Abstract
Background
Long-term exposure to stress has been demonstrated to cause neuroinflammation through a sustained overproduction of free radicals, including nitric oxide, via an increased inducible nitric oxide synthase activity. We previously demonstrated that inducible nitric oxide synthase activity and mRNA are significantly upregulated in the rat hippocampus following just 4 hours of restraint stress. Similar to nitric oxide, endocannabinoids are synthesized on demand, with preclinical observations suggesting that cannabinoid receptor agonists and endocannabinoid enhancers inhibit nitrergic activity. Specifically, previous work has shown that enhancement of endocannabinoids via inhibition of fatty acid amide hydrolase with PF-3845 reduced inducible nitric oxide synthase-expressing microglia following traumatic brain injury. However, this describes cannabinoid modulation following physical injury, and therefore the present study aimed to examine the effects of PF-3845 in the modulation of nitrergic and inflammatory-related genes within the hippocampus after acute stress exposure.
Methods
Following vehicle or PF-3845 injections (5 mg/kg; i.p.), male Wistar rats were exposed to 0 (control), 60, 240, or 360 minutes of restraint stress after which plasma and dorsal hippocampus were isolated for further biochemical and gene expression analysis.
Results
The results demonstrate that pretreatment with PF-3845 rapidly ameliorates plasma corticosterone release at 60 minutes of stress. An increase in endocannabinoid signalling also induces an overall attenuation in inducible nitric oxide synthase, tumor necrosis factor-alpha convertase, interleukin-6, cyclooxygenase-2, peroxisome proliferator-activated receptor gamma mRNA, and the transactivation potential of nuclear factor kappa-light-chain-enhancer of activated B cells in the hippocampus.
Conclusions
These results suggest that enhanced endocannabinoid levels in the dorsal hippocampus have an overall antinitrosative and antiinflammatory effect following acute stress exposure.
Keywords: acute stress, endocannabinoids, fatty acid amide hydrolase inhibitor, inducible nitric oxide synthase, neuroinflammatory response
Significance Statement
This study employed an enzyme inhibitor, PF-3845, to pharmacologically enhance endocannabinoid content that demonstrated antiinflammatory properties by decreasing the expression of iNOS and other inflammatory genes following acute psychological stress in rats.
Introduction
Glucocorticoids are the final hormonal response of an activated hypothalamic-pituitary-adrenal (HPA) axis, which exerts a plethora of physiological functions through the ubiquitously expressed glucocorticoid receptors. One of the essential roles of glucocorticoids along with protecting the host from noxious insults is the regulation of immune homeostasis (Cancedda et al., 2002; Cain and Cidlowski, 2017). Stress and glucocorticoids are generally regarded to be antiinflammatory, as numerous studies have demonstrated their ability to suppress gene transcription of several proinflammatory cytokines (Barnes, 1998; Coutinho and Chapman, 2011). Nevertheless, in addition to the antiinflammatory actions, it has become increasingly apparent that the initial rise in glucocorticoids following stress exposure primes the immune effector cells through innate immune signalling pathways such as toll-like receptor upregulation (de Pablos et al., 2006; Frank et al., 2007, 2012; Wohleb et al., 2011). This is considered to be an important adaptive action by glucocorticoids, with dissipation of the fight/flight response heightening immune defences and vigilance beyond basal levels and promoting more vigorous reactions to persisting or impending threats (Sapolsky et al., 2000; Johnson et al., 2002; Sorrells et al., 2009). Specifically, within the CNS, it has been demonstrated that short-term stress can modulate the microglial immunophenotype to sensitize a neuroinflammatory response that may be exaggerated in subsequent inflammatory challenges (Blandino et al., 2006; Frank et al., 2007; Sugama et al., 2007, 2009, 2011; Blandino et al., 2009). Activated microglia upregulate antigen presenting molecules such as major histocompatibility complex, increase phagocytic activity, generate proinflammatory cytokines and reactive nitrogen species, and overall induce a neuroinflammatory response (de Pablos et al., 2006; Frank et al., 2007; Colton, 2009; Chen et al., 2016).
Proinflammatory cytokines including tumor necrosis factor alpha (Tnf-α) and interleukin-1β are among the principle messengers responsible for initiating and coordinating the acute phase inflammatory response, with interleukin-1β acting through autocrine/paracrine signalling to further potentiate interleukin-6 production (Dinarello, 1998). Furthermore, it has been reported that following acute immobilization stress, the increase in soluble Tnf-α was released by a zinc-dependent, modular cell surface protein known as a disintegrin and metalloproteinase 17 (Adam17), or commonly as Tnf-α convertase (TACE), in the rat brain cortex (Madrigal et al., 2002). In recent years, it has become clear that interleukin-1 and Tnf-α triggered the canonical nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway involving RelA and C-Rel, subsequently initiating downstream inflammatory, oxidative, and nitrosative signaling (Mercurio and Manning, 1999; Albrecht et al., 2007; Solt et al., 2007). An alternative NF-κB pathway can be activated by Tnf receptor superfamily members resulting in activation of RelB and p52 complexes (Sun, 2011). Another important inflammatory signaling cascade involves cyclooxygenase-2 (Cox-2), a constitutively expressed enzyme in the brain that converts arachidonic acid into prostaglandin. A number of studies have demonstrated its involvement in HPA axis activation, with inhibition of Cox-2 demonstrating antidepressant-like properties without affecting major cytokine responses following lipopolysaccharide-injection (de Paiva et al., 2010; Teeling et al., 2010; Ma et al., 2013).
Recently, cannabinoids have received extensive research interest due to their roles in increasing sleep and fat storage while attenuating the inflammatory response and stress-induced behaviors (Pacher et al., 2006). The 2 major endogenous cannabinoids, N-arachidonoylethanolamine (commonly known as anandamide) and 2-arachidonoylglycerol (2-AG), are produced on demand postsynaptically as neuromodulators in response to stress and generally function in opposition to the HPA response. For example, the selective cannabinoid receptor type 1 (CB1R) antagonist has been reported to modulate corticosterone release and exert anxiogenic effects (Wade et al., 2006). Hill and colleagues (2011) demonstrated that endocannabinoid signaling plays an important role in suppressing corticosteroid secretion following stress through inhibition of GABAergic neurotransmission. Furthermore, pharmacological inhibition of fatty acid amide hydrolase (FAAH), an enzyme responsible for rapid physiological degradation of anandamide, robustly blocked stress-induced corticosterone release and anxiety-like behaviors (Patel et al., 2004; Bluett et al., 2014). In addition to a critical involvement in stress modulation, endocannabinoids have demonstrated antiinflammatory properties, as FAAH knockout mice have a reduced lipopolysaccharide-induced hyperalgesia and oedema, an effect mediated mainly through cannabinoid receptor type 2 (Naidu et al., 2010). Accumulating evidence has also suggested that endocannabinoids and their derivatives mediate antiinflammatory effects by serving as potent agonists for the peroxisome proliferator-activated receptors (PPARs), a subfamily of nuclear receptors (Ulrich-Lai and Ryan, 2013; O’Sullivan, 2016). Interestingly, Tchantchou and colleagues (2014) have recently demonstrated effective suppression of traumatic brain injury-induced neuroinflammation, including iNOS and Cox-2 expression, in microglia/macrophages by PF-3845, an irreversible FAAH inhibitor with high sensitivity and long duration. However, this represents modulation occurring in a condition of chronic injury, while the effects of endocannabinoid modulation by FAAH inhibition on nitrergic and inflammatory indicators following a short-term stress remain unclear. Therefore, the present study has examined the effects of PF-3845 on nitrergic indicators and inflammatory-related gene expression changes in the dorsal hippocampus following differing durations of acute restraint stress.
Materials And Methods
Ethical Approval and Experimental Animals
All experimental procedures were in accordance with regulations and policies outlined by The University of Queensland Animal Ethics Committee under AEC approval number SBS/456/14/URG. Outbred male Wistar rats (Rattus norvegicus) aged 5 to 6 weeks postnatal weighing 206.6±2.04 g were sourced from The University of Queensland Biological Resources breeding colony. Rats were housed individually under controlled laboratory conditions (22°C±2°C; 55±5% humidity) with a 12-hour-light/dark cycle (lights off at 12:30 pm). Standard rat chow and water were available ad libitum.
Experimental Protocol
Part A
To evaluate the effectiveness of PF-3845 on FAAH antagonism following acute stress, an experiment was conducted using 5 mg/kg of PF-3845 dissolved in 2% dimethyl sulfoxide in normal saline (0.9% sodium chloride) as adapted from Ahn and colleagues (2009). Rats were habituated to human handling for 10 min/d, 6 days prior to experimentation. On the day of the treatment, rats were transported in individual home cages from the colony room to an experimental room within the same animal facility. The FAAH inhibitor PF-3845 was injected i.p. with an injection volume of 10 mL/kg 1 hour prior to a 60-minute acute restraint stress (stress starts at 1:30 pm) using wire mesh restrainers. During this period, rats were acclimatized to the novel experimental room under low light and noise. To isolate the effects of restraint stress, all rats were deprived of food and water during the 1-hour habituation and stress treatment period. Rats were then randomly allocated to 0 (control) or 60 minute stress groups (n=4 per group). At the end of each treatment period, rats were weighed and killed with sodium pentobarbital (100 mg/kg i.p. injection; Lethabarb, Virbac), and the whole brain was rapidly removed and snap frozen. Frozen brains were sectioned on a cryostat, and the dorsal hippocampus was cryo-dissected from sections according to Banasr and colleagues (2006) and a rat brain atlas (Paxinos and Watson, 2007). The tissue was stored at -80oC for FAAH activity assay and markers of NO and nitration including total nitrite and nitrate and 3-nitrotyrosine.
Hippocampal tissues were homogenized with 10 volumes (w/v) of homogenizing buffer (0.1 M Tris-HCl, 20 µM of aprotinin, 100 µM of leupeptin, and 1µM of pepstatin A), and an aliquot of whole homogenate was removed for 3-nitrotrosine determination. A commercially available ELISA kit (ab116691, Abcam) was used for the quantitative measurement of 3-nitrotyrosine according to the manufacturer’s instructions. The remaining crude homogenate was centrifuged at 10000×g for 10 minutes at 4°C, and the resultant supernatant was used in the measurement of nitrite and nitrate and FAAH activity. The total NO metabolites, NOx, were measured in ultra-filtered supernatant using a commercially available total nitrate/nitrite colorimetric assay kit (Cayman Chemical Company). A fluorescence-based assay was used to detect FAAH activity using a novel substrate for FAAH, arachidonyl 7-amino, 4-methyl coumarin amide (AAMCA), modified from Ramarao and colleagues (2005). The enzyme FAAH hydrolyses AAMCA, resulting in the liberation of the highly fluorescent 7-amino 4-methyl coumarin (AMC) that can be monitored at an excitation wavelength of 355 nm and emission wavelength at 460 nm. In brief, the assay was carried out in black-walled plates containing 50 µL of supernatant diluted in 0.05 M Tris-HCl (pH=9.0) with 50 µL of 10 µM AAMCA solution in assay buffer (0.05 M Tris-HCl, 2 mM EDTA, 100 mM HEPES, 0.04% Triton X-100, 2 mg/mL BSA; pH=9.0). The assay plates were incubated at room temperature for 2.5 hours with readings taken every 15 minutes using the POLARstar OPTIMA microplate reader (BMG Labtechnologies) to kinetically monitor AAMCA hydrolysis by FAAH in the sample. Data were finally adjusted with corresponding protein concentrations.
Part B
Rats were habituated as described earlier and were randomly allocated to 8 treatment groups (n=5–7) with control, or restraint stress (starts at 1:30 PM) for 60, 240, and 360 minutes with either vehicle or PF-3845 injection. The FAAH inhibitor, PF-3845 (5 mg/kg; Sigma), dissolved in 2% dimethyl sulfoxide in normal saline (0.9% sodium chloride) was injected 60 minutes before stress treatment with an injection volume of 10 mL/kg (i.p.). Following each allocated treatment, a blood sample was collected in sodium heparin (20 IU/mL blood) via tail-tipping. Heparinized blood samples were centrifuged at 2000 x g for 5 minutes immediately after collection, and plasma glucose levels were determined using standard glucometer (FreeStyle Optium Neo, Abbott). The resulting plasma supernatant was stored at -80 oC for later determination of corticosterone concentrations. Rats were then overdosed with 100 mg/kg of sodium pentobarbital (i.p.). The whole brain was rapidly removed and frozen on powdered dry ice for storage at -80oC. Dorsal hippocampus was isolated and stored at -80oC for later relative gene expression analysis. We have included plasma measures for one animal from which we were unable to obtain hippocampal tissue and hence was excluded from analysis of gene expression.
Plasma Corticosterone Assay
Corticosterone concentrations were measured by an in-house radioimmunoassay using anti-rat corticosterone polyclonal antibody (Sapphire Bioscience Pty. Ltd.) and tritiated [1, 2, 6, 7- 3H]-corticosterone tracer as previously described in Spiers and colleagues (2016). Radioactivity was counted in liquid scintillation cocktail (Ultima Gold, Perkin Elmer) using a Liquid Scintillation Spectrometer (Tri-Carb 3100 TR, Perkin Elmer). Dichloromethane extraction recovery was 79.14% and intra- and inter-assay coefficients of variation were 5.79% and 2.12%, respectively.
Real-Time PCR
Total RNA was extracted from the dorsal hippocampus using a RNeasy mini kit (Qiagen) treated with deoxyribonuclease I and reversed transcribed into cDNA using iScript Reverse Transcription Supermix (Bio-Rad Laboratories) according to the manufacturer’s instructions. Taqman gene expression assay-on-demand kits (Life Technologies) with optimized primers and FAM-labelled probes were used to detect gene expression of Nos2 (inducible nitric oxide synthase; Rn00561646_m1), Nos1 (neuronal nitric oxide synthase; Rn00583793_m1), Tnf (tumor necrosis factor alpha; Rn01525859_g1), Adam17 (ADAM metallopeptidase domain 17; Rn00571880_m1), Slc39a14 (solute carrier family 39 (zinc transporter), member 14; Rn01468336_m1), Il1b (interleukin 1 beta; Rn00580432_m1), Il6 (interleukin 6; Rn01410330_m1), Ptgs2 (prostaglandin-endoperoxide synthase 2; Rn01483828_m1), Nfkbia (nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; Rn01473657_g1), and Pparγ (peroxisome proliferator activated receptor gamma; Rn00440945_m1). The ∆∆CT method was used for all expression assays, which were run in a multiplex reaction with VIC-labelled GAPDH as endogenous control using the QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems).
Statistical Analysis
Data were analyzed using statistical software GraphPad Prism (Version 7.03, GraphPad Software Inc.). All data sets were first checked for normality using the Brown-Forsythe test. Normally distributed data were subsequently analyzed using 2-way ANOVA with Fisher’s Least Significant Difference test to make comparisons between all groups with the vehicle control and between vehicle and PF-3845 data at corresponding time-points. Results were expressed as mean±SEM and P values < .05 were considered statistically significant.
Results
Systemic PF-3845 Treatment Decreased FAAH Activity and 3-Nitrotyrosine Formation in the Dorsal Hippocampus
Treatment with PF-3845 at a dose of 5 mg/kg was effective at decreasing FAAH activity in the dorsal hippocampus measured by AAMCA hydrolysis when injected 1 hour prior to stress treatment (Table 1). Two-way ANOVA demonstrated a significant main effect of PF-3845 treatment [F(1, 12) = 25.94, P=.0003] on FAAH activity with no significant effects of time and the interaction between drug treatment and stress duration. There was a significant reduction in AMC liberation indicative of reduced FAAH activity in PF-3845-treated groups at 0 (P<.01) and 60 (P<.05) minutes of stress compared with the corresponding vehicle groups. Two-way ANOVA also showed a main effect of PF-3845 treatment [F(1, 12)=19.32, P=.0009] on 3-nitrotyrosine levels. Posttest analysis further revealed that PF-3845 treatment reduced hippocampal 3-nitrotyrosine compared with vehicle-treated groups at 0 (P<.01) and 60 (P<.05) minutes of restraint stress exposure. No significant difference was observed in total NOx between the vehicle- and PF-3845-treated groups.
Table 1.
The Effect of the Fatty Acid Amide Hydrolase Inhibitor PF-3845 (5 mg/kg, i.p. injection) on Fatty Acid Amide Hydrolase Activity Measured by AMC fluorescence, NOx, and 3-Nitrotyrosine from Control and Stressed Rats (n=4/group)
| Duration of Stress (min) | Treatment | ||
|---|---|---|---|
| Vehicle | PF-3845 | ||
| Hippocampal AMC fluorescence (AU/mg protein) |
0 | 15.19±0.98 | 9.70±1.05** |
| 60 | 14.10±0.85 | 10.11±0.82* | |
| Hippocampal NOx (µM/mg protein) |
0 | 2.95±0.45 | 2.04±0.40 |
| 60 | 2.85±0.47 | 1.72±0.25 | |
| Hippocampal 3-nitrotyrosine (ng/mg protein) |
0 | 43.67±1.67 | 31.93±3.54** |
| 60 | 38.22±2.45 | 29.29±0.90* | |
Isolated dorsal hippocampus was collected from rats exposed to 0 (control) and 60 minute of restraint stress.
Two-way ANOVA followed by Fisher’s least significant difference test. Data are expressed as mean±SEM. *P<.05 and **P<.01 vs vehicle-treated group at each respective time-point.
Inhibition of FAAH Dampens Acute Stress-Induced Corticosterone Release and Glucose Mobilization
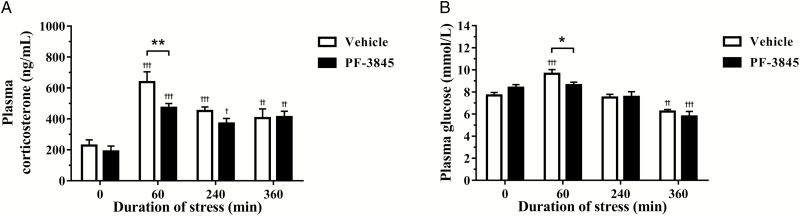
To determine whether increased endocannabinoid signaling modulates HPA output, we determined concentrations of the plasma stress hormone, corticosterone. Pretreatment with the FAAH inhibitor, PF-3845, significantly attenuated corticosterone release [F(1, 36)=5.863, P=.0201] following restraint stress exposure in a time-dependent manner [F(3, 36)=24.42, P<.0001]. Compared with vehicle controls, restraint effectively elevated plasma corticosterone in all groups regardless of drug treatment. However, significantly lower corticosterone concentrations were observed in the PF-3845-treated group (P<.01) compared with the corresponding vehicle group at 60 minutes of stress (Figure 1a). Stress exposure triggers physiological processes, including hepatic glycolysis and gluconeogenesis, that acutely increase circulating glucose, making this a good downstream indicator of stress induction. The accompanying changes in plasma glucose following treatments demonstrated a time-dependent effect [F(3, 36)=27.4, P<.0001]. Compared with vehicle controls, posttest analysis showed a significant increase at 60 minutes of restraint in the vehicle-treated group alone, while both vehicle and PF-3845 treated animals displayed decreased glucose by 360 minutes of restraint exposure. Moreover, PF-3845 treatment significantly decreased glucose mobilization compared with the corresponding vehicle group following 60 minutes (P<.05) of restraint stress (Figure 1b).
Figure 1.
The effect of the fatty acid amide hydrolase inhibitor, PF-3845 (5 mg/kg, i.p. injection), on plasma (A) corticosterone and (B) glucose levels from control and stressed rats (n=5–7/group). Plasma corticosterone and glucose concentration was determined in blood samples collected via tail-tipping from rats exposed to 0 (control), 60, 240, and 360 minutes of acute restraint stress. Data are presented as mean±SEM. †P<.05, ††P<.01, and †††P<.001 vs vehicle-treated group at 0 (control) minute; *P<.05 and **P<.01 between vehicle- and PF-3845-treated groups at each respective time-point.
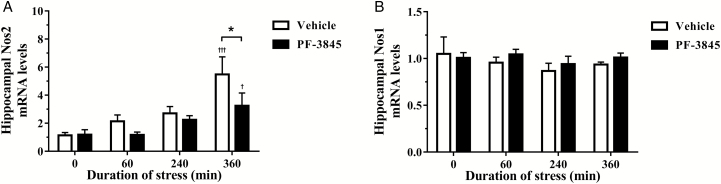
Upregulation of iNOS Following Exposure to Restraint was Attenuated by PF-3845
Figure 2a demonstrates a progressive time-dependent [F(3, 40)=10.17, P<.0001] increase in hippocampal iNOS mRNA levels in both vehicle and PF-3845-treated groups [F(1, 40)=4.191, P=.047] that became significant following exposure to 360 minutes of restraint. Posttest analysis also revealed PF-3845 treatment significantly ameliorated the increase in inducible NOS mRNA expression compared with the corresponding vehicle-treated group at 360 minutes (P<.05) of restraint exposure. No significant changes were observed in hippocampal neuronal NOS (Nos1) mRNA levels between vehicle- and PF-3845-treated groups (Figure 2b).
Figure 2.
The effect of the fatty acid amide hydrolase inhibitor, PF-3845 (5 mg/kg, i.p. injection), on hippocampal (A) inducible nitric oxide synthase (Nos2) and (B) neuronal nitric oxide synthase (Nos1) mRNA expression from control and stressed rats (n=5–6/group). The relative expression was determined in the dorsal hippocampus collected from rats exposed to 0 (control), 60, 240, and 360 minutes of acute restraint stress. Data are presented as mean±SEM †P<.05 and †††P<.001 vs vehicle-treated group at 0 (control) minute; *P<.05 between vehicle- and PF-3845-treated groups at each respective time-point.
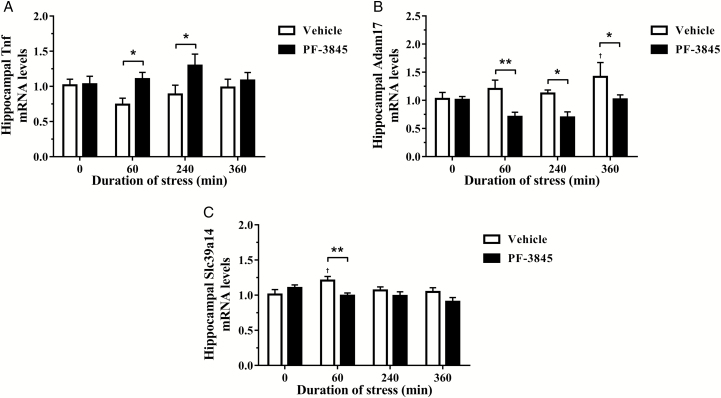
PF-3845 Alters Tnf-α Expression and Ameliorates TACE and ZIP14 Upregulation Following Exposure to Restraint Stress
Treatment with FAAH inhibitor altered hippocampal Tnf-α mRNA expression [F(1, 36)=6.963, P=.0122] following 60 and 240 (P<.05) minutes of restraint exposure compared with the corresponding vehicle groups, which displayed mild but nonsignificant decreases compared with the vehicle-treated control group (Figure 3a). The proinflammatory cytokine, Tnf-α, is synthesized as a 32-kDa transmembrane anchored precursor that is cleaved by TACE, yielding a nonglycosylated soluble 17-kDa protein. A 2-way ANOVA demonstrated that administration of PF-3845 significantly reduced Adam17 mRNA levels [F(1, 36)=15.21, P=.0004] in the dorsal hippocampus. Posttest analysis revealed vehicle treatment alone resulted in a progressive increase in Adam17 mRNA that became significant following 360 minutes of exposure to restraint compared with vehicle controls. This increase was attenuated by treatment with PF-3845 at 60 (P<.05), 240, and 360 [P<.01] minutes of restraint stress compared with the corresponding vehicle-treated animals (Figure 3b). It is known that proteolytically active ADAM enzymes contain zinc binding sequences in their catalytic domain and the activity of TACE can be reduced by zinc chelators. Treatment with PF-3845 significantly reduced [F(1, 36)=4.71, P=.0367] mRNA expression of the zinc importer Slc39a14 (Zip14). Posttest analysis revealed vehicle treatment caused a transient increase in Slc39a14 mRNA levels following 60 minutes (P<.05) of restraint stress compared with the vehicle controls. This increase at 60 minutes was attenuated in PF-3845-treated animals compared with the corresponding vehicle group (Figure 3c).
Figure 3.
The effect of the fatty acid amide hydrolase inhibitor, PF-3845 (5 mg/kg, i.p. injection), on hippocampal (A) tumor necrosis factor (Tnf), (B) a disintegrin and metalloprotease 17 (Adam17), and (C) solute carrier family 39 member 14 (Slc39a14) mRNA expression from control and stressed rats (n=5–6/group). The relative mRNA expression was determined in the dorsal hippocampus from rats exposed to 0 (control), 60, 240, and 360 minutes of acute restraint stress. Data are presented as mean±SEM. †P<.05 vs vehicle-treated group at 0 (control) minute; *P<.05 and **P<.01 between vehicle- and PF-3845-treated groups at each respective time-point.
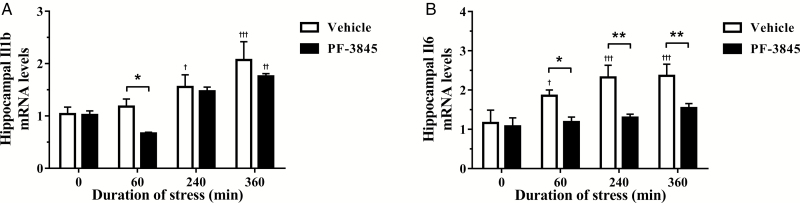
Inhibition of FAAH Constrains Upregulation of Interleukin-1β and Interleukin-6 Following Acute Stress
Inflammatory cytokines are rapidly upregulated following activation of immune cells that reside in the CNS, including microglia, CNS macrophages, and astrocytes. Previous studies have revealed the antiinflammatory properties exerted by endocannabinoids are partly through attenuation of microglial activation. In the present study, there was a significant time-dependent [F(3, 36)=17.21, P<.0001] and PF-3845 treatment-dependent [F(1, 36)=4.358, P=.044] effect on Il1b gene expression following restraint stress exposure. Posttest analysis showed a progressive increase in both treatment groups that significantly differed from vehicle controls following 240 (P<.05) and 360 (P<.001) minutes of restraint in vehicle-treated and 360 minutes of restraint in PF-3845-treated groups (Figure 4a). Treatment with PF-3845 also reduced interleukin-1β mRNA (P<.05) compared with the corresponding vehicle-treated group following 60 minutes of restraint stress. Furthermore, FAAH inhibition [F(1, 36)=18.95, P=.0001] also attenuated the stress-induced upregulation of interleukin-6 mRNA in a time-dependent manner [F(3, 36)=6.049, P=.002]. Exposure to restraint robustly upregulated interleukin-6 mRNA in vehicle-treated groups at 60 (P<.05), 240, and 360 (P<.001) minutes of restraint compared with the vehicle controls (Figure 4b). Treatment with PF-3845 effectively attenuated this increase, with further posttest analysis revealing significant reductions compared with corresponding vehicle-treated groups at 60 (P<.05), 240, and 360 (P<.01) minutes of restraint exposure.
Figure 4.
The effect of the fatty acid amide hydrolase inhibitor, PF-3845 (5 mg/kg, i.p. injection), on hippocampal (A) interleukin-1β (Il1b) and (B) interleukin-6 (Il6) mRNA expression from control and stressed rats (n=5–6/group). The relative mRNA expression was determined in the dorsal hippocampus from rats exposed to 0 (control), 60, 240, and 360 minutes of acute restraint stress. Data are presented as mean±SEM. †P<.05, ††P<.01, and †††P<.001 vs vehicle-treated group at 0 (control) minute; *P<.05 and **P<.01 between vehicle- and PF-3845-treated groups at each respective time-point.
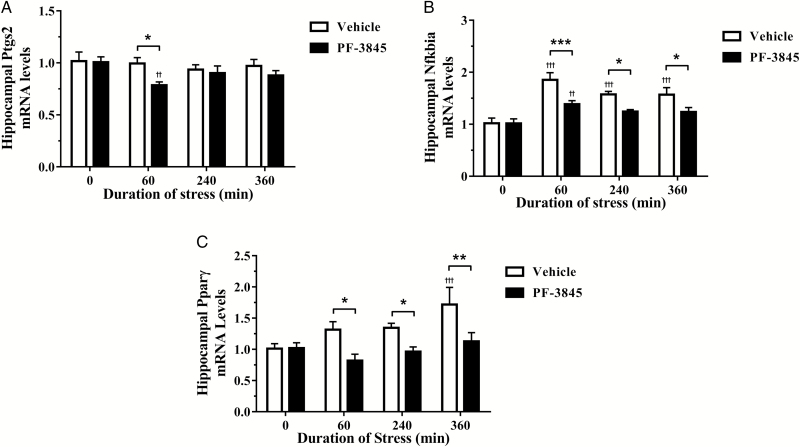
PF-3845 Acutely Suppressed Expression of Cox-2 and Attenuated the Upregulation of IκB-α and PPARγ mRNA in the Dorsal Hippocampus
It has been demonstrated that Cox-2 is constitutively expressed in the brain and is primarily responsible for the production of prostanoids, including prostaglandin E2, which is involved in pathological inflammatory conditions. Notably in Figure 5a, PF-3845 treatment [F(1, 36)=4.476, P=.041] resulted in decreased Cox-2 mRNA levels in animals exposed to 60 minutes of stress compared with both the vehicle-treated controls (P<.01) and the corresponding vehicle-treated group at 60 minutes (P<.05) of restraint. The transcription factor NF-кB plays a central role in the proinflammatory signaling pathway by regulating the expressions of proteins involved in oxidative stress and inflammation, including iNOS, pro-inflammatory cytokines, and Cox-2. To examine the NF-кB transactivation potential, expression of the inhibitory subunit Nfkbia (or IκB-α) was monitored as described by Bottero and colleagues (2003). Two-way ANOVA demonstrated PF-3845 was highly effective [F(1, 36)=19.16, P<.001] in ameliorating stress-induced IκB-α mRNA expression over the time course of the experiment [F(3, 36)=15.05, P<.001] (Figure 5b). Compared with vehicle-treated controls, posttest analysis revealed restraint stress induced a sustained increase in vehicle-treated animals following 60, 240, and 360 (P<.001) minutes, an effect only transiently observed in PF-3845-treated animals at 60 minutes of restraint. Moreover, PF-3845 treatment significantly reduced IκB-α mRNA compared with the corresponding vehicle-treated groups at 60 (P<.001), 240, and 360 (P<.05) minutes of restraint stress exposure. In addition to preventing the breakdown of endocannabinoids, inhibitors of FAAH also prevent breakdown of the ligands for PPARγ. Treatment with PF-3845 effectively attenuated increases in hippocampal PPARγ mRNA [F(3, 36)=3.82, P<.0179] following exposure to restraint stress in a time-dependent manner [F(1, 36)=15.51, P<001] (Figure 5c). There was a progressive increase in vehicle-treated groups that became significant following 360 minutes (P<.05) of restraint exposure compared with vehicle-treated controls. Posttest analysis also revealed PF-3845 treatment reduced PPARγ mRNA expression at 60, 240 (P<.05), and 360 (P<.01) minutes of restraint stress compared with the corresponding vehicle-treated groups.
Figure 5.
The effect of the fatty acid amide hydrolase inhibitor, PF-3845 (5 mg/kg, i.p. injection), on hippocampal (A) prostaglandin-endoperoxide synthase 2 (Ptgs2; also known as cyclooxygenase-2 or Cox-2), (B) nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (Nfkbia), and (C) peroxisome proliferator activated receptor gamma (Pparγ) mRNA expression from control and stressed rats (n=5–6/group). The relative mRNA expression was determined in the dorsal hippocampus from rats exposed to 0 (control), 60, 240, and 360 minutes of acute restraint stress. Data are presented as mean±SEM. ††P<.01 and †††P<.001 vs vehicle-treated group at 0 (control) minute; *P<.05, **P<.01, and ***P<.001 between vehicle- and PF-3845-treated groups at each respective time-point.
Discussion
This study has demonstrated that administration of the FAAH inhibitor, PF-3845, robustly suppresses acute stress-induced upregulation of nitrergic and proinflammatory responses due to blockade of anandamide degradation within the dorsal hippocampus. The results show that pretreatment with PF-3845 rapidly dampens restraint stress-induced plasma corticosterone release and glucose liberation. Treatment with this FAAH inhibitor also attenuated iNOS, TACE, interleukin-6, Cox-2, and PPARγ mRNA upregulation and ameliorated the transactivation potential of NF-κB, suggesting that enhanced endocannabinoid levels in the dorsal hippocampus have an overall antinitrosative and antiinflammatory effect following acute stress exposure.
Intraperitoneal injection of 5 mg/kg PF-3845 effectively lowered tonic FAAH activity at 0 and 60 minutes of restraint stress (1 and 2 hours postdosing) measured by a selective FAAH substrate AAMCA (Ramarao et al., 2005). It has been demonstrated previously that PF-3845-mediated FAAH inhibition rapidly elevated anandamide and other endogenous fatty acid ethanolamides such as oleoylethanolamide and palmitoylethanolamide, which peaked at 2 to 3 hours postinjection (Ahn et al., 2009). It was also confirmed that FAAH inhibition with PF-3845 decreased nitric oxide signaling compared with vehicle-treated groups using 3-nitrotyrosine as a collective index of protein nitration (Schopfer et al., 2003; Radi, 2004). This occurred in the absence of increased nitric oxide metabolism and irrespective of stress exposure, indicating increased endocannabinoid signalling may potentiate the metabolism of existing nitrated proteins.
The importance of endocannabinoid signalling in regulation of the stress system has been demonstrated by several studies (Patel et al., 2004; Wade et al., 2006; Cota et al., 2007; Steiner et al., 2008). Enhanced FAAH-mediated anandamide hydrolysis has been observed in response to acute stress, and therefore the rapid loss of anandamide levels in the basolateral amygdala may play an important role in the disinhibition of HPA axis following stress (Hill et al., 2009). Moreover, due to this physiological role of anandamide, administration of PF-3845 to mice exposed to chronic corticosterone treatment has anxiolytic effects through activation of astroglial CB1R, which in turn triggers AMPA receptor internalization, resulting in long-term depression in the basolateral amygdala (Duan et al., 2017). Thus, it is very likely that the attenuation in stress-induced corticosterone observed in the present study was due to FAAH inhibition within the amygdala, which in turn reduced the overall drive through the HPA axis and consequently decreased plasma glucose at 60 minutes of stress. This is in agreement with results from Rivera and colleagues (2015) demonstrating decreased levels of plasma glucose, triglycerides, and total cholesterol following acute or repeated treatment with the selective FAAH inhibitor [3-(3-Carbamoylphenyl)phenyl] N-cyclohexylcarbamate (URB597).
Previous reports have demonstrated that the expression of the calcium-independent NOS isoform can become rapidly elevated within astrocytes and microglia following stimulation by a variety of factors, including proinflammatory cytokines (Aktan, 2004; Calabrese et al., 2007; Chen et al., 2015). We have previously demonstrated a 10.5-fold increase in whole hippocampal iNOS mRNA following 240 minutes of restraint stress, which corresponded well with the 2-fold increase in iNOS activity (Chen et al., 2016). In comparison, the increase in iNOS mRNA was not as robust in the present study using only the dorsal hippocampus, increasing by approximately 3-fold at the equivalent restraint duration and approximately 6-fold at the longest restraint duration tested, and we observed no significant changes in expression of the calcium-dependent isoform, nNOS. This increase in iNOS mRNA was significantly attenuated by FAAH inhibition at 360 minutes of restraint compared with the vehicle group and is likely a combination of the early reduction in corticosterone secretion by PF-3845 administration and a direct antiinflammatory action of the drug. Previous reports have shown that selective CB1R agonists inhibit nitrite production and iNOS protein expression in an astroglia-derived cell line following lipopolysaccharide and interferon gamma stimulation (Esposito et al., 2001). Thus, activation of the cannabinoid receptor that is negatively coupled to adenylate cyclase and subsequent altered cAMP signaling appears to be one likely mechanism mediating the inhibition of iNOS expression (Esposito et al., 2001; Won et al., 2004; Turu and Hunyady, 2010).
The transcriptional activation of iNOS has previously been shown to rely heavily on Tnf-α-induced NF-κB transactivation. Madrigal and colleagues (2002) demonstrated that 1 hour of stress caused an increase in cortical TACE activity and Tnf-α level, leading to translocation of NF-κB into the nucleus by 4 hours and a subsequent increase in iNOS activity by 6 hours of stress exposure. Moreover, as little as 30 minutes of stress was sufficient to increase TACE activity with no change in corresponding protein levels. Pharmacological inhibition of TACE activity strongly attenuated these changes, indicating that Tnf-α production is involved in NF-κB activation prior to iNOS transcription. In the present study, stress exposure induced delayed differences in Adam17 mRNA, with PF-3845 treatment-dependent changes in Tnf/Adam17 resulting in differences that related more to the antiinflammatory nature of the drug. Although this may seem paradoxical comparison with the results obtained by Madrigal and colleagues (2002) in the cortex, it is likely that the hippocampus undergoes a constrained increase in secreted Tnf-α at the protein level via increased TACE-induced cleavage of pro-Tnf protein already present in the cytosol, and changes at the mRNA level may occur earlier than the time-points examined in the present study (Madrigal et al., 2002; Gooz, 2010). Cleavage of pro-Tnf may be facilitated by increased zinc availability, as it has been shown previously that TACE is a zinc-dependent metalloprotease and its catalytic activity can be inhibited by zinc chelating agents (Moss et al., 2001; Udechukwu et al., 2017). In this study, we demonstrated that exposure to 60 minutes of restraint stress increased expression of the zinc transporter (Zip14) in the dorsal hippocampus of vehicle-treated animals, an effect that was ameliorated by PF-3845 treatment. Increased expression of this cell membrane-localized zinc importer in vehicle-treated animals could function to support TACE in terms of providing zinc for structural stabilization and substrate catalysis. In addition to the Tnf/TACE system, stress exposure also altered hippocampal interleukin-1β and interleukin-6 mRNA in a time-dependent manner. Both interleukin-1β and interleukin-6 mRNA increased in the hippocampus following stress exposure in vehicle-treated animals. Converging evidence indicates that the immediate cellular source of these proinflammatory cytokines derives from the sensitization of microglia by glucocorticoids and norepinephrine (Blandino et al., 2006; Frank et al., 2007, 2012; Chen et al., 2016). Furthermore, previous studies have suggested that microglia most likely constitute the main cellular source of endocannabinoid under neuroinflammatory conditions (Stella, 2009). In the present study, increased endocannabinoid signalling by PF-3845 caused an early inhibition of the increase in interleukin-1β that was only significantly different from vehicle treatment following 60 minutes of stress. However, PF-3845 markedly attenuated increases in hippocampal interleukin-6 mRNA at all durations of stress exposure. The magnitude of these changes may indicate that the endocannabinoid system preferentially interacts with interleukin-6 during a normal response to stress. However, interleukin-6 also exhibits a positive feed-forward expression, and therefore reductions in corticosterone by PF-3845 may prevent transcription and further potentiation of interleukin-6 mRNA in the hippocampus (Bethin et al., 2000; Spooren et al., 2011).
There was a subtle but significant reduction in hippocampal Cox-2 following 60 minutes of stress in the PF-3845-treated group. While Cox-2 has been heavily linked to the proinflammatory pathway, it also mediates inactivation of anandamide signaling by catalyzing the formation of prostaglandins from arachidonic acid (Hermanson et al., 2014). Several studies have demonstrated that selective Cox-2 inhibitors can elevate anandamide and 2-AG concentrations in addition to prolonging the depolarization-induced suppression of inhibition representing a protracted action of endocannabinoids (Kim and Alger, 2004; Hermanson et al., 2014). In the present study, the status of NF-κB following stress exposure and drug treatment was determined by monitoring the inhibitory subunit IκB-α, responsible for binding and inactivating NF-κB. Although NF-κB can be activated by several different upstream pathways, Madrigal and colleagues (2002) demonstrated that nuclear translocation of this transcription factor was required for stress-induced upregulation of iNOS. The IκB-α expression in this present work showed early and persistent increases following exposure to stress. Inhibition of FAAH strongly attenuated this increase at all time points, which likely reflects the combined action of decreased corticosterone and reduced proinflammatory cytokines. Anandamide and other cannabinoid-like fatty acid amides, including palmitoylethanolamide and oleoylethanolamide, have been implicated in the induction of antiinflammatory proteins such as IkB-α while repressing expression of Tnf by acting on PPARs (D’Agostino et al., 2007; Rettori et al., 2012; Paterniti et al., 2013; Alhouayek and Muccioli, 2014; O’Sullivan, 2016). In the present study, the steady increase in hippocampal PPARγ mRNA was greatly attenuated by inhibition of FAAH. In agreement with these results, 6 hours of stress exposure has previously been demonstrated to increase both the activity and protein expression of PPARγ in neurons and most astrocytes of the brain cortex (García-Bueno et al., 2005). Using specific pharmacological tools, García-Bueno and colleagues (2008) further demonstrated that PPARγ activation following restraint stress is mediated by the stress-induced increase in catecholamines, glucocorticoids, and glutamatergic signalling.
In conclusion, psychological stress produces a characteristic temporal profile in several proinflammatory cytokines and nitrosative activity in the dorsal hippocampus. Reductions in corticosterone and direct antiinflammatory action of enhanced endocannabinoid signaling resulted in decreased proinflammatory markers following psychological stress exposure. Although this present work was limited to measurements of mRNA levels, we have previously shown iNOS mRNA correlates well with enzymatic activity and thus, PF-3845-mediated reductions in iNOS mRNA following stress exposure likely attenuate stress-induced nitrosative stress on a biochemical level. By establishing the importance of the endocannabinoid system in the neuroinflammatory response following stress, this study opens up potential therapeutic targets for conditions exacerbated or caused by prolonged stress exposure.
Funding
This work was supported by a University of Queensland Research Grant. H.-J.C. Chen and J.G. Spiers were recipients of APA and UQRS scholarships. These funding bodies have no further role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Statement of Interest
None.
Acknowledgments
We thank Professor Karen M. Moritz for her continuous support and sharing of reagents. We also thank the animal technicians from the University of Queensland Biological Resources animal house for animal care and husbandry.
References
- Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, Weerapana E, Sadagopan N, Liimatta M, Smith SE, Lazerwith S, Stiff C, Kamtekar S, Bhattacharya K, Zhang Y, Swaney S, Van Becelaere K, Stevens RC, Cravatt BF(2009)Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem Biol 16:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktan F.(2004)Inos-mediated nitric oxide production and its regulation. Life Sci 75:639–653. [DOI] [PubMed] [Google Scholar]
- Albrecht U, Yang X, Asselta R, Keitel V, Tenchini ML, Ludwig S, Heinrich PC, Häussinger D, Schaper F, Bode JG(2007)Activation of NF-kappab by IL-1beta blocks IL-6-induced sustained STAT3 activation and STAT3-dependent gene expression of the human gamma-fibrinogen gene. Cell Signal 19:1866–1878. [DOI] [PubMed] [Google Scholar]
- Alhouayek M, Muccioli GG(2014)Harnessing the anti-inflammatory potential of palmitoylethanolamide. Drug Discov Today 19:1632–1639. [DOI] [PubMed] [Google Scholar]
- Banasr M, Soumier A, Hery M, Mocaër E, Daszuta A(2006)Agomelatine, a new antidepressant, induces regional changes in hippocampal neurogenesis. Biol Psychiatry 59:1087–1096. [DOI] [PubMed] [Google Scholar]
- Barnes PJ.(1998)Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond) 94:557–572. [DOI] [PubMed] [Google Scholar]
- Bethin KE, Vogt SK, Muglia LJ(2000)Interleukin-6 is an essential, corticotropin-releasing hormone-independent stimulator of the adrenal axis during immune system activation. Proc Natl Acad Sci U S A 97:9317–9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandino P Jr, Barnum CJ, Deak T(2006)The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1beta responses to stress. J Neuroimmunol 173:87–95. [DOI] [PubMed] [Google Scholar]
- Blandino P Jr, Barnum CJ, Solomon LG, Larish Y, Lankow BS, Deak T(2009)Gene expression changes in the hypothalamus provide evidence for regionally-selective changes in IL-1 and microglial markers after acute stress. Brain Behav Immun 23:958–968. [DOI] [PubMed] [Google Scholar]
- Bluett RJ, Gamble-George JC, Hermanson DJ, Hartley ND, Marnett LJ, Patel S(2014)Central anandamide deficiency predicts stress-induced anxiety: behavioral reversal through endocannabinoid augmentation. Transl Psychiatry 4:e408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottero V, Imbert V, Frelin C, Formento JL, Peyron JF(2003)Monitoring NF-kappa B transactivation potential via real-time PCR quantification of I kappa B-alpha gene expression. Mol Diagn 7:187–194. [DOI] [PubMed] [Google Scholar]
- Cain DW, Cidlowski JA(2017)Immune regulation by glucocorticoids. Nat Rev Immunol 17:233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM(2007)Nitric oxide in the central nervous system: neuroprotection vs neurotoxicity. Nat Rev Neurosci 8:766–775. [DOI] [PubMed] [Google Scholar]
- Cancedda C, Filaci G, Puppo F, Ghio M, Contini P, Indiveri F(2002)Immune homeostasis requires several biologic factors including glucocorticoid hormones. Ann N Y Acad Sci 966:49–63. [DOI] [PubMed] [Google Scholar]
- Chen HJ, Spiers JG, Sernia C, Lavidis NA(2015)Response of the nitrergic system to activation of the neuroendocrine stress axis. Front Neurosci 9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HJ, Spiers JG, Sernia C, Lavidis NA(2016)Acute restraint stress induces specific changes in nitric oxide production and inflammatory markers in the rat hippocampus and striatum. Free Radic Biol Med 90:219–229. [DOI] [PubMed] [Google Scholar]
- Colton CA.(2009)Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol 4:399–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Steiner MA, Marsicano G, Cervino C, Herman JP, Grübler Y, Stalla J, Pasquali R, Lutz B, Stalla GK, Pagotto U(2007)Requirement of cannabinoid receptor type 1 for the basal modulation of hypothalamic-pituitary-adrenal axis function. Endocrinology 148:1574–1581. [DOI] [PubMed] [Google Scholar]
- Coutinho AE, Chapman KE(2011)The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol 335:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino G, La Rana G, Russo R, Sasso O, Iacono A, Esposito E, Raso GM, Cuzzocrea S, Lo Verme J, Piomelli D, Meli R, Calignano A(2007)Acute intracerebroventricular administration of palmitoylethanolamide, an endogenous peroxisome proliferator-activated receptor-alpha agonist, modulates carrageenan-induced paw edema in mice. J Pharmacol Exp Ther 322:1137–1143. [DOI] [PubMed] [Google Scholar]
- de Pablos RM, Villarán RF, Argüelles S, Herrera AJ, Venero JL, Ayala A, Cano J, Machado A(2006)Stress increases vulnerability to inflammation in the rat prefrontal cortex. J Neurosci 26:5709–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paiva VN, Lima SN, Fernandes MM, Soncini R, Andrade CA, Giusti-Paiva A(2010)Prostaglandins mediate depressive-like behaviour induced by endotoxin in mice. Behav Brain Res 215:146–151. [DOI] [PubMed] [Google Scholar]
- Dinarello CA.(1998)Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int Rev Immunol 16:457–499. [DOI] [PubMed] [Google Scholar]
- Duan T, Gu N, Wang Y, Wang F, Zhu J, Fang Y, Shen Y, Han J, Zhang X(2017)Fatty acid amide hydrolase inhibitors produce rapid anti-anxiety responses through amygdala long-term depression in male rodents. J Psychiatry Neurosci 42:230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Izzo AA, Di Rosa M, Iuvone T(2001)Selective cannabinoid CB1 receptor-mediated inhibition of inducible nitric oxide synthase protein expression in C6 rat glioma cells. J Neurochem 78:835–841. [DOI] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF(2007)Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun 21:47–59. [DOI] [PubMed] [Google Scholar]
- Frank MG, Thompson BM, Watkins LR, Maier SF(2012)Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav Immun 26:337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Bueno B, Madrigal JL, Lizasoain I, Moro MA, Lorenzo P, Leza JC(2005)Peroxisome proliferator-activated receptor gamma activation decreases neuroinflammation in brain after stress in rats. Biol Psychiatry 57:885–894. [DOI] [PubMed] [Google Scholar]
- García-Bueno B, Madrigal JL, Pérez-Nievas BG, Leza JC(2008)Stress mediators regulate brain prostaglandin synthesis and peroxisome proliferator-activated receptor-gamma activation after stress in rats. Endocrinology 149:1969–1978. [DOI] [PubMed] [Google Scholar]
- Gooz M.(2010)ADAM-17: the enzyme that does it all. Crit Rev Biochem Mol Biol 45:146–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanson DJ, Gamble-George JC, Marnett LJ, Patel S(2014)Substrate-selective COX-2 inhibition as a novel strategy for therapeutic endocannabinoid augmentation. Trends Pharmacol Sci 35:358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Morrish AC, Viau V, Floresco SB, Hillard CJ, Gorzalka BB(2009)Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology 34:2733–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Pan B, Fitzgerald ML, Roberts CJ, Lee TT, Karatsoreos IN, Mackie K, Viau V, Pickel VM, McEwen BS, Liu QS, Gorzalka BB, Hillard CJ(2011)Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci 31:10506–10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Deak T, Stark M, Watkins LR, Maier SF(2002)Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun 16:461–476. [DOI] [PubMed] [Google Scholar]
- Kim J, Alger BE(2004)Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat Neurosci 7:697–698. [DOI] [PubMed] [Google Scholar]
- Ma Y, Matsuwaki T, Yamanouchi K, Nishihara M(2013)Cyclooxygenase-2-related signaling in the hypothalamus plays differential roles in response to various acute stresses. Brain Res 1508:23–33. [DOI] [PubMed] [Google Scholar]
- Madrigal JL, Hurtado O, Moro MA, Lizasoain I, Lorenzo P, Castrillo A, Boscá L, Leza JC(2002)The increase in TNF-alpha levels is implicated in NF-kappab activation and inducible nitric oxide synthase expression in brain cortex after immobilization stress. Neuropsychopharmacology 26:155–163. [DOI] [PubMed] [Google Scholar]
- Mercurio F, Manning AM(1999)NF-kappab as a primary regulator of the stress response. Oncogene 18:6163–6171. [DOI] [PubMed] [Google Scholar]
- Moss ML, White JM, Lambert MH, Andrews RC(2001)TACE and other ADAM proteases as targets for drug discovery. Drug Discov Today 6:417–426. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Kinsey SG, Guo TL, Cravatt BF, Lichtman AH(2010)Regulation of inflammatory pain by inhibition of fatty acid amide hydrolase. J Pharmacol Exp Ther 334:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan SE.(2016)An update on PPAR activation by cannabinoids. Br J Pharmacol 173:1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Bátkai S, Kunos G(2006)The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 58:389–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ(2004)Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology 145:5431–5438. [DOI] [PubMed] [Google Scholar]
- Paterniti I, Impellizzeri D, Crupi R, Morabito R, Campolo M, Esposito E, Cuzzocrea S(2013)Molecular evidence for the involvement of PPAR-δ and PPAR-γ in anti-inflammatory and neuroprotective activities of palmitoylethanolamide after spinal cord trauma. J Neuroinflammation 10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C(2007)The rat brain in stereotaxic coordinates, 6th ed Amsterdam, Boston: Elsevier. [Google Scholar]
- Radi R.(2004)Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A 101:4003–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramarao MK, Murphy EA, Shen MW, Wang Y, Bushell KN, Huang N, Pan N, Williams C, Clark JD(2005)A fluorescence-based assay for fatty acid amide hydrolase compatible with high-throughput screening. Anal Biochem 343:143–151. [DOI] [PubMed] [Google Scholar]
- Rettori E, De Laurentiis A, Zorrilla Zubilete M, Rettori V, Elverdin JC(2012)Anti-inflammatory effect of the endocannabinoid anandamide in experimental periodontitis and stress in the rat. Neuroimmunomodulation 19:293–303. [DOI] [PubMed] [Google Scholar]
- Rivera P, Bindila L, Pastor A, Pérez-Martín M, Pavón FJ, Serrano A, de la Torre R, Lutz B, Rodríguez de Fonseca F, Suárez J(2015)Pharmacological blockade of the fatty acid amide hydrolase (FAAH) alters neural proliferation, apoptosis and gliosis in the rat hippocampus, hypothalamus and striatum in a negative energy context. Front Cell Neurosci 9:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU(2000)How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89. [DOI] [PubMed] [Google Scholar]
- Schopfer FJ, Baker PR, Freeman BA(2003)NO-dependent protein nitration: a cell signaling event or an oxidative inflammatory response?Trends Biochem Sci 28:646–654. [DOI] [PubMed] [Google Scholar]
- Solt LA, Madge LA, Orange JS, May MJ(2007)Interleukin-1-induced NF-kappab activation is NEMO-dependent but does not require IKKbeta. J Biol Chem 282:8724–8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Caso JR, Munhoz CD, Sapolsky RM(2009)The stressed CNS: when glucocorticoids aggravate inflammation. Neuron 64:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers JG, Chen HJ, Cuffe JS, Sernia C, Lavidis NA(2016)Acute restraint stress induces rapid changes in central redox status and protective antioxidant genes in rats. Psychoneuroendocrinology 67:104–112. [DOI] [PubMed] [Google Scholar]
- Spooren A, Kolmus K, Laureys G, Clinckers R, De Keyser J, Haegeman G, Gerlo S(2011)Interleukin-6, a mental cytokine. Brain Res Rev 67:157–183. [DOI] [PubMed] [Google Scholar]
- Steiner MA, Marsicano G, Wotjak CT, Lutz B(2008)Conditional cannabinoid receptor type 1 mutants reveal neuron subpopulation-specific effects on behavioral and neuroendocrine stress responses. Psychoneuroendocrinology 33:1165–1170. [DOI] [PubMed] [Google Scholar]
- Stella N.(2009)Endocannabinoid signaling in microglial cells. Neuropharmacology 56:244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugama S, Fujita M, Hashimoto M, Conti B(2007)Stress induced morphological microglial activation in the rodent brain: involvement of interleukin-18. Neuroscience 146:1388–1399. [DOI] [PubMed] [Google Scholar]
- Sugama S, Takenouchi T, Fujita M, Conti B, Hashimoto M(2009)Differential microglial activation between acute stress and lipopolysaccharide treatment. J Neuroimmunol 207:24–31. [DOI] [PubMed] [Google Scholar]
- Sugama S, Takenouchi T, Fujita M, Kitani H, Hashimoto M(2011)Cold stress induced morphological microglial activation and increased IL-1β expression in astroglial cells in rat brain. J Neuroimmunol 233:29–36. [DOI] [PubMed] [Google Scholar]
- Sun SC.(2011)Non-canonical NF-κb signaling pathway. Cell Res 21:71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchantchou F, Tucker LB, Fu AH, Bluett RJ, McCabe JT, Patel S, Zhang Y(2014)The fatty acid amide hydrolase inhibitor PF-3845 promotes neuronal survival, attenuates inflammation and improves functional recovery in mice with traumatic brain injury. Neuropharmacology 85:427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeling JL, Cunningham C, Newman TA, Perry VH(2010)The effect of non-steroidal anti-inflammatory agents on behavioural changes and cytokine production following systemic inflammation: implications for a role of COX-1. Brain Behav Immun 24:409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turu G, Hunyady L(2010)Signal transduction of the CB1 cannabinoid receptor. J Mol Endocrinol 44:75–85. [DOI] [PubMed] [Google Scholar]
- Udechukwu MC, Tsopmo A, Mawhinney H, He R, Kienesberger PC, Udenigwe CC(2017)Inhibition of ADAM17/TACE activity by zinc-chelating rye secalin-derived tripeptides and analogues. RSC Advances 7:26361–26369. [Google Scholar]
- Ulrich-Lai YM, Ryan KK(2013)PPARγ and stress: implications for aging. Exp Gerontol 48:671–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade MR, Degroot A, Nomikos GG(2006)Cannabinoid CB1 receptor antagonism modulates plasma corticosterone in rodents. Eur J Pharmacol 551:162–167. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF(2011)Β-adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci 31:6277–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won JS, Im YB, Singh AK, Singh I(2004)Dual role of cAMP in iNOS expression in glial cells and macrophages is mediated by differential regulation of p38-MAPK/ATF-2 activation and inos stability. Free Radic Biol Med 37:1834–1844. [DOI] [PubMed] [Google Scholar]