictors of coding for CKD
A breakdown of coding status by key characteristics is given in Table 1 for a total of 256 433 patients with Stages 3–5 biochemical CKD, among which 78 156 (30%) did not have a read code for CKD. There was considerable interpractice variation in the proportion of biochemical CKD cases that were coded, ranging from 4 to 100%.
Table 1.
Coding for CKD by patient characteristics among those with biochemical evidence of CKD
| n with biochemical CKD at round 1 | % of these who are coded for CKD | Univariable OR for coding (95% CI) | Multivariablea OR for coding (95% CI) | |
|---|---|---|---|---|
| Sex | ||||
| Female | 152 194 | 68.8 | 1 | 1 |
| Male | 104 239 | 70.6 | 1.09 (1.06–1.11) | 1.04 (1.02–1.07) |
| Age (years) | ||||
| <50 | 5371 | 57.7 | 0.82 (0.77–0.87) | 0.82 (0.78–0.88) |
| 50–59 | 12 612 | 55.9 | 0.79 (0.76–0.82) | 0.86 (0.83–0.89) |
| 60–69 | 39 520 | 62.1 | 1 | 1 |
| 70–79 | 82 776 | 69.7 | 1.38 (1.34–1.42) | 1.23 (1.20–1.27) |
| 80–89 | 90 209 | 73.9 | 1.69 (1.64–1.75) | 1.37 (1.32–1.41) |
| ≥90 | 25 945 | 74.2 | 1.72 (1.64–1.80) | 1.34 (1.28–1.40) |
| IMDb | ||||
| <10 000 | 41 051 | 71.4 | 1 | 1 |
| 10 000–14 999 | 28 079 | 71.2 | 0.98 (0.95–1.02) | 1.00 (0.97–1.04) |
| 15 000–19 999 | 30 222 | 70.4 | 0.95 (0.92–0.99) | 0.98 (0.94–1.02) |
| 20 000–24 999 | 31 815 | 70.1 | 0.94 (0.91–0.97) | 0.99 (0.95–1.02) |
| ≥25 000 | 40 230 | 70.1 | 0.95 (0.91–0.99) | 1.01 (0.97–1.05) |
| Missing | 85 036 | 67.2 | 0.86 (0.78–0.95) | 1.09 (0.96–1.23) |
| Last known CKD stagec | ||||
| Stage 3a | 160 100 | 60.8 | 1 | 1 |
| Stage 3b | 75 855 | 82.5 | 3.01 (2.90–3.12) | 2.71 (2.62–2.80) |
| Stage 4 | 17 224 | 89.7 | 5.64 (5.25–6.06) | 5.03 (4.70–5.38) |
| Stage 5 | 3254 | 90.3 | 5.94 (5.22–6.77) | 5.81 (5.13–6.58) |
| Diabetes | ||||
| No | 187 716 | 67.5 | 1 | 1 |
| Yes | 68 717 | 75.2 | 1.45 (1.41–1.49) | 1.11 (1.08–1.14) |
| Hypertension | ||||
| No | 74 817 | 59.2 | 1 | 1 |
| Yes | 181 616 | 73.8 | 1.83 (1.79–1.87) | 1.50 (1.47–1.53) |
| Statin offered | ||||
| No | 84 885 | 62.0 | 1 | 1 |
| Yes | 171 548 | 73.3 | 1.64 (1.60–1.67) | 1.38 (1.35–1.40) |
| Country | ||||
| Wales | 85 308 | 67.1 | 1 | 1 |
| England | 171 125 | 70.7 | 1.25 (1.12–1.40) | 1.42 (1.18–1.71) |
Simultaneous adjustment for all characteristics in the table.
Low IMD rank corresponds to higher deprivation.
Based on last eGFR measurement. Stage 3a: eGFR 46–59 mL/min/1.73 m2; Stage 3b: eGFR 31–45 mL/min/1.73 m2; Stage 4: eGFR 16–30 mL/min/1.73 m2; Stage 5: eGFR <15 mL/min/1.73 m2.
Being male, being older, having later stage CKD, lower IMD (more deprived), diabetes or hypertension and being offered statins were all associated with increased odds of coding in unadjusted analyses. In a mutually adjusted analysis, all these associations remained except for IMD (Table 1). Belonging to an English practice rather than a Welsh practice also seemed to increase the odds of coding in both the unadjusted and adjusted analyses. There was evidence that the difference between males and females was only present in Wales {multivariable OR for males in Wales 1.11 [95% confidence interval (CI) 1.07–1.14]; in England, multivariable OR 1.01 (95% CI 0.99–1.04); P-value for interaction <0.0005}.
Around half of those with uncoded Stage 5 CKD (164/317) also had a renal disorder code (Table 2). Of the 153 who did not, 45 had either a dialysis or a transplant code, and a further 70 had a nephrologist referral code (either in the audit data or HES data). This left 38/3254 (1%) patients with biochemical evidence of Stage 5 CKD who were not coded for CKD, had no other renal code and who also had no referral, dialysis or transplant code.
Table 2.
Percentage of coding for renal disorder by CKD coding status and stage among those with biochemical evidence of CKD at round 1 of the National CKD Audit
| CKD coded |
CKD not coded |
|||
|---|---|---|---|---|
| Last known CKD stagea | n | % with renal disorder code | n | % with renal disorder code |
| Stage 3a | 97 352 | 13.7 | 62 748 | 6.2 |
| Stage 3b | 62 543 | 19.3 | 13 312 | 12.0 |
| Stage 4 | 15 445 | 34.6 | 1779 | 27.7 |
| Stage 5 | 2937 | 59.1 | 317 | 51.7 |
Based on last eGFR measurement. Stage 3a: eGFR 46–59 mL/min/1.73 m2; Stage 3b: eGFR 31–45 mL/min/1.73 m2; Stage 4: eGFR 16–30 mL/min/1.73 m2; Stage 5: eGFR <15 mL/min/1.73 m2.
Predictors of coding improvement at round 2
Among those with uncoded biochemical CKD at round 1 who also had biochemical evidence of CKD at round 2, 5211 patients [of 54 000 (9.7%)] were found to have been coded at round 2 (Table 3).
Table 3.
Coding improvement among those with biochemical evidence of CKD at R1 (uncoded) and R2 of the National CKD Audit
| n with biochemical evidence of CKD at R1 (uncoded) and R2 | % of these coded at R2 | Multivariablea OR for coding improvement at R2 (95% CI) | |
|---|---|---|---|
| Sex | |||
| Female | 32 661 | 9.0 | 1 |
| Male | 21 339 | 10.7 | 1.14 (1.09–1.19) |
| Age (years) | |||
| <50 | 1507 | 8.2 | 0.83 (0.72–0.97) |
| 50–59 | 3636 | 7.5 | 0.85 (0.78–0.94) |
| 60–69 | 10 243 | 9.7 | 1 |
| 70–79 | 17 844 | 10.4 | 1.04 (0.99–1.10) |
| 80–89 | 16 537 | 9.6 | 0.96 (0.90–1.02) |
| ≥90 | 4233 | 9.2 | 0.86 (0.79–0.95) |
| IMDb | |||
| <10 000 | 7481 | 11.7 | 1 |
| 10 000–14 999 | 5286 | 11.8 | 1.01 (0.92–1.12) |
| 15 000–19 999 | 5816 | 13.0 | 0.97 (0.87–1.07) |
| 20 000–24 999 | 6514 | 10.8 | 0.93 (0.85–1.02) |
| ≥25 000 | 8088 | 10.9 | 0.94 (0.87–1.03) |
| Missing | 20 815 | 6.6 | 0.85 (0.63–1.16) |
| Last known CKD stagec | |||
| Stage 3a | 42 405 | 8.7 | 1 |
| Stage 3b | 10 085 | 12.5 | 1.50 (1.42–1.59) |
| Stage 4 | 1302 | 16.9 | 2.02 (1.78–2.30) |
| Stage 5 | 208 | 13.9 | 1.53 (1.08–2.17) |
| Diabetes status | |||
| No | 41 934 | 9.0 | 1 |
| Yes | 12 066 | 11.8 | 1.21 (1.15–1.28) |
| Hypertension status | |||
| No | 20 784 | 8.1 | 1 |
| Yes | 33 216 | 10.6 | 1.24 (1.17–1.31) |
| Statin offered | |||
| No | 21 850 | 8.8 | 1 |
| Yes | 32 150 | 10.2 | 1.08 (1.03–1.13) |
| Country | |||
| Wales | 20 917 | 6.7 | 1 |
| England | 33 083 | 11.5 | 1.21 (0.81–1.79) |
R1, round 1; R2, round 2.
Simultaneous adjustment for all characteristics in the table.
Low IMD rank corresponds to higher deprivation.
Based on the last eGFR measurement at R1. Stage 3a: eGFR 46–59 mL/min/1.73 m2; Stage 3b: eGFR 31–45 mL/min/1.73 m2; Stage 4: eGFR 16–30 mL/min/1.73 m2; Stage 5: eGFR <15 mL/min/1.73 m2.
After adjusting for other factors, those <60 years of age had a 15–20% reduction in the odds of coding improvement compared with those ≥60 years of age, although those aged ≥90 years of age also had a 15% reduction in the odds of coding improvement compared with those 60–69 years of age [OR 0.86 (95% CI 0.79, 0.95)] (Table 3). Those with CKD Stages 3b–5 all had a 1.5- to 2-fold increase in the odds of coding compared with those with biochemical evidence for CKD Stage 3a. Furthermore, there was evidence of higher odds of coding improvement among males and those with diabetes, hypertension and on statins. There was no evidence of a difference in improvement by IMD.
Associations of coding with CKD management
Receipt of all primary care management interventions was highest in those who were coded for CKD (Table 4). The odds of receiving each intervention were greatest in those with coded CKD; the odds of intervention were comparatively reduced among those with uncoded CKD and a renal disorder code (except for statins, where the adjusted odds were similar to those with coded CKD), and reduced even further for those with uncoded CKD and no renal disorder code.
Table 4.
Management outcomes for those with biochemical evidence of CKD at round 1, by coding status
| Coded CKD | Uncoded CKD with renal disorder code | Uncoded CKD without renal disorder code | |
|---|---|---|---|
| n | 178 277 | 6176 | 71 980 |
| Met blood pressure target in past yeara | |||
| % achieving outcome | 51.5 | 41.7 | 46.8 |
| Adjusted OR (95% CI)b | 1 | 0.83 (0.78–0.87) | 0.78 (0.76–0.79) |
| Statins offered | |||
| % achieving outcome | 70.5 | 69.2 | 57.8 |
| Adjusted OR (95% CI)c | 1 | 1.04 (0.97–1.11) | 0.69 (0.67–0.71) |
| ACR/PCR test in the past year | |||
| % achieving outcome | 49.7 | 32.7 | 15.9 |
| Adjusted OR (95% CI)d | 1 | 0.50 (0.45–0.55) | 0.20 (0.18–0.22) |
| ACR/PCR test in the past 2 years | |||
| % achieving outcome | 73.8 | 49.4 | 25.1 |
| Adjusted OR (95% CI)d | 1 | 0.35 (0.32–0.39) | 0.12 (0.11–0.13) |
| Flu vaccination in the past year | |||
| % achieving outcome | 79.3 | 72.9 | 69.6 |
| Adjusted OR (95% CI)b | 1 | 0.83 (0.77–0.88) | 0.75 (0.73–0.77) |
| Pneumococcus vaccination in the past 5 years, Stages 4–5 only (based on last eGFR)e | |||
| % achieving outcome | 16.1 | 15.5 | 11.3 |
| Adjusted OR (95% CI)b | 1 | 0.79 (0.63–1.00) | 0.73 (0.62–0.86) |
Measurements taken in the past year and systolic blood pressure (SBP) <130 mmHg and diastolic blood pressure (DBP) <80 mmHg (for those with diabetes or proteinuria) or SBP <140 mmHg and DBP <90 mmHg for everyone else.
Adjusted for IMD group, sex, age group, country, last known CKD stage, diabetes, hypertension, CVD and statins offered.
Adjusted for sex, age group, last known CKD stage, hypertension and CVD (due to model convergence).
Adjusted for age group and last known CKD stage only (due to model convergence).
Numbers in Stages 4–5 in each coding category: coded CKD, n = 19 076; uncoded CKD with renal code, n = 755; uncoded CKD without renal code, n = 1739.
Blood pressure targets had only been met in the previous year in ∼50% of patients with coded CKD; the odds of meeting the target were even lower in those with uncoded CKD, with a 15% reduction in those with a renal code and a 20% reduction in those without a renal disease code. Proteinuria testing was also low, at ∼50% of coded CKD patients in the previous year; this was considerably lower in those with uncoded CKD, with an ∼50% and 80% reduction in odds for those with and without renal codes, respectively. Around 70% of those patients with coded CKD had been offered a statin at some time in the past, with substantially reduced odds for those with uncoded CKD and no renal disease code. There was an ∼20% reduction in the odds of receiving both vaccinations for those with uncoded CKD and a renal code compared with those with coded CKD, and a 25% reduction in odds for those with uncoded CKD and no renal code.
Referrals to secondary care
Referrals recorded on either the GP record or HES databases accounted for 27.9% of those with uncoded CKD at round 1 with a renal code but only 5.3% of those with uncoded CKD and no renal code (compared with 19.0% of those with coded CKD).
DISCUSSION
Main findings
Younger patients, females and those without major co-morbidities (diabetes, hypertension) who have biochemical evidence of CKD are least likely to have a CKD (Stages 3–5) code in their primary care record. Patients with biochemical CKD without a CKD code were less likely to be offered a statin, receive flu and pneumococcal vaccination and have their blood pressure controlled to target or have undergone proteinuria testing. Those who have biochemical evidence of CKD and a renal code were more likely to have received some interventions, but not to the same level as people with biochemical CKD who were coded.
Main findings in context
Among the 256 433 cases with biochemical evidence of CKD at round 1 of the national CKD audit, only 70% (178 277) are included on the QOF register as CKD Stages 3–5. This compares to 72% reported by Jain et al. [6] in a sample of similar size from 2005 to 2009. Although broadly similar, some of the difference could be explained by the definitions of biochemical CKD used (we use two eGFRs at least 3 months apart, versus their two measurements 7 days apart) and our use of re-calculated eGFRs.
Among those patients with biochemical evidence of CKD, males, older patients, those with lower eGFR (more severe CKD stage), diabetes, hypertension or receiving statins and those in English practices had increased odds of being coded for CKD. Another study previously reported similar relationships with sex and co-morbidities, but not with age [12]. Our results suggest that even for patients with the same CKD stage and comorbidities, younger patients have reduced odds of coding compared with older patients. Furthermore, we have shown that these same characteristics (except country) were associated with coding improvement following audit among those patients with uncoded biochemical CKD at round 1 who still had evidence of biochemical CKD at round 2. Although others have demonstrated that QI tools can be useful in improving intervention outcomes [13], such studies have taken a more direct approach to improve specific interventions, such as blood pressure control, rather than through coding improvements, which may be more wide-reaching.
Our findings on management interventions for patients with coded CKD in primary care are also broadly similar to those reported elsewhere [14, 15], but we have also demonstrated the positive relationship between coding and patient management.
Interpretation and implication
There are many reasons why an individual with biochemical evidence of CKD may not have a corresponding code, including uncertainty about guidelines for testing and diagnosis and concern about medicalizing a natural ageing process [15]. However, the relevance of the absence of coding lies in its potential impact on a range of patient measures in primary care. Here, this is substantiated by the reported differences in the application of key management interventions between coded and uncoded groups with biochemical CKD. Among those with uncoded CKD, having a renal disorder code is associated with higher application of all these interventions, though not to the same level as those with a CKD code. We examined the possibility that the observed differences are, at least in part, due to differences in recording of these interventions and whether those with renal disorder codes can be managed in secondary rather than primary care, with a corresponding lack of recording on GP databases. Investigation of referrals to secondary care suggests that although the difference in interventions in patients with coded CKD and those with uncoded CKD and a renal disorder may be explained in part by differences in referrals, there is no evidence that patients without a CKD or a renal disorder code are receiving interventions in secondary care. However, it is also possible that some referrals were not captured here, for example, for joint specialist outpatient clinics, which may be coded under the non-nephrology speciality code in HES (e.g. joint diabetic–renal specialist clinic or joint urology–renal clinic).
Our findings suggest that practices and local health authorities should take a more active approach to ensuring CKD coding and resultant patient review for those with CKD and that implementation is encouraged using active QI techniques. In the UK, this is of particular importance, as the renal QOF indicators have now been retired [16].
Strengths and limitations
We used data from a large, population-based study to investigate relationships between coding, patient characteristics and care. Although large and with good coverage of Welsh practices (76%), the study includes only 9% of English GP practices. It is likely that practices with higher ethnic minorities are under-represented in this sample (in England, the non-white population is ∼9.1%, compared with 4.4% in Wales [17]). Previous work has suggested that CKD prevalence varies across ethnic minorities [18], but also that management outcomes may be reduced in these minority subgroups [19]. Furthermore, participating English practices chose to install the audit software and therefore were more likely to have an interest in QI. In light of this, for England, the underlying proportion of uncoded biochemical CKD cases may be even higher than we report and management outcomes may be lower in some or all of the groups of patients with CKD.
Limitations of the audit include the use of routinely collected clinical data. There will inevitably be inaccuracies in the clinical data set and it is likely that there will be underrecording of at least some morbidities; however, underrecording would mean that the GP also is not aware of the respective morbidity. The ‘missingness’ in hypertension, diabetes and statin prescriptions is not known, since this would occur where there is an absence of a recorded date, making it indistinguishable from individuals without these events. However, as recording of hypertension and diabetes has been incentivized by the QOF through a number of measures, there is little reason to assume that a majority of cases would have been missed.
CONCLUSIONS
Electronic QI initiatives, which alert practitioners to uncoded CKD cases, with in-consultation prompts and patient lists requiring action, produce a small but important improvement in coding. However, this improvement tends to be focused on older patients and those with well-established risk factors for CKD. Further efforts to improve coding for younger patients who have much to gain from regular CKD review, blood pressure and CVD risk management are needed.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
FUNDING
The NCKDA is commissioned by the Healthcare QI Partnership (HQIP) and funded by NHS England, as part of the National Clinical Audit and Patient Outcomes Programme (NCAPOP), and the Welsh Government.
CONFLICT OF INTEREST STATEMENT
D.C.W. has received honoraria from Akebia, Amgen, AstraZeneca, Boehringer Ingelheim, Vifor Fresenius Medical Care, Janssen, Otsuka and ER Squibb.
Supplementary Material
REFERENCES
- 1. Nitsch D, Caplin B, Hull SA. et al. National Chronic Kidney Disease Audit: National Report (Part 1), 2017. http://www.ckdaudit.org.uk/files/4614/8429/6654/08532_CKD_Audit_Report_Jan_17_FINAL.pdf (19 January 2017, date last accessed)
- 2. Iwagami M, Tomlinson LA, Mansfield KE. et al. Validity of estimated prevalence of decreased kidney function and renal replacement therapy from primary care electronic health records compared to national survey and registry data in the UK. Nephrol Dial Transplant 2017; 32: 142–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lv J, Ehteshami P, Sarnak MJ. et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ 2013; 185: 949–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lusignan S, Gallagher H, Jones S. et al. Audit-based education lowers systolic blood pressure in chronic kidney disease: the Quality Improvement in CKD (QICKD) trial results. Kidney Int 2013; 84: 609–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roland M, Gurthrie B.. Quality and outcomes framework: what have we learnt? BMJ 2016; 354: i4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jain P, Calvert M, Cockwell P. et al. The need for improved identification and accurate classification of stages 3–5 chronic kidney disease in primary care: retrospective cohort study. PLoS One 2014; 9: e100831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim LG, Caplin B, Cleary F. et al. Accounting for overdispersion when determining primary care outliers for the identification of chronic kidney disease: learning from the NCKDA. Nephrol Dial Transplant 2017; 32: 151–158 [DOI] [PubMed] [Google Scholar]
- 8. Jameson K, Jick S, Hagberg KW. et al. Prevalence and management of chronic kidney disease in primary care patients in the UK. Int J Clin Pract 2014; 68: 1110–1121 [DOI] [PubMed] [Google Scholar]
- 9. Walker N, Bankart J, Brunskill N. et al. Which factors are associated with higher rates of chronic kidney disease recording in primary care? A cross-sectional survey of GP practices. Br J Gen Pract 2011; 61: 203–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kidney Disease: Improving Global Outcomes (KDIGO) CKD work group. Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [Google Scholar]
- 11.National Clinical Guideline Centre. National institute for health and care excellence. chronic kidney disease in adults: assessment and management. 2014, http://www.nice.org.uk/guidance/cg182 (30 November 2015, date last accessed). [PubMed]
- 12. Fraser SD, Parkes J, Culliford D. et al. Timeliness in chronic kidney disease and albuminuria identification: a retrospective cohort study. BMC Fam Pract 2015; 16: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nihat A, de Lusignan S, Thomas N. et al. What drives quality improvement in chronic kidney disease (CKD) in primary care: process evaluation of the Quality Improvement in Chronic Kidney Disease (QICKD) trial. BMJ Open 2016; 6: e008480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Gelder VA, Scherpbier-De Haan ND, De Grauw WJ. et al. Quality of chronic kidney disease management in primary care: a retrospective study. Scand J Prim Health Care 2016; 34: 73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fraser SD, Blakeman T.. Chronic kidney disease: identification and management in primary care. Pragmat Obs Res 2016; 7: 21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.General Medical Services Contract Changes 2016/17, 2016. (13 February 2017). https://www.pcc-cic.org.uk/article/201617-gms-contract-changes
- 17. Office of National Statistics. UK Census 2011. (4 May 2017). https://www.ons.gov.uk/peoplepopulationandcommunity/culturalidentity/ethnicity/articles/ethnicityandnationalidentityinenglandandwales/2012-12-11
- 18. Aitken GR, Roderick PJ, Fraser S. et al. Change in prevalence of chronic kidney disease in England over time: comparison of nationally representative cross-sectional surveys from 2003 to 2010. BMJ Open 2014; 4: e005480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hull S, Dreyer G, Badrick E. et al. The relationship of ethnicity to the prevalence and management of hypertension and associated chronic kidney disease. BMC Nephrol 2011; 12: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.