Abstract
Background
Membranoproliferative glomerulonephritis (MPGN) with immune complexes and C3 glomerulopathy (C3G) in children are rare and have a variable outcome, with some patients progressing to end-stage renal disease (ESRD). Mutations in genes encoding regulatory proteins of the alternative complement pathway and of complement C3 (C3) have been identified as concausative factors.
Methods
Three children with MPGN type I, four with C3G, i.e. three with C3 glomerulonephritis (C3GN) and one with dense deposit disease (DDD), were followed. Clinical, autoimmune data, histological characteristics, estimated glomerular filtration rate (eGFR), proteinuria, serum C3, genetic and biochemical analysis were assessed.
Results
The median age at onset was 7.3 years and the median eGFR was 72 mL/min/1.73 m2. Six children had marked proteinuria. All were treated with renin–angiotensin–aldosterone system (RAAS) blockers. Three were given one or more immunosuppressive drugs and two eculizumab. At the last median follow-up of 9 years after diagnosis, three children had normal eGFR and no or mild proteinuria on RAAS blockers only. Among four patients without remission of proteinuria, genetic analysis revealed mutations in complement regulator proteins of the alternative pathway. None of the three patients with immunosuppressive treatment achieved partial or complete remission of proteinuria and two progressed to ESRD and renal transplantation. Two patients treated with eculizumab revealed relevant decreases in proteinuria.
Conclusions
In children with MPGN type I and C3G, the outcomes of renal function and response to treatment modality show great variability independent from histological diagnosis at disease onset. In case of severe clinical presentation at disease onset, early genetic and biochemical analysis of the alternative pathway dysregulation is recommended. Treatment with eculizumab appears to be an option to slow disease progression in single cases.
Keywords: C3 glomerulopathy, complement dysregulation, eculizumab, MPGN, paediatrics
Introduction
Membranoproliferative glomerulonephritis (MPGN) with immune complexes is a rare chronic glomerulonephritis in childhood characterized by proteinuria (up to the nephrotic range), haematuria, hypertension and often impaired renal function at disease onset [1]. In up to 50% of affected children, MPGN leads to renal failure within 10 years [2]. Impaired renal function after 1 year of onset is considered a risk factor for poor renal outcome and end-stage renal disease (ESRD) [1]. The recurrence rate after renal transplantation (RTPL) is high (up to 45%) [1–4]. MPGN may occur as a primary genetic disorder or secondary to chronic diseases, including infections (e.g. hepatitis B or C), systemic lupus erythematosus, liver disease and malignancies.
In the past, MPGN was diagnosed and classified by renal histological features and grouped into three pathological subtypes with different aetiologies and pathogenesis, types I, II [(dense deposit disease (DDD)] and III [5]. Activation of the alternative complement pathway has repeatedly been observed in conjunction with low serum levels of complement C3 (C3) [6–8]. A link between dysregulation of the alternative complement pathway and the pathogenesis of MPGN was assumed [7] and has recently been confirmed by findings of mutations in the genes of complement factor H (CFH) and CF-related proteins (CFHR) in DDD [9–13].
Therefore the histological classification has been reconsidered recently on the basis of pathogenesis and with division into those cases in which the glomerular immune deposits stain for immunoglobulins and complement and those cases that are characterized by C3 deposition alone [5, 14–16]. The term ‘C3 glomerulopathy’ (C3G) encompassed complement-mediated renal disease, and therefore incorporates disease entities where the presence of a disease-associated complement mutation is causally associated with the underlying renal pathology. Examples include DDD and C3 glomerulonephritis (C3GN) [5]. The term C3GN was coined to describe glomerular lesions in which there is glomerular accumulation of C3 with little or no immunoglobulin in the absence of the characteristic highly electron-dense transformation seen in DDD [16]. The incidence of C3G is estimated to be 1–2 per 106 children [17, 18], with disease recurrence after RTPL reported at between 30 and 77% and a graft failure due to recurrence in 17–50% of the recipients [19, 20]. MPGN associated with the presence of immunoglobulins and complement has been termed immune complex–mediated MPGN by Sethi and Fervenza [21]. Immune complex-mediated MPGN is commonly associated with autoimmune disease and chronic infection and can be associated with mixed cryoglobulinaemia or monoclonal gammopathy [5, 21]. These associations were excluded in our study. In our series, histological MPGN I is a case of so-called idiopathic MPGN. MPGN I and C3G are regarded as heterogeneous diseases, with several studies reporting complement mutations in complement genes [15, 22]. So far there are no evidence-based guidelines for treatment of MPGN I [1, 2] and C3G. The mainstay of treatment in MPGN I and C3G is based on single-centre studies and expert opinions. Clinical trials in adults and children with different treatment modality propositions [e.g. immunosuppressant agents, antiplatelet drugs and plasmapheresis (PEX)] are described [23–29]. Treatment with renin–angiotensin–aldosterone system (RAAS) blockers are described to induce a decrease in proteinuria and delays progression to ESRD in many glomerular diseases in adults [30, 31] and in some glomerulopathies in children [32, 33]; however, there is no evidence for beneficial effect in children with MPGN I [34] and spontaneous recovery in C3GN can also not be excluded [22].
Eculizumab is a monoclonal antibody binding to C5 and thereby inhibiting the complement system and preventing activation of the alternative pathway. Recently, promising results were shown in selected patients with MPGN and C3GN characterized by the presence of alterations in regulatory proteins of the alternative complement pathway [22, 35–37].
We evaluated the outcome of seven children with the diagnosis of MPGN I, C3GN and DDD. All were screened for the presence of genetic mutations of the alternative complement pathway. We analysed the benefit of different administered treatments.
Materials and methods
All patients with histologically diagnosed MPGN I, DDD and C3GN who were seen at the University Children’s Hospital Zurich between 2003 and 2015 were included in a retrospective data analysis. Clinical assessment included diagnosis, sex, medical history, clinical examination, blood pressure (BP) and analysis to exclude potentially secondary forms of MPGN.
Biochemical and genetic analysis
Plasma creatinine, total serum protein and albumin and urinary protein:creatinine ratio (UPCR) were analysed at disease onset and at the last follow-up. Blood samples for complement regulator protein analysis were centrifuged immediately after collection and frozen at−80°C prior to analysis. C3 serum levels were evaluated by kinetic nephelometry (Immage 800, Beckman Coulter, Brea, CA, USA) with a normal value >0.7 g/L (reference value of the immunology laboratory of Zurich University Children’s Hospital). The soluble complement components C5b-9 (membrane attack complex), C3d and C3 nephritic factor (C3NeF) were measured from plasma samples at the Institute for Immunology of Heidelberg University (Heidelberg, Germany). The following biochemical analyses were performed at follow-up from plasma and serum, as well as ethylenediaminetetraacetic acid blood samples for genetic analysis (samples at the Leibniz Institute for Natural Product Research and Infection Biology, Jena, Germany): CFH antibody, C3-convertase antibody, CFI, CFB, CFHR1, CFHR2, CFHR3, CFHR4, CFHR5, MCP (CD46) and by next-generation sequencing (NGS) at the Center for Human Genetics at Bioscientia in Ingelheim (Germany), as described in detail below.
Targeted NGS using a customized multigene panel for atypical hemolytic uremic syndrome (aHUS) and related disorders was performed in all patients [38]. In brief, we utilized a customized sequence capture library that targets exons and additionally 35 bp of flanking intronic sequence (20–23). Genomic DNA was fragmented and the coding exons of the analysed genes, as well as the corresponding exon–intron boundaries, were enriched using the Roche/NimbleGen sequence capture approach (NimbleGen, Madison, WI, USA), amplified and sequenced simultaneously by Illumina NGS sequencing-by-synthesis technology using a HiSeq 1500 system (Illumina, San Diego, CA, USA). Target regions were usually sequenced with an average coverage of ∼ 400–500-fold. With this method, 20-fold coverage is obtained for >99.5% of the regions of interest. NGS data analysis was performed using bioinformatic analysis tools and JSI Medical Systems software (version 4.1.2; JSI Medical Systems, Kippenheim, Germany). Identified variants and indels were filtered against external and internal databases and depending on allelic frequency. The focus was on rare variants with a minor allele frequency of ≤1%. Nonsense, frameshift and canonical splice site variants were considered a priori as likely to be pathogenic. Pathogenicity of identified non-synonymous variants was assessed using bioinformatic prediction programs, such as Mutation Taster, Polyphen-2, MutationAssessor and FATHMM. Only those variants predicted by the majority of algorithms used to be probably damaging were considered likely to be pathogenic. In silico analysis of splice site effects was performed using bioinformatic programs such as Fruitfly, NetGene2, Human Splicing Finder, Mutation Taster and ESEFinder. Mapping and coverage statistics were generated from the mapping output files using GATK. The resulting sequence data were compared with the reference sequence of the RefSeq database. High coverage enabled copy number variation analysis. Potential copy number alterations were initially identified by VarScan on mapped reads. In this way, coverage of every target region of the sample was internally normalized and compared with normalized control data of other samples of the same run by VarScan copy number mode and standard settings. Putative pathogenic differences between the wild-type sequence (human reference genome according to the University of California, Santa Cruz Genome Browser: hg19, GRCh37) and the patient’s sequence were validated by conventional Sanger sequencing and, in the case of copy number variation, by multiplex ligation-dependent probe amplification.
Renal biopsy
All patients underwent native kidney biopsy at disease onset. Repeated renal biopsy was performed in two children, one of the native kidneys and one in the transplanted kidney. The biopsy diagnosis was based on the current classification and nomenclature for C3G and MPGN [5, 16] on findings by light microscopy (LM), immunofluorescence (IF) and electron microscopy (EM).
Definitions
The following definitions were used: nephrotic-range proteinuria: UPCR >250 g/mol; nephrotic syndrome: serum albumin <25 g/L, nephrotic-range proteinuria and generalized oedema; remission: UPCR <20 g/mol; partial remission: UPCR >20–<80 g/mol; normal renal function: estimated glomerular filtration rate (eGFR) >90 mL/min/1.73 m2, as calculated by the Schwartz formula using a local k-factor of 40 [39]; hypertension: casual systolic BP >95th percentile for sex and height [40].
Treatment
All patients were given RAAS blockers as first-line antiproteinuric therapy. Additional treatment consisted of prednisolone (PDN), in accordance with the dosage regimen for treatment of children with steroid-resistant nephrotic syndrome [41, 42]. Further immunosuppressive treatment [cyclosporine A (CSA) and mycofenolate mofetile (MMF)] [25, 42] was administered to patients who failed to attain partial or full remission of proteinuria. Eculizumab has been available in our hospital since 2013, but costs were not covered by all insurances, as this drug is still off-label for MPGN/C3G treatment. Eculizumab was given to two patients with nephrotic-range proteinuria despite immunosuppressive and RAAS blocker treatment. The dosage followed current recommendations for aHUS [43]. Patients treated with eculizumab were immunized with meningococcal vaccine (Menveo, GlaxoSmithKline Biologicals, Rixensart, Belgium) 4 weeks prior to administration. Informed consent was obtained from all parents of included patients for medical data collection, genetic analysis and treatment with eculizumab.
Results
Seven children (four boys and three girls) fulfilled the inclusion criteria (Table 1): three patients suffered from MPGN I, three from C3GN and one from DDD. The median age at onset was 7.3 years (range 2.5–12.5) with a median eGFR of 72 mL/min/1.73 m2 (range 41–140) at diagnosis.
Table 1.
Patient characteristics at disease onset
| Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 |
|---|---|---|---|---|---|---|---|
| Age (years) | 7.3 | 2.5 | 6 | 12.5 | 5.9 | 10.5 | 8.8 |
| Sex | Male | Male | Female | Female | Male | Female | Male |
| Hypertension | No | No | No | No | No | Yes | Yes |
| Haematuria | Micro | Micro | Macro | Micro | Macro | Micro | Micro |
| UPCR (g/mol) (reference <20) | 634 | 126 | 530 | 855 | 580 | 1500 | 1000 |
| Nephrotic syndrome (UPCR >250 g/mol) | No | No | No | Yes | No | Yes | Yes |
| Serum albumin (g/L) (reference 35–51) | 27 | 32 | 26 | 11 | 30 | 12 | 12 |
| eGFR (mL/min/1.73 m2) (reference >90) | 72 | 140 | 41 | 67 | 88 | 65 | 95 |
Analysis of the alternative complement pathway: CFs and genetic testing
All children showed persistently low serum C3 (Table 2). In addition, C3NeF was negative in all but one patient and C3b antibodies were positive in two children with C3GN.
Table 2.
Genetic, complement and histology testing
| Test | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | |
|---|---|---|---|---|---|---|---|---|
| Genetic testing |
|
|
|
|
|
|
|
|
| C3 antibody positivity | No | No | No | No | No | Yes | Yes | |
| C3NeF | Negative | Negative | Negative | Negative | Positivea | Negative | Negativea | |
| C3 g/L (reference 0.7–1.76)b | 0.4 | <0.06 | 0.23 | 0.26 | 0.3 | 0.24 | 0.6 | |
| Renal histology biopsy | MPGN I | MPGN I | C3 GN | MPGN I | C3-GN | cC3-GN | DDD | |
| LM | Mesangial and endocapillary proliferation. Double contours of GBM | Mesangial and endocapillary proliferation. Double contours of GBM | Mesangial and mild endocapillary proliferation, double contours of GBM, humps | Mesangial and mild endocapillary proliferation, double contours of GBM, crescents, 36% glomeruli hyalinized, 50% interstitial fibrosis and tubular atrophy. | Mesangial and mild endocapillary proliferation | Mesangial and endocapillary proliferation, double contours of GBM, 40% hyalinized glomeruli, segmental sclerosis, crescents, >95% interstitial fibrosis and tubular atrophy | Mesangial and mild endocapillary proliferation, 25% hyalinized glomeruli, crescents in 50% of glomeruli, thickended, glassy GBM | |
| IF | Dominant GBM and mesangial positivity for IgG (2+) and C3 (2+) | Dominant GBM and mesangial positivity for C3 (3+), IgG (2+), IgM (3+) | GBM and mesangial positivity for C3 (3+), IgM (1+). IgG is negative | Dominant GBM and mesangial positivity for IgG (3+) and C3 (3+) | Dominant mesangial and GBM positivity for C3 (3+). IgG is negative | Mesangial and GBM positivity for C3 (3+), less IgG (1+) | GBM positivity for C3 (3+), IgM (3+), IgG (1+) | |
| Electron microscopy | Subendothelial and mesangial electron-dense deposits | Subendothelial, mesangial and rare small subepithelial and intramembranous electron-dense deposits | Mesangial, subendothelial, intramembranous and subepithelial electron-dense deposits with humps | Mesangial, subendothelial, intramembranous and rare subepithelial electron-dense deposits | Granular, not very dense intramembranous and mesangial electron-dense deposits | Mesangial and intramembranous, not very dense granular electron-dense deposits. Rare small subepithelial deposits. | Highly osmiophilic segmental electron-dense deposits in lamina densa of GBM |
Hom, homozygous; het, heterozygous; del, deletion; GBM, glomerular basement membrane. aResult performed only under treatment with eculizumab in Patient 5 and with PEX in Patient 7. bValues of serum complement C3 at disease onset. cSecond biopsy 7 years after onset biopsy.
At the last follow-up (Tables 3–5), C3d was elevated in five patients and sC5b-9 levels were increased in four children, both in MPGN I and C3GN.
Table 3.
Patients with RAAS blockers only: values at last follow-up
| Characteristic | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Maintained renal function | Native kidney | Native kidney | Native kidney |
| Treatment | Enalapril | Enalapril | Enalapril |
| Losartan | Losartan | ||
| Duration of observation (years) | 9.7 | 2.2 | 4.8 |
| eGFR | 143 | 97 | 180 |
| C3 (g/L) (reference 0.7–1.76) | 0.6 | <0.06 | 0.13 |
| UPCR (g/mol) (reference < 20) | 41 | 41 | <20 |
| sC3d (mU/L) (reference < 40) | 50 | 103 | 35 |
| sC5b-9 (ng/mL) (reference < 320) | 239 | 2538 | 164 |
eGFR rate according to Schwartz formula in mL/min/1.73 m2.
Renal biopsy
Renal biopsy findings (MPGN I, C3GN and DDD) (Figures 1–4) are presented in Table 2. Repeated renal biopsy was performed in Patient 4 (native kidney, before embarking on MMF) and Patient 7 (renal graft for suspected acute graft rejection). The diagnoses were made based on IF findings. As the IF slides were no longer available, the figures contain pictures of immunohistochemistry performed for the purpose of the publication.
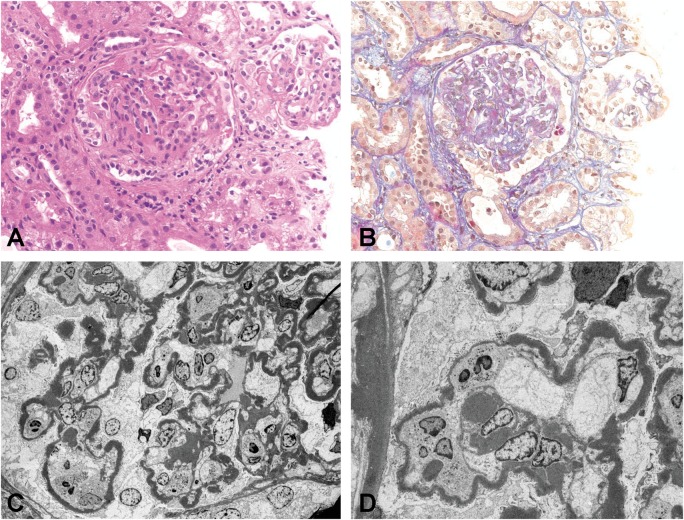
Fig. 1.
DDD native kidney (Patient 7). (A) Glomeruli with mesangial and endocapillary proliferation and a fibrocellular crescent [hematoxylin and eosin (H&E) stain, original magnification ×200]. (B) Intramembranous and mesangial deposits (acid fuchsin orange G stain, original magnification ×200). (C and D) EM with highly osmiophilic electron-dense deposits in the lamina densa of the glomerular basement membrane and mesangium (original magnification ×1100 and ×1950).
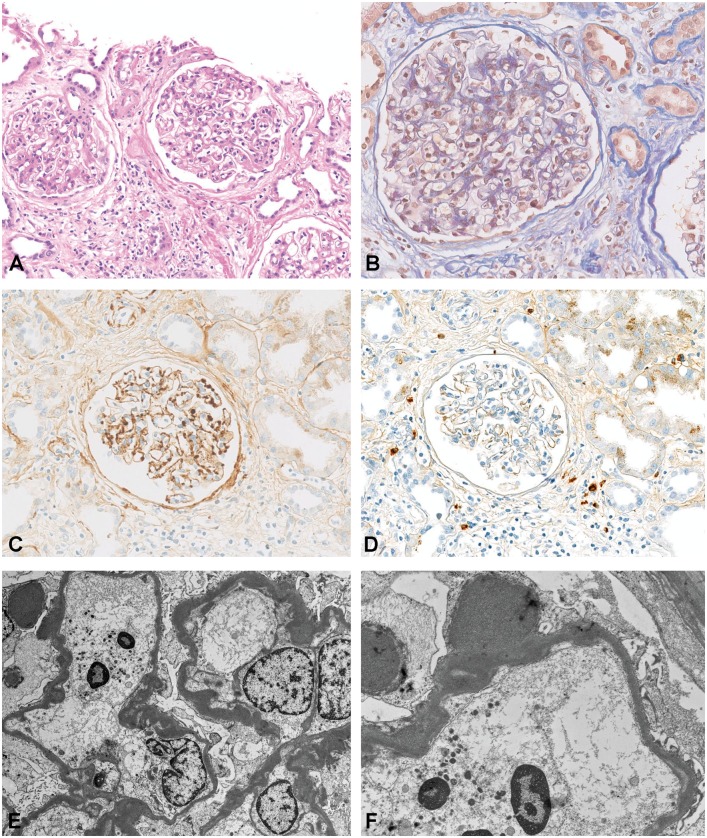
Fig. 2.
DDD recurrence in transplant biopsy (Patient 7). (A) Thickened, glassy GBM and endocapillary hypercellularity with mononuclear cells (H&E stain, original magnification ×200). (B) Intramembranous deposits and endocapillary proliferation (AFOG stain, original magnification ×324). (C) Immunohistochemistry positive for C3 (original magnification ×200). (D) Immunohistochemistry negative for immunoglobulin G (original magnification ×200). (E and F) EM with highly osmiophilic electron-dense deposits in the lamina densa and subepithelial humps (original magnification ×1000 and ×2500).
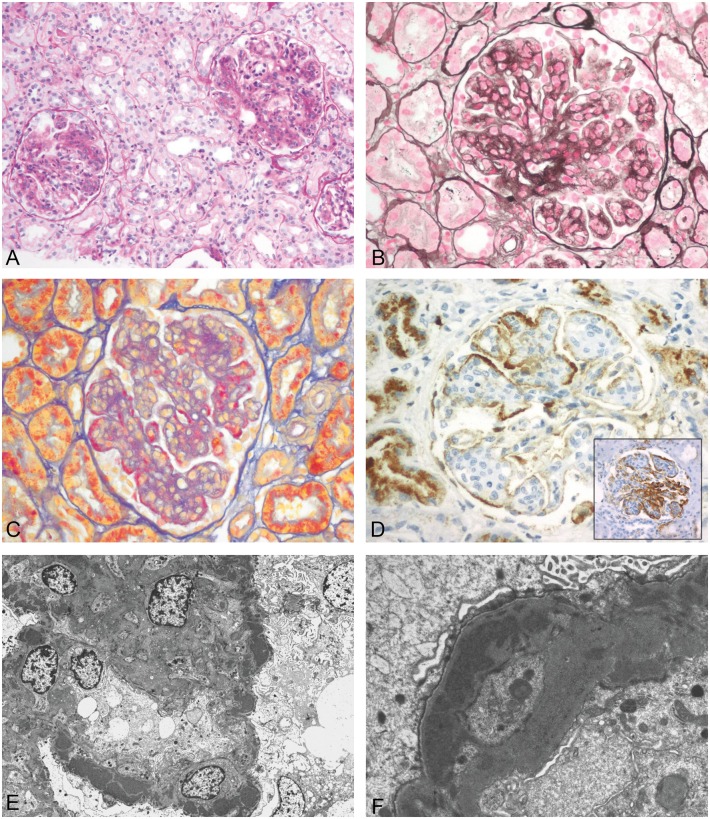
Fig. 3.
MPGN I (Patient 1). (A) Mesangial and endocapillary proliferation (periodic acid–Schiff stain, original magnification ×110). (B) Splitting of the GBM (silver methenamine stain, original magnification ×220). (C) Subendothelial deposits (AFOG stain, original magnification ×200). (D) Immunhistochemistry positive for IgG (original magnification ×280), inset: positivity for C3 (original magnification ×220). (E) EM with mostly subendothelial and some subepithelial and mesangial electron-dense deposits (original magnification ×1950). (F) Subendothelial and small subepithelial electron-dense deposits (original magnification ×10 500).
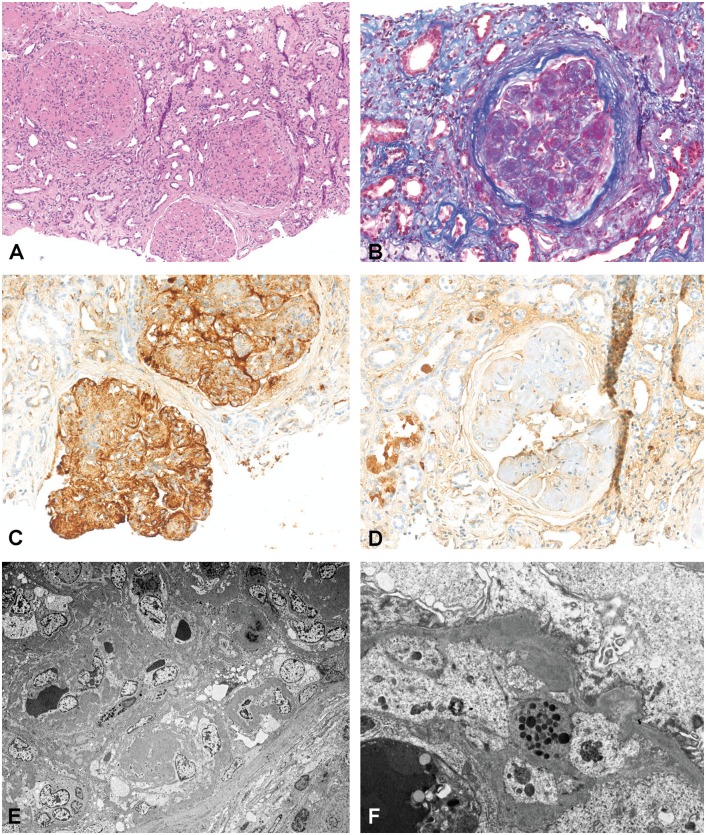
Fig. 4.
C3GN (Patient 6). (A) Mesangial and endocapillary proliferation (H&E stain, original magnification ×100). (B) Mesangial and GBM deposits (AFOG stain, original magnification ×200). (C) Immunohistochemistry positive for C3 (original magnification ×200). (D) Immunohistochemistry negative for IgG (original magnification ×200). (E) EM with mostly intramembranous and mesangial not very dense electron-dense deposits (original magnification ×300). (F) EM with subepithelial, intramembranous and subendothelial deposits (original magnification ×2000).
Treatment regimens and follow-up
The median follow-up was 9 years (range 2.2–11.5). Five children maintained renal function of their native kidneys, with a median eGFR of 107 mL/min/1.73 m2 (range 40–180), including four patients with normal eGFR. Two children reached ESRD and underwent RTPL with initially good renal graft function. All children had BP < 95th percentile, with six on RAAS blockers: in one child, RAAS blockers were stopped after RTPL. All children exhibited persistent haematuria. Three patients with nephrotic syndrome (Patients 4, 6 and 7) exhibited progression of proteinuria on PDN and were given additional immunosuppressive treatment.
Patients with RAAS blockers only
Patients 1–3 (MPGN I, n = 2; C3GN, n = 1) (Table 3)
All had normal renal function at onset without signs of a nephrotic syndrome. Follow-up was uneventful, with partial or complete remission of proteinuria and maintained normal renal function. All had persistently low C3. Genetic testing revealed a risk haplotype for the membrane cofactor protein (MCP/CD46) gene in all three children. In addition, Patient 2 (MPGN I) had elevated sC5b-9 and a heterozygous mutation in the CFHR5 gene.
Patients with additional immunosuppressive treatments, including eculizumab
Patient 4 (MPGN I) (Tables 4)
Table 4.
Patients with eculizumab: values before eculizumab and at last follow-up
| Characteristic (original disease) | Patient 4 (MPGN I) | Patient 5 (C3GN) |
|---|---|---|
| Maintained renal function | Native kidney | Native kidney |
| Additional treatments to RAAS blockers | PDN | |
| MMF | ||
| Before eculizumab | ||
| Duration of observation (years) | 6.9 | 9.5 |
| eGFR (mL/min/1.73 m2) | 40 | 100 |
| C3 (g/L) (reference 0.7–1.76) | 0.09 | 0.06 |
| UPCR (g/mol) (reference <20) | 510 | 750 |
| sC3d (mU/L) (reference <40) | 63 | 95 |
| sC5b-9 (ng/mL) (reference >320) | 4100 | 6500 |
| Observation on eculizumab | ||
| Duration of therapy (months) | 6 | 11 |
| eGFR | 45 | 90 |
| UPCR (g/mol) | 270 | 227 |
| sC5b-9 (ng/mL) | 282 | 639 |
| C3 (g/L) | 0.12 | 0.14 |
Because of progressive proteinuria on PDN, MMF was started [25]. Daily proteinuria initially decreased from 4 to 2 g. However, impaired renal function and nephrotic-range proteinuria persisted with elevated alternative pathway activity. A second renal biopsy confirmed the diagnosis of MPGN I. Therefore MMF was discontinued and eculizumab was started. Six months after starting eculizumab, a significant decrease in proteinuria and sC5b-9 was observed with stabilization of eGRF.
Patient 5 (C3GN) (Tables 4)
Because of increasing proteinuria with normal renal function and persistently elevated alternative pathway activity despite RAAS blockade, the treatment with eculizumab was initiated [43]. After 11 months, proteinuria and sC5b-9 level decreased, whereas C3NeF remained positive. Renal function remained stable at a normal level.
Patient 6 (C3GN) (Table 5)
Table 5.
Patients undergoing RTPL: values before RTPL and at last follow-up after RTPL
| Characteristic (original disease) | Patient 6 (C3GN) | Patient 7 (DDD) |
|---|---|---|
| Before RTPL | ||
| Additional treatments to RAAS blockers | CSA | CSA |
| Duration of observation (years) | 9 | 11.5 |
| C3 (g/L) (reference 0.7–1.76) | 0.2 | 0.55 |
| UPCR (g/mol) (reference <20) | 1350 | 400 |
| sC3d (mU/L) (reference <40) | 27 | 61 |
| sC5b-9 (ng/mL) (reference <320) | 1359 | Not done |
| Observation after RTPL (m) | ||
| Duration (months) | 7 | 48 (2nd RTPL) |
| Recurrence of original disease (m) | No | Yes (15) |
| Treatment for original disease recurrence | No | PEX/FFP |
| Graft rejection | No | Yes (ABMR) |
| eGFR | 51 | 42 |
| UPCR (g/mol) | <20 | 400 |
| sC5b-9 (ng/mL) | 770 | 121 |
| C3 (g/L) | 0.25 | 0.9 |
| Renal graft biopsy after RTPL | No | Yes |
| Treatment after RTPL | Induction with basiliximab for RTPL | Induction with thymeoglobulin for RTPL |
| Prednisolone | Prednisolone | |
| Tacrolimus | Tacrolimus | |
| MMF | MMF |
Nephrotic-range proteinuria persisted despite additional treatment with CSA for 6 years. The child reached ESRD and underwent peritoneal dialysis 6.5 years after disease onset. Deceased-donor RTPL was performed; immunosuppression included tacrolimus, MMF, PDN and induction therapy with basiliximab. RAAS blocker was stopped. Seven months after transplantation, sC5b-9 was still slightly elevated. Renal graft function remained stable without proteinuria.
Patient 7 (DDD) (Table 5)
Additional treatment with CSA was started due to persistent nephrotic-range proteinuria. The child reached ESRD 4.2 years after disease onset. Living paternal-donor RTPL was performed; immunosuppression included CSA, MMF and PDN. The child experienced acute/active antibody-mediated rejection (aABMR) and recurrence of DDD in the graft 3.3 years after transplantation Therefore treatment with CSA was switched to tacrolimus and PEX was started [1.5-fold plasma volume exchange with fresh frozen plasma (FFP)], but ESRD occurred 3.9 years after RTPL. A deceased-donor RTPL was then performed. Immunosuppression included tacrolimus, MMF, PDN and induction with thymeoglobulin. Again, ABMR and recurrence of the disease with nephrotic proteinuria occurred 15 months after retransplantation. Weekly treatment with PEX reduced proteinuria, but proteinuria increased when the PEX interval was extended to every second week. Measurement of sC5b-9 (after PEX session) showed normal values. Renal graft function was impaired (eGFR 42 mL/min/1.73 m2) at the last follow-up.
Discussion
MPGN and C3G are rare diseases with chronic progressive glomerulonephritis and children may have an unfavourable course leading to ESRD [17, 18, 44, 45]. Studies in adults and children have shown that proteinuria is a major risk factor for developing ESRD [1, 46, 47]. In the last decade, new insights are emerging to improve genetic and biochemical investigations of these diseases.
Currently there is no established treatment for MPGN and C3G. Patients appear to respond differently to various therapy modalities [27, 29, 48, 49]. The majority of treatment regimens and case series have been reported in adults, not in children [23, 26], and are often associated with significant side effects. Recently, treatment with eculizumab, a monoclonal antibody binding to C5 of the alternative pathway, has shown promising results in the treatment of some cases of MPGN and C3G [22, 35–37, 50].
Our analysis revealed a dysregulation of the complement alternative pathway and mutation/variation in genes of CF proteins in children with MPGN, C3GN and DDD. Three patients of our series showed a favourable outcome. These were two patients with MPGN I and one with C3GN, the latter without any genetic variation in CFHR proteins. Five of seven children had a heterozygous mutation/deletion or variation in CFHR proteins 1, 2, 3 or 5. Recent findings show that dysregulation of the complement pathway by CFHR2–CFHR5 hybrid protein leads to enhanced C3-convertase activation of the alternative complement pathway and other genetic complement abnormalities associated with MPGN [12, 17, 51]. In our patients, genetic alterations, including variation or polymorphisms, may at least in part explain the different outcomes and responses to the various treatment modalities. Similar findings are described by other authors, where the clinical presentation and the measurement of plasma C3, C3d and sC5b-9 do not allow differentiation between C3G and MPGN I [22]. Recent knowledge suggests that in idiopathic MPGN—an immune complex Rixensart, Belgium mediated disease—involvement of the alternative pathway plays an important role [17, 52].
Three of seven of our patients (two with MPGN and one with C3GN) without nephrotic syndrome showed remission of proteinuria on RAAS blocker therapy only. No significant reduction in proteinuria is described in children with MPGN treated with RAAS blocker only. Other data revealed that patients with less parenchymal damage in their initial renal biopsy benefit the most from sole treatment with RAAS blocker [1]; however, spontaneous remission may not be excluded [22]. Consistent with other authors [1], those children of our series with nephrotic syndrome at onset showed a more severe course, with two progressing to ESRD followed by RTPL.
Eculizumab has been described as a successful treatment of patients with MPGN [35, 50, 53–55] and C3G [19, 22, 56] in several reports. Elevated sCb5-9 levels may be a predictor of response to treatment with eculizumab, but other factors affecting response to therapy are poorly understood [53, 55]. Two patients of our series, one with C3GN and one with MPGN I, exhibited a significant decrease in proteinuria on eculizumab in their native kidneys, but elevated activity of the alternative pathway persisted. This is consistent with observations of other paediatric patients with MPGN [50] suggesting that eculizumab is not completely effective in suppressing sC5b-9 activity in C3G. They hypothesized that sC5b-9 alone may not reflect disease activity. The presence of mutations alone does not significantly increase the risk of developing idiopathic MPGN or C3G, but they do so when combined with common susceptibility variants [i.e. in CD46, CFH or Thrombomodulin (THBD)] [17, 52].
C3NeF, an auto-antibody directed against the alternative C3-convertase, was positive at follow-up in only one child of our series. Published reports on the impact of C3NeF on outcome in C3G patients are inconsistent, with reported patients ranging from complete remission to ESRD [57, 58]. Apparently C3NeF can fluctuate during the clinical course independent of the disease treatment [22]. Others observed a higher risk of progressing to ESRD in patients without complement gene mutation or C3NeF, stabilizing the alternative pathway C3-convertase [52]. In our patient, C3NeF and a heterozygous variation of CFHR1 and CFHR1/3 was found. In addition, sC5b-9 was significantly elevated. Other treatments, for example, rituximab and PEX, are controversially discussed in the literature with different results [52, 58, 59]. Therefore, after a risk–benefit evaluation of these treatment options, we decided to apply eculizumab in our young child. The outcome was favourable, with stable, normal renal function and a significant decrease in proteinuria 11 months after eculizumab treatment was started.
In a patient undergoing RTPL twice, C3NeF and sC5b-9 were analysed after PEX treatment with FFP had been started, so detection of both C3NeF and sC5b-9 might have been missed [58]. This patient experienced aABMR with recurrence of DDD 3 years after the first RTPL and was therefore treated with PEX. However, he reached ESRD after the first RTPL, with recurrence of the disease in the second renal graft. Treatment with PEX was started at weekly intervals but led to only a moderate decrease in proteinuria with persistently impaired graft function. The insurance refused to pay for treatment with eculizumab. As complement-mediated dysregulation is involved in the pathophysiology of aABMR [60], it is conceivable that DDD recurrence may have triggered aABMR by inducing complement activation. In a recent study, MPGN recurrence after RTPL was detected in 18 of 40 transplants and in 3 cases disease recurrence preceded aABMR and led to graft loss [4]. Registries have also reported recurrence rates on renal graft for DDD and C3G of 50% and 43–67%, respectively [22].
There are some limitations to our study: (i) its retrospective character; (ii) the individual treatment approach based on the clinical course; (iii) due to the different time period at disease manifestation of each patient, no uniformity of treatment was possible and (iv) measurement of activity of the alternative pathway (i.e. C3d, sC5b-9) and C3NeF was not available at disease onset.
Our results reveal that early examination of the alternative complement pathway may aid to define a more individually tailored treatment. However, we observed heterogeneity of clinical and biological features in MPGN and C3G and therefore the difficulty of interpretation of both genetic abnormalities and biochemical analysis. Long-term observation is necessary in order to draw conclusions about the results of treatments and renal function at follow-up.
Conflict of interest statement
None declared.
References
- 1. Cansick JC, Lennon R, Cummins CL. et al. Prognosis, treatment and outcome of childhood mesangiocapillary (membranoproliferative) glomerulonephritis. Nephrol Dial Transplant 2004; 19: 2769–2777 [DOI] [PubMed] [Google Scholar]
- 2. Smith RJ, Alexander J, Barlow PN. et al. New approaches to the treatment of dense deposit disease. J Am Soc Nephrol 2007; 18: 2447–2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ponticelli C, Glassock RJ.. Posttransplant recurrence of primary glomerulonephritis. Clin J Am Soc Nephrol 2010; 5: 2363–2372 [DOI] [PubMed] [Google Scholar]
- 4. Alasfar S, Carter-Monroe N, Rosenberg AZ. et al. Membranoproliferative glomerulonephritis recurrence after kidney transplantation: using the new classification. BMC Nephrol 2016; 17: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cook HT, Pickering MC.. Histopathology of MPGN and C3 glomerulopathies. Nat Rev Nephrol 2015; 11: 14–22 [DOI] [PubMed] [Google Scholar]
- 6. West CD. Idiopathic membranoproliferative glomerulonephritis in childhood. Pediatr Nephrol 1992; 6: 96–103 [DOI] [PubMed] [Google Scholar]
- 7. Daha MR, Fearon DT, Austen KF.. Formation in the presence of C3 nephritic factor (C3NeF) of an alternative pathway C3 convertase containing uncleaved B. Immunology 1976; 31: 789–796 [PMC free article] [PubMed] [Google Scholar]
- 8. Licht C, Schlotzer-Schrehardt U, Kirschfink M. et al. MPGN II – genetically determined by defective complement regulation? Pediatr Nephrol 2007; 22: 2–9 [DOI] [PubMed] [Google Scholar]
- 9. Licht C, Heinen S, Jozsi M. et al. Deletion of Lys224 in regulatory domain 4 of factor H reveals a novel pathomechanism for dense deposit disease (MPGN II). Kidney Int 2006; 70: 42–50 [DOI] [PubMed] [Google Scholar]
- 10. Skerka C, Lauer N, Weinberger AA. et al. Defective complement control of factor H (Y402H) and FHL-1 in age-related macular degeneration. Mol Immunol 2007; 44: 3398–3406 [DOI] [PubMed] [Google Scholar]
- 11. Dragon-Durey MA, Fremeaux-Bacchi V, Loirat C. et al. Heterozygous and homozygous factor h deficiencies associated with hemolytic uremic syndrome or membranoproliferative glomerulonephritis: report and genetic analysis of 16 cases. J Am Soc Nephrol 2004; 15: 787–795 [DOI] [PubMed] [Google Scholar]
- 12. Chen Q, Wiesener M, Eberhardt HU. et al. Complement factor H-related hybrid protein deregulates complement in dense deposit disease. J Clin Invest 2014; 124: 145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen Q, Manzke M, Hartmann A. et al. Complement factor H-related 5-hybrid proteins anchor properdin and activate complement at self-surfaces. J Am Soc Nephrol 2016; 27: 1413–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walker PD, Ferrario F, Joh K. et al. Dense deposit disease is not a membranoproliferative glomerulonephritis. Mod Pathol 2007; 20: 605–616 [DOI] [PubMed] [Google Scholar]
- 15. Sethi S, Nester CM, Smith RJ.. Membranoproliferative glomerulonephritis and C3 glomerulopathy: resolving the confusion. Kidney Int 2012; 81: 434–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pickering MC, D’Agati VD, Nester CM. et al. C3 glomerulopathy: consensus report. Kidney Int 2013; 84: 1079–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Servais A, Noël L-H, Roumenina LT. et al. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int 2012; 82: 454–464 [DOI] [PubMed] [Google Scholar]
- 18. Medjeral-Thomas N, Malik TH, Patel MP. et al. A novel CFHR5 fusion protein causes C3 glomerulopathy in a family without Cypriot ancestry. Kidney Int 2014; 85: 933–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lebreton C, Bacchetta J, Dijoud F. et al. C3 glomerulopathy and eculizumab: a report on four paediatric cases. Pediatr Nephrol 2017; 32: 1023–1028 [DOI] [PubMed] [Google Scholar]
- 20. Bacchetta J, Cochat P.. Primary disease recurrence-effects on paediatric renal transplantation outcomes. Nat Rev Nephrol 2015; 11: 371–384 [DOI] [PubMed] [Google Scholar]
- 21. Sethi S, Fervenza FC.. Membranoproliferative glomerulonephritis–a new look at an old entity. N Engl J Med 2012; 366: 1119–1131 [DOI] [PubMed] [Google Scholar]
- 22. Riedl M, Thorner P, Licht C.. C3 Glomerulopathy. Pediatr Nephrol 2017; 32: 43–57 [DOI] [PubMed] [Google Scholar]
- 23. Levin A. Management of membranoproliferative glomerulonephritis: evidence-based recommendations. Kidney Int 1999; 55 (Suppl 70) S41–S46 [DOI] [PubMed] [Google Scholar]
- 24. Somers M, Kertesz S, Rosen S. et al. Non-nephrotic children with membranoproliferative glomerulonephritis: are steroids indicated? Pediatr Nephrol 1995; 9: 140–144 [DOI] [PubMed] [Google Scholar]
- 25. De S, Al-Nabhani D, Thorner P. et al. Remission of resistant MPGN type I with mycophenolate mofetil and steroids. Pediatr Nephrol 2009; 24: 597–600 [DOI] [PubMed] [Google Scholar]
- 26. Jones G, Juszczak M, Kingdon E. et al. Treatment of idiopathic membranoproliferative glomerulonephritis with mycophenolate mofetil and steroids. Nephrol Dial Transplant 2004; 19: 3160–3164 [DOI] [PubMed] [Google Scholar]
- 27. Bagheri N, Nemati E, Rahbar K. et al. Cyclosporine in the treatment of membranoproliferative glomerulonephritis. Arch Iran Med 2008; 11: 26–29 [PubMed] [Google Scholar]
- 28. Appel GB, Cook HT, Hageman G. et al. Membranoproliferative glomerulonephritis type II (dense deposit disease): an update. J Am Soc Nephrol 2005; 16: 1392–1403 [DOI] [PubMed] [Google Scholar]
- 29. Rabasco C, Cavero T, Roman E. et al. Effectiveness of mycophenolate mofetil in C3 glomerulonephritis. Kidney Int 2015; 88: 1153–1160 [DOI] [PubMed] [Google Scholar]
- 30. Ruggenenti P, Perna A, Gherardi G. et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 1999; 354: 359–364 [DOI] [PubMed] [Google Scholar]
- 31. Brenner BM, Cooper ME, de Zeeuw D. et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869 [DOI] [PubMed] [Google Scholar]
- 32. Yang Y, Ohta K, Shimizu M. et al. Treatment with low-dose angiotensin-converting enzyme inhibitor (ACEI) plus angiotensin II receptor blocker (ARB) in pediatric patients with IgA nephropathy. Clin Nephrol 2005; 64: 35–40 [DOI] [PubMed] [Google Scholar]
- 33. Camacho Diaz JA, Gimenez Llort A, Garcia Garcia L. et al. [Long-term effect of angiotensin-converting inhibitors in children with proteinuria]. An Esp Pediatr 2001; 55: 219–224 [PubMed] [Google Scholar]
- 34. Butani L. Angiotensin blockade in children with chronic glomerulonephritis and heavy proteinuria. Pediatr Nephrol 2005; 20: 1651–1654 [DOI] [PubMed] [Google Scholar]
- 35. Vivarelli M, Pasini A, Emma F.. Eculizumab for the treatment of dense-deposit disease. N Engl J Med 2012; 366: 1163–1165 [DOI] [PubMed] [Google Scholar]
- 36. Daina E, Noris M, Remuzzi G.. Eculizumab in a patient with dense-deposit disease. N Engl J Med 2012; 366: 1161–1163 [DOI] [PubMed] [Google Scholar]
- 37. McCaughan JA, O’Rourke DM, Courtney AE.. Recurrent dense deposit disease after renal transplantation: an emerging role for complementary therapies. Am J Transplant 2012; 12: 1046–1051 [DOI] [PubMed] [Google Scholar]
- 38. Bachmann NGM, Hiersche M, Häffner K. et al. Comprehensive genetic analysis by next-generation sequencing (NGS) in a cohort of 264 patients with aHUS. Oral presentation at the 5th International Conference on HUS & Related Disorders, Innsbruck, Austria, 2015. http://www.hus-online.at/de/Conference2_en.html
- 39. Schwartz GJ, Munoz A, Schneider MF. et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 2009; 20: 629–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: a working group report from the National High Blood Pressure Education Program. National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents. Pediatrics 1996; 98: 649–658 [PubMed] [Google Scholar]
- 41. Ehrich JH, Brodehl J, Arbeitsgemeinschaft fur Padiatrische Nephrologie. Long versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Eur J Pediatr 1993; 152: 357–361 [DOI] [PubMed] [Google Scholar]
- 42. Lombel RM, Gipson DS, Hodson EM. et al. Treatment of steroid-sensitive nephrotic syndrome: new guidelines from KDIGO. Pediatr Nephrol 2013; 28: 415–426 [DOI] [PubMed] [Google Scholar]
- 43. Schmidtko J, Peine S, El-Housseini Y. et al. Treatment of atypical hemolytic uremic syndrome and thrombotic microangiopathies: a focus on eculizumab. Am J Kidney Dis 2013; 61: 289–299 [DOI] [PubMed] [Google Scholar]
- 44. Ault BH. Factor H and the pathogenesis of renal diseases. Pediatr Nephrol 2000; 14: 1045–1053 [DOI] [PubMed] [Google Scholar]
- 45. Schwertz R, de Jong R, Gretz N. et al. Outcome of idiopathic membranoproliferative glomerulonephritis in children. Acta Paediatr 1996; 85: 308–312 [DOI] [PubMed] [Google Scholar]
- 46. Warady BA, Abraham AG, Schwartz GJ. et al. Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: the chronic kidney disease in children (CKiD) cohort. Am J Kidney Dis 2015; 65: 878–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sandsmark DK, Messe SR, Zhang X. et al. Proteinuria, but not eGFR, predicts stroke risk in chronic kidney disease: chronic renal insufficiency cohort study. Stroke 2015; 46: 2075–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nester CM, Smith RJ.. Treatment options for C3 glomerulopathy. Curr Opin Nephrol Hypertens 2013; 22: 231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nasr SH, Valeri AM, Appel GB. et al. Dense deposit disease: clinicopathologic study of 32 pediatric and adult patients. Clin J Am Soc Nephrol 2009; 4: 22–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oosterveld MJ, Garrelfs MR, Hoppe B. et al. Eculizumab in pediatric dense deposit disease. Clin J Am Soc Nephrol 2015; 10: 1773–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Abrera-Abeleda MA, Nishimura C, Smith JL. et al. Variations in the complement regulatory genes factor H (CFH) and factor H related 5 (CFHR5) are associated with membranoproliferative glomerulonephritis type II (dense deposit disease). J Med Genet 2006; 43: 582–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Iatropoulos P, Noris M, Mele C. et al. Complement gene variants determine the risk of immunoglobulin-associated MPGN and C3 glomerulopathy and predict long-term renal outcome. Mol Immunol 2016; 71: 131–142 [DOI] [PubMed] [Google Scholar]
- 53. Bomback AS. Eculizumab in the treatment of membranoproliferative glomerulonephritis. Nephron Clin Pract 2014; 128: 270–276 [DOI] [PubMed] [Google Scholar]
- 54. Inman M, Prater G, Fatima H. et al. Eculizumab-induced reversal of dialysis-dependent kidney failure from C3 glomerulonephritis. Clin Kidney J 2015; 8: 445–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rodriguez-Osorio L, Ortiz A.. Timing of eculizumab therapy for C3 glomerulonephritis. Clin Kidney J 2015; 8: 449–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vivarelli M, Emma F.. Treatment of C3 glomerulopathy with complement blockers. Semin Thromb Hemost 2014; 40: 472–477 [DOI] [PubMed] [Google Scholar]
- 57. Nicolas C, Vuiblet V, Baudouin V. et al. C3 nephritic factor associated with C3 glomerulopathy in children. Pediatr Nephrol 2014; 29: 85–94 [DOI] [PubMed] [Google Scholar]
- 58. Haffner K, Michelfelder S, Pohl M.. Successful therapy of C3Nef-positive C3 glomerulopathy with plasma therapy and immunosuppression. Pediatr Nephrol 2015; 30: 1951–1959 [DOI] [PubMed] [Google Scholar]
- 59. Rousset-Rouviere C, Cailliez M, Garaix F. et al. Rituximab fails where eculizumab restores renal function in C3nef-related DDD. Pediatr Nephrol 2014; 29: 1107–1111 [DOI] [PubMed] [Google Scholar]
- 60. Valenzuela NM, McNamara JT, Reed EF.. Antibody-mediated graft injury: complement-dependent and complement-independent mechanisms. Curr Opin Organ Transplant 2014; 19: 33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]