Abstract
Rotavirus vaccines were introduced in the United States in 2006, and in the years since they have fundamentally altered the seasonality of rotavirus infection and have shifted disease outbreaks from annual epidemics to biennial epidemics. We investigated whether season and year of birth have emerged as risk factors for rotavirus or have affected vaccine performance. We constructed a retrospective birth cohort of US children under age 5 years using the 2001–2014 MarketScan database (Truven Health Analytics, Chicago, Illinois). We evaluated the associations of season of birth, even/odd year of birth, and interactions with vaccination. We fitted Cox proportional hazards models to estimate the hazard of rotavirus hospitalization according to calendar year of birth and season of birth assessed for interaction with vaccination. After the introduction of rotavirus vaccine, we observed monotonically decreasing rates of rotavirus hospitalization for each subsequent birth cohort but a biennial incidence pattern by calendar year. In the postvaccine period, children born in odd calendar years had a higher hazard of rotavirus hospitalization than those born in even years. Children born in winter had the highest hazard of hospitalization but also had greater vaccine effectiveness than children born in spring, summer, or fall. With the emergence of a strong biennial pattern of disease following vaccine introduction, the timing of a child’s birth has become a risk factor for rotavirus infection.
Keywords: administrative data, cohort studies, hospitalization, pediatric infectious disease, rotavirus, seasonality, vaccines
In late 2006, rotavirus vaccines were introduced into the routine infant immunization program in the United States. Both licensed rotavirus vaccines (RotaTeq (Merck & Co., Kenilworth, New Jersey) and Rotarix (GlaxoSmithKline UK Ltd., Brentford, United Kingdom)) are approximately 80% efficacious against rotavirus hospitalization (1–3). By 2015, vaccine coverage had reached 73.2% among children aged 19–35 months—below coverage for well-established vaccines such as poliovirus, hepatitis B, measles-mumps-rubella, and varicella, for which coverage is greater than 90% (4).
In addition to providing direct protection for vaccinated individuals, the rotavirus immunization program in the United States appears to have disrupted the epidemiology of rotavirus by interrupting the transmission process, providing indirect protection to unvaccinated persons (3, 5–7). Consequently, despite relatively modest coverage, vaccine impacts have been considerable, with approximately 60%–90% reductions in numbers of rotavirus-coded or laboratory-confirmed hospitalizations (3, 8). In addition, the seasonality of rotavirus has shifted in the United States from a highly regular annual pattern with peaks in February and March during the prevaccine period to a biennial pattern, with less consistent peak timing and irregular local patterns (9–11). Introduction of vaccination can increase the interepidemic period (12), which was predicted by some to occur following mass rotavirus immunization (11). In the postvaccination era in the United States, it appears that it takes 2 years of new births for there to be sufficient numbers of unimmunized children to sustain transmission. These changes in seasonal patterns could affect the timing of exposure to rotavirus for postvaccination birth cohorts.
While vaccine efficacy (VE) is relatively high in low-mortality settings in North America, Europe, and Asia, at 72%–100% (2, 3), there is heterogeneity in the VE of live oral vaccines (13). VE may vary by time of administration, as we observed in the Americas (14), perhaps driven by seasonal factors such as 1) the cocirculation of other pathogens that might interfere with vaccine immunogenicity; 2) the length of the interval from birth to rotavirus exposure, given seasonal disease and some waning of vaccine immunity; and 3) the different levels of maternal antibody, depending on when children were born relative to the onset of the rotavirus season.
We investigated whether season and year of birth have emerged as risk factors for rotavirus as the seasonality and periodicity of disease have shifted in the wake of vaccination. We also considered whether season or timing of birth interacts with vaccine performance.
METHODS
Data
We constructed a retrospective birth cohort of commercially insured US children under 5 years of age, using data from the 2001–2014 Truven Health MarketScan Commercial Claims and Encounters Database (Truven Health Analytics, Chicago, Illinois) (15). MarketScan data were extracted from health insurance claims and contained deidentified, individual-level health-care information from various public and private employer-sponsored health plans. The MarketScan database includes more than 100 million patients from over 100 insurance companies (15), and the data come primarily from large employers; medium and small firms and Medicaid claims are not represented. MarketScan data come from all 50 states.
Exposure and outcomes
Records from children who were enrolled in MarketScan at birth and were continuously enrolled for at least 3 months were included in the cohort, to ensure that any rotavirus vaccine administered to this cohort was captured in the database. We identified rotavirus-associated hospitalizations using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code 008.61 for rotavirus. A discharge was defined as rotavirus hospitalization if 00.861 was coded as the primary diagnosis or listed in any of the 15 diagnosis categories from the inpatient-admission table. Current Procedural Terminology codes 90680 and 90681 were used to identify children who received pentavalent rotavirus vaccine and monovalent rotavirus vaccine, respectively, among the eligible cohort. Recording of vaccine administration was independent of recording of hospitalizations.
Cohort construction
Children whose births were included in the MarketScan data set were considered to be enrolled and eligible for follow-up in the cohort. In order to minimize missing information on vaccination or hospitalizations, we did not include any children who were enrolled in MarketScan at a time after birth. From birth until receipt of the first dose of vaccine (for those receiving at least 1 dose of vaccine), children were considered unvaccinated and were considered to have had 1 dose until receipt of a second dose. Because many studies have shown similar vaccine performance in the United States, we did not distinguish between monovalent rotavirus vaccine and pentavalent rotavirus vaccine in analysis and grouped together children who had received 2 or more doses, regardless of the vaccine. A rotavirus hospitalization was considered a failure event, after which children were excluded from the cohort (i.e., only a single failure event was permitted). Unenrollment from a MarketScan-captured insurance plan or reaching 5 years of age was considered a censoring event.
Statistical analysis
We calculated rates of rotavirus hospitalization according to calendar year of birth, in odd- and even-numbered calendar years, by season of birth. We fitted Cox proportional hazards models with the aim of examining the hazard of rotavirus hospitalization by calendar year of birth, season of birth (winter = January–March; spring = April–June; summer = July–September; fall = October–December), and number of doses of vaccine. We used the Breslow method for handling tie failures in the calculation of the log partial likelihood. We calculated vaccine effectiveness as 100% × (1 – hazard ratio). Analyses were performed in Stata 14.1 (StataCorp LLC, College Station, Texas). Vaccination was considered a time-varying exposure covariate, and only children born in the postvaccination era (i.e., those who were age-eligible) were included in VE estimation. To search for evidence of seasonal differences in vaccine performance, we tested for evidence of an interaction between vaccine effectiveness and timing of birth by performing a Wald test for the interaction term.
RESULTS
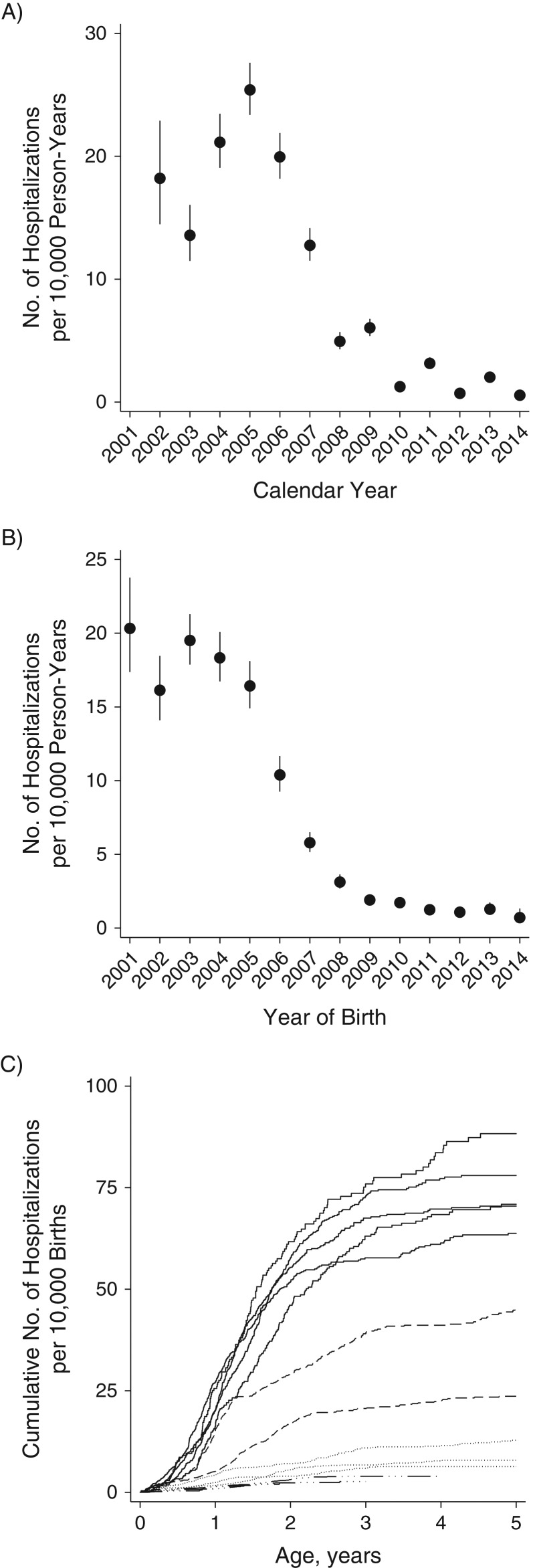
Among 2.9 million children, we analyzed 3.3 million person-years of follow-up, which included 2,774 first rotavirus hospitalizations (Table 1). Fourteen (0.5%) and 3 (0.1%) of the rotavirus hospitalizations occurred within 14 days of the first and second vaccine administration, respectively. Rotavirus hospitalization rates ranged from 13.6 per 10,000 person-years to 25.4 per 10,000 person-years in the prevaccine period (2002–2006), after which rates declined to 0.5–6.0 per 10,000 person-years and a biennial pattern of disease incidence emerged, with increases in 2009, 2011, and 2013 relative to the previous year (Figure 1A).
Table 1.
Rates of Rotavirus Hospitalization Among Prevaccine and Postvaccine Birth Cohorts Under Age 5 Years, by Age, Season of Birth, Even/Odd Calendar Year of Birth, and Individual Vaccination Status, United States, 2002–2014
| Stratum | Prevaccine Birth Cohorts (2002–2006 Births) | Postvaccine Birth Cohorts (2007–2014 Births) | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of Rotavirus Hospitalizationsa | Tens of Thousands of Person-Yearsa | Rate per 10,000 Person-Years | 95% CI | No. of Rotavirus Hospitalizationsa | Tens of Thousands of Person-Yearsa | Rate per 10,000 Person-Years | 95% CI | |
| Total | 2,017 | 119 | 17 | 16, 18 | 757 | 206 | 3.7 | 3.4, 3.9 |
| Age, months | ||||||||
| 0–11 | 813 | 42 | 19 | 18, 21 | 341 | 117 | 2.9 | 2.6, 3.2 |
| 12–23 | 854 | 28 | 31 | 29, 33 | 288 | 41 | 7.0 | 6.3, 7.9 |
| 24–59 | 350 | 50 | 7 | 6, 8 | 128 | 48 | 2.7 | 2.2, 3.2 |
| Season of birth | ||||||||
| Winter | 569 | 32 | 18 | 17, 19 | 268 | 56 | 4.8 | 4.2, 5.4 |
| Spring | 558 | 32 | 17 | 16, 19 | 184 | 57 | 3.2 | 2.6, 3.8 |
| Summer | 492 | 30 | 16 | 15, 18 | 150 | 54 | 2.8 | 2.8, 3.7 |
| Fall | 398 | 25 | 16 | 14, 18 | 155 | 39 | 3.9 | 3.3, 4.6 |
| Even/odd calendar year | ||||||||
| Even | 955 | 61 | 16 | 15, 17 | 293 | 96 | 3.0 | 2.7, 3.4 |
| Odd | 1,062 | 58 | 18 | 17, 19 | 464 | 109 | 4.2 | 3.9, 4.6 |
| No. of doses of vaccine received | ||||||||
| 0 | 2,007 | 116 | 17 | 16, 18 | 581 | 80 | 7.3 | 6.7, 7.9 |
| 1 | 44 | 21 | 2.1 | 1.6, 2.8 | ||||
| ≥2 | 132 | 105 | 1.3 | 1.1, 1.5 | ||||
Abbreviation: CI, confidence interval.
a Numbers in subgroups may not sum to total because of missing data.
Figure 1.
Rates of rotavirus hospitalization according to calendar year (A), year of birth (B), and cumulative risk up to age 5 years (C) among children under 5 years of age, United States, 2001–2014. In panels A and B, the vertical lines represent 95% confidence intervals. In panel C, the solid black lines represent the prevaccine birth cohort (2001–2005); the dashed lines represent transitional birth cohorts (2006 and 2007); the dotted lines represent postvaccine birth cohorts with complete 5-year follow-up (2008–2010); and the dashed-dotted lines represent postvaccine birth cohorts with incomplete censored follow-up (2011–2014).
For cohorts born prior to the introduction of rotavirus vaccine (2002–2006), rates of rotavirus hospitalization varied little by year of birth (ranging from 16.1 per 10,000 person-years to 20.3 per 10,000 person-years). Rotavirus hospitalization rates then fell monotonically for each subsequent year’s birth cohort, from 12.3 per 10,000 person-years for 2007 to 2.9 per 10,000 person-years for 2010 when vaccine coverage was increasing; they then stabilized at a low level (1.0–1.6 per 10,000 person-years) for children born after 2010 (Figure 1B and 1C).
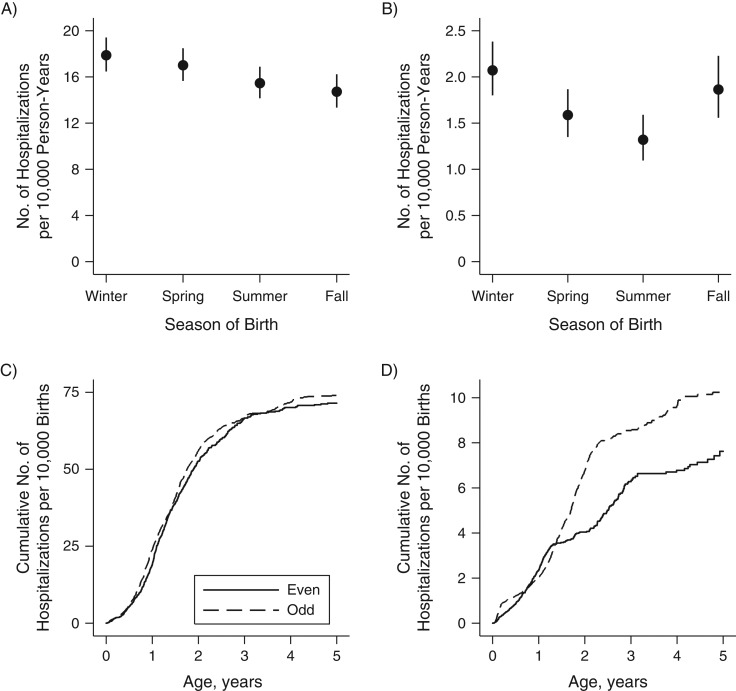
Prior to vaccine introduction, season of birth had little bearing on rates of rotavirus hospitalization (Figure 2A), and a child’s hazard of rotavirus hospitalization was not dependent on his or her year of birth (hazard ratio (HR) = 1.05, 95% confidence interval (CI): 0.95, 1.15) (Figure 2C).
Figure 2.
Cumulative incidence of rotavirus hospitalization before and after the introduction of rotavirus vaccine, by season of birth (per 10,000 person-years; top row) and even/odd calendar year of birth (per 10,000 births; bottom row), among children under 5 years of age, United States, 2001–2014. Rotavirus vaccine was introduced in late 2006, so we considered children born in 2007 and later as having been born during the postvaccination period. Note the differences in scale between the pre- and postvaccination periods. A) Children born prior to vaccine introduction (2002–2006); B) children born after vaccine introduction (2007–2014); C) children born prior to vaccine introduction, by even/odd calendar year of birth; D) children born after vaccine introduction, by even/odd calendar year of birth. Bars, 95% confidence intervals.
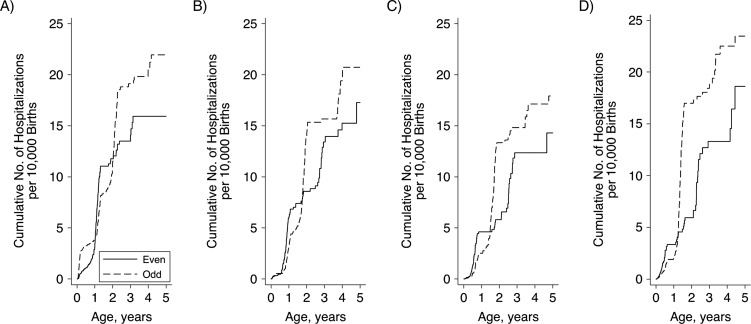
However, in the postvaccine period (2007–2014), children born in spring (HR = 0.68, 95% CI: 0.57, 0.82), summer (HR = 0.59, 95% CI: 0.48, 0.72), and fall (HR = 0.81, 95% CI: 0.66, 0.99) had lower hazards of rotavirus hospitalization than children born in winter, after controlling for even/odd calendar year of birth (Figure 2B). Following vaccine introduction, children born in odd calendar years had significantly increased hazards relative to those born in even calendar years (HR = 1.35, 95% CI: 1.16, 1.57) (Figure 2D). This association was largely driven by high rates among children born in the winter months of odd calendar years (per 10,000 person-years, HR = 5.8, 95% CI: 5.0, 6.7), who experienced relatively high rates of rotavirus hospitalization over their first 3 winters (i.e., up to age 2.5 years). Fall births in odd calendar years had the second highest overall rates (per 10,000 person-years, HR = 4.4, 95% CI: 3.6, 5.3), but these children experienced the majority of their hospitalizations between 12 and 17 months of age, when they passed through an odd–calendar-year rotavirus season (Figure 3).
Figure 3.
Cumulative numbers of rotavirus hospitalizations per 10,000 births among children under 5 years of age after the introduction of rotavirus vaccine, according to calendar year of birth (even (low-rotavirus-incidence year) or odd (high-rotavirus-incidence year)) and season of birth, United States, 2007–2014. A) Winter; B) spring; C) summer; D) fall.
Vaccine effectiveness
Overall, we found a high VE in line with previous estimates from this data set and others (Table 2). VE against rotavirus hospitalization was 74% (95% CI: 66, 81) for 1 dose of rotavirus vaccine and 90% (95% CI: 87, 92) for 2 or more doses of rotavirus vaccine. We saw no evidence of differential VE by age: VE was 87% (95% CI: 80, 91) for children aged <12 months, 90% (95% CI: 86, 94) for children aged 12–23 months, and 93% (95% CI: 86, 97) for children aged 24–59 months. We found evidence that 2-dose VE was marginally lower for children born in spring (84%, 95% CI: 75, 89; P = 0.002), summer (89%, 95% CI: 81, 96; P = 0.056), or fall (88%, 95% CI: 78, 94; P = 0.031) than for children born in winter (96%, 95% CI: 91, 98). However, this significantly lower VE was mainly restricted to children born from April to December in even calendar years, and perhaps children born in spring in odd calendar years.
Table 2.
Effectiveness of 2 or More Doses of Rotavirus Vaccine (Compared With No Vaccination) Among Children Under Age 5 Years, by Age, Even/Odd Calendar Year of Birth, and Season of Birth, United States, 2002–2014
| Stratum | VE | 95% CI | P Value |
|---|---|---|---|
| Age, months | |||
| ≤11 | 87 | 80, 91 | |
| 12–23 | 90 | 86, 94 | |
| 24–59 | 93 | 86, 97 | |
| ≤59 (total) | 90 | 87, 92 | |
| Even/odd calendar year of birth | |||
| Even | 87 | 82, 91 | |
| Odd | 92 | 88, 95 | 0.092a |
| Season of birthb | |||
| Winter | 96 | 91, 98 | |
| Spring | 84 | 75, 89 | 0.002 |
| Summer | 89 | 81, 96 | 0.056 |
| Fall | 88 | 78, 94 | 0.031 |
| Season of birth in even calendar yearsc | |||
| Winter | 97 | 89, 99 | |
| Spring | 82 | 68, 90 | 0.016 |
| Summer | 85 | 70, 93 | 0.041 |
| Fall | 80 | 60, 91 | 0.015 |
| Season of birth in odd calendar yearsc | |||
| Winter | 95 | 87, 98 | 0.480 |
| Spring | 85 | 70, 92 | 0.029 |
| Summer | 93 | 81, 97 | 0.312 |
| Fall | 95 | 83, 98 | 0.475 |
Abbreviations: CI, confidence interval; VE, vaccine effectiveness.
aP value is from a Wald test for comparison with VE among 2-dose births in even calendar years (2008, 2010, and 2012).
bP values are from a Wald test for comparison with VE among 2-dose winter births.
cP values are from a Wald test for comparison with VE among 2-dose winter births in even calendar years (2008, 2010, and 2012).
DISCUSSION
Our analysis demonstrated that in addition to reducing rates of rotavirus hospitalization, vaccination has substantially altered the epidemiology of rotavirus disease in the United States. With the emergence of a strong biennial pattern of disease, the timing of a child’s birth has become a risk factor for disease. Children born in odd calendar years (2009, 2011, and 2013) had substantially higher disease risks than children born in even calendar years (2008, 2010, and 2012). Children born in odd years had relatively high incidence in the postvaccine era, so these patterns reflect the levels of exposure incurred early in life (9). Disease severity has long been understood to decrease with each subsequent infection (16, 17), though recent analysis has suggested that age (rather than previous infection) is the determinant of severity (18). With severity being strongly associated with age, children infected later in life will be less likely to be hospitalized. Thus, children born in odd (i.e., high-activity) calendar years may be exposed in their first year of life, and then before their third birthday they may be exposed again in a relatively large season—that is, a season with high incidence of rotavirus gastroenteritis (odd calendar year). Additionally, children born in even (i.e., low-activity) calendar years are only exposed to a single relatively large season (odd calendar year) before their third birthday, after which hospitalization becomes less likely.
A second important finding of our analysis is that timing of birth may also be a determinant of the degree of protection conferred by vaccination. We found that children born in winter who received 2 or more doses of vaccine had significantly greater protection than children born in spring, summer, or fall. This was an observational study without a strong a priori hypothesis, but we might have expected to see lower protection for children born in winter, because of 1) possible interference of natural infection with vaccine immunogenicity, 2) waning vaccine protection due to a longer gap between vaccination and exposure to their first rotavirus season, 3) seasonal maternal antibodies that interfere with vaccine immunogenicity (19), or 4) seasonal variations in the susceptibility of the human host (20). Coinfections may interfere with the immune response to and efficacy of live enteric vaccines. For example, having a concurrent nonpoliomyelitis enterovirus at the time of oral poliovirus vaccination significantly reduces seroconversion and vaccine shedding (another indicator of immunogenicity), as does diarrhea at the time of oral polivirus vaccine administration (21). Similarly, other enteric viruses interfere with wild rotavirus replication (22). In a previous study, we found marginally lower VE during months in which rotavirus naturally circulated, pooling data mainly from Latin America but also from the United States (14). Our present findings stand in contrast to those, however, since we found higher VE in the historical rotavirus months (January–March) in the United States. Perhaps children born in the winter had the lowest levels of maternal antibody, since they would have been in utero during the lowest-incidence months. Maternal antibodies are known to interfere with rotavirus vaccine immunogenicity. Alternatively, perhaps pathogens that have a seasonality out of phase with that of rotavirus (e.g., Campylobacter species and Salmonella species) (23) interfere with vaccine immunogenicity in the spring/summer months. Without a sound biological explanation for these patterns in variation in VE, we advocate for more research from other settings where rotavirus is seasonal and vaccine has been introduced.
The MarketScan data set analyzed here presented us with a unique opportunity to construct a birth cohort study, free from some recruitment-related biases. We were able to identify children born into the data set, continuously followed for a specified period of time, and were able to link their key time-varying exposures (dates of vaccination) and outcomes (rotavirus-coded hospitalizations). Accordingly, we suspect that there was little opportunity for bias in recording the key variables in our study: dates of birth, vaccination, and rotavirus hospitalization. However, the MarketScan data set is built on private health insurance claims, so it is potentially biased in that it does not include children covered by Medicaid, for example.
For our analysis of vaccine performance, we were comparing VE among different groups (akin to a comparative effectiveness analysis) (24). Therefore, we were looking for small differences in vaccine performance, which we had limited statistical power to detect. Second, it is likely that the case-hospitalization ratio is highly dependent on age, such that children who are infected at a young age are more likely to be hospitalized. Because rotavirus is highly seasonal, teasing apart the potential effects of season of birth, season of vaccination, and age-specific risks is extraordinarily difficult, since these factors are themselves correlated. However, given the size of this cohort and the independence of measurement of birth timing, vaccination, and disease outcome, MarketScan was a robust data source with which to assess these questions. Finally, diagnostic testing and, therefore, coding for rotavirus is probably more frequent at young ages, so any differences observed regarding younger age distributions in high-incidence years could have been accentuated by biased testing of infants. More generally, ICD-9-CM coding is an imperfect measure of rotavirus hospitalizations; the code 008.61 is highly specific (25) but insensitive, so many true rotavirus hospitalizations were probably excluded. In addition, if the sensitivity or (especially) specificity varied over time, VE estimates could have been biased, but we have no reason to think that occurred over the course of the study period or that these parameters would vary for some reason in even and odd calendar years.
A related issue is that misclassification of rotavirus hospitalization probably increased over time as prevalence decreased, resulting in a decreasing trend in the positive predictive value of the rotavirus ICD-9-CM code. This would have served to bias downward VE estimates for lower-incidence strata. This could be one contributor to the lower VE estimate for even-year births compared with odd-year births, for example. We found no association between season of birth and risk of confirmed rotavirus infection in the prevaccine era in the United States. This contrasts with data from England and Wales, where, overall, winter births were at lower risk, but this association was driven by the first year of life, and the association was reversed (winter births at higher risk) in the second-to-fourth years of life (26). This observed contrast between the United States and the United Kingdom could be due to different age-specific testing practices; for example, if very young children (i.e., age <3 months) are less likely to be tested in the United Kingdom, it may falsely appear that winter births are at lower risk.
In summary, we found that being born in an odd-numbered (high-activity) calendar year, especially in the winter or fall season, emerged as a risk factor for rotavirus infection in the postvaccine era in the United States. These results point out the dynamic nature of rotavirus in the United States and how vaccination has brought about a new endemic pattern of disease. Likely, the postvaccination dynamics have not yet stabilized, and the biennial pattern may continue to evolve. There are already recognized inequities in that children living in households below the poverty line have 10% lower rotavirus vaccine coverage (27); here, we have identified other heterogeneities in risk that are unrelated to individual behavior, including vaccine receipt. While season of birth is not a modifiable risk factor, these findings suggest a heighted importance of vaccinating children born in advance of a relatively large forthcoming rotavirus season. In addition, further research into the causes of seasonally varying VE may lead to identification of individual factors resulting in poorer vaccine performance in lower-income settings. We hypothesize that further increasing vaccine coverage may further reduce incidence and the biennial patterns, and in doing so could diminish birth timing as a risk factor for rotavirus infection.
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia (Benjamin Lopman); and Division of Viral Diseases, National Center for Immunizations and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia (Benjamin Lopman, Rebecca Dahl, Minesh Shah, Umesh D. Parashar).
This project was supported by grants from the National Institute of Allergy and Infectious Diseases (grants R01AI112970 and R01AI125842) to B.L.
The findings and conclusions presented in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflict of interest: none declared.
Abbreviations
- CI
confidence interval
- HR
hazard ratio
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- VE
vaccine efficacy.
REFERENCES
- 1. Payne DC, Boom JA, Staat MA, et al. Effectiveness of pentavalent and monovalent rotavirus vaccines in concurrent use among US children <5 years of age, 2009–2011. Clin Infect Dis. 2013;57(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Soares-Weiser K, Maclehose H, Bergman H, et al. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev. 2012;11:CD008521. [DOI] [PubMed] [Google Scholar]
- 3. Yen C, Tate JE, Hyde TB, et al. Rotavirus vaccines: current status and future considerations. Hum Vaccin Immunother. 2014;10(6):1436–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hill HA, Elam-Evans LD, Yankey D, et al. Vaccination coverage among children aged 19–35 months—United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(39):1065–1071. [DOI] [PubMed] [Google Scholar]
- 5. Fine P, Eames K, Heymann DL. “Herd immunity”: a rough guide. Clin Infect Dis. 2011;52(7):911–916. [DOI] [PubMed] [Google Scholar]
- 6. Gastañaduy PA, Curns AT, Parashar UD, et al. Gastroenteritis hospitalizations in older children and adults in the United States before and after implementation of infant rotavirus vaccination. JAMA. 2013;310(8):851–853. [DOI] [PubMed] [Google Scholar]
- 7. Buttery JP, Lambert SB, Grimwood K, et al. Reduction in rotavirus-associated acute gastroenteritis following introduction of rotavirus vaccine into Australia’s national childhood vaccine schedule. Pediatr Infect Dis J. 2011;30(1 suppl):S25–S29. [DOI] [PubMed] [Google Scholar]
- 8. Leshem E, Tate JE, Steiner CA, et al. Acute gastroenteritis hospitalizations among US children following implementation of the rotavirus vaccine. JAMA. 2015;313(22):2282–2284. [DOI] [PubMed] [Google Scholar]
- 9. Aliabadi N, Tate JE, Haynes AK, et al. Sustained decrease in laboratory detection of rotavirus after implementation of routine vaccination—United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2015;64(13):337–342. [PMC free article] [PubMed] [Google Scholar]
- 10. Curns AT, Panozzo CA, Tate JE, et al. Remarkable postvaccination spatiotemporal changes in United States rotavirus activity. Pediatr Infect Dis J. 2011;30(1 suppl):S54–S55. [DOI] [PubMed] [Google Scholar]
- 11. Pitzer VE, Viboud C, Simonsen L, et al. Demographic variability, vaccination, and the spatiotemporal dynamics of rotavirus epidemics. Science. 2009;325(5938):290–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. Oxford, United Kingdom: Oxford University Press; 1991. [Google Scholar]
- 13. Pasetti MF, Simon JK, Sztein MB, et al. Immunology of gut mucosal vaccines. Immunol Rev. 2011;239(1):125–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Premkumar PS, Parashar UD, Gastanaduy PA, et al. Reduced rotavirus vaccine effectiveness among children born during the rotavirus season: a pooled analysis of 5 case-control studies from the Americas. Clin Infect Dis. 2015;60(7):1075–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. IBM Corporation (Truven Health Analytics) IBM MarketScan research. 2018. https://marketscan.truvenhealth.com/marketscanportal/ . Accessed July 1, 2016.
- 16. Velázquez FR, Matson DO, Calva JJ, et al. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med. 1996;335(14):1022–1028. [DOI] [PubMed] [Google Scholar]
- 17. Gladstone BP, Ramani S, Mukhopadhya I, et al. Protective effect of natural rotavirus infection in an Indian birth cohort. N Engl J Med. 2011;365(4):337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lewnard JA, Lopman BA, Parashar UD, et al. Naturally acquired immunity against rotavirus infection and gastroenteritis in children: paired reanalyses of birth cohort studies. J Infect Dis. 2017;216(3):317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cadranel S, Zeglache S, Jonckheer T, et al. Factors affecting antibody response of newborns to repeated administrations of the rotavirus vaccine RIT 4237. J Pediatr Gastroenterol Nutr. 1987;6(4):525–528. [DOI] [PubMed] [Google Scholar]
- 20. Dowell SF. Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerg Infect Dis. 2001;7(3):369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parker EP, Kampmann B, Kang G, et al. Influence of enteric infections on response to oral poliovirus vaccine: a systematic review and meta-analysis. J Infect Dis. 2014;210(6):853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang H, Moon S, Wang Y, et al. Multiple virus infection alters rotavirus replication and expression of cytokines and toll-like receptors in intestinal epithelial cells. Virus Res. 2012;167(1):48–55. [DOI] [PubMed] [Google Scholar]
- 23. Gould LH, Walsh KA, Vieira AR, et al. Surveillance for foodborne disease outbreaks—United States, 1998–2008. MMWR Surveill Summ. 2013;62(2):1–34. [PubMed] [Google Scholar]
- 24. O’Leary TJ, Slutsky JR, Bernard MA. Comparative effectiveness research priorities at federal agencies: the view from the Department of Veterans Affairs, National Institute on Aging, and Agency for Healthcare Research and Quality. J Am Geriatr Soc. 2010;58(6):1187–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hsu VP, Staat MA, Roberts N, et al. Use of active surveillance to validate International Classification of Diseases code estimates of rotavirus hospitalizations in children. Pediatrics. 2005;115(1):78–82. [DOI] [PubMed] [Google Scholar]
- 26. Atchison CJ, Tam CC, Lopman BA. Season of birth and risk of rotavirus diarrhoea in children aged <5 years. Epidemiol Infect. 2009;137(7):957–960. [DOI] [PubMed] [Google Scholar]
- 27. Centers for Disease Control and Prevention National, state, and local area vaccination coverage among children aged 19–35 months—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(36):1171–1177. [PubMed] [Google Scholar]