Cytokine release syndrome grade 3 or higher was independently associated with increased risk of subsequent infection and in particular with bloodstream infection in patients with relapsed B-cell acute lymphoblastic leukemia treated with CD19 chimeric antigen receptor T-cell therapy.
Keywords: cytokine release syndrome, CAR T-cell therapy, relapsed acute lymphoblastic leukemia, early infections, late infections
Abstract
Background
Chimeric antigen receptor (CAR)–modified T cells that target the CD19 antigen present a novel promising therapy for the treatment of relapsed B-cell acute lymphoblastic leukemia (B-ALL). Although cytokine release syndrome (CRS) and neurotoxicity have emerged as predominant noninfectious complications of CD19 CAR T-cell therapy, infections associated with this treatment modality have not been well documented.
Methods
We analyzed infectious complications that followed CD19 CAR T-cell therapy in 53 adult patients with relapsed B-ALL enrolled in a phase I clinical trial at Memorial Sloan Kettering Cancer Center (NCT01044069).
Results
Overall, 22 patients (42%) experienced 26 infections (17 bacterial, 4 fungal, and 5 viral) within the first 30 days of CAR T-cell infusion. In 10 of 32 (31%) patients in whom complete remission was achieved, 15 infections developed between days 31 and 180; the majority of these late infections were due to respiratory viruses. In general, bacterial, fungal, and viral infections were detected at a median of 18, 23, and 48 days, respectively, after CAR T-cell infusion. CRS grade 3 or higher was independently associated with increased risk of subsequent infection (adjusted hazard ratio [HR], 2.67; P = .05) and in particular with bloodstream infection (adjusted HR, 19.97; P < .001). Three of 53 patients (6%) died of an infection-related cause.
Conclusions
Infections in adult patients with relapsed B-ALL are common after CD19 CAR T-cell therapy. Understanding the infectious complications that are temporally coincident with CD19 CAR T-cell therapy is critical for developing effective prophylactic and other supportive care measures to improve clinical outcomes.
Clinical Trials Registration
Because adults with relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL) have a poor prognosis, there has been keen interest in exploring other therapeutic modalities [1]. One such approach is to genetically modify autologous T cells to express a chimeric antigen receptor (CAR) that targets a specific tumor antigen [2]. CAR-modified T cells targeting the B cell-specific antigen CD19 have been studied in several clinical trials and demonstrated high rates of complete remission in patients with relapsed or refractory B-ALL [3–7]. Based on these encouraging results, a CAR T-cell product was recently approved by the US Food and Drug Administration for treatment of relapsed or refractory B-ALL in children and young adults.
A number of adverse effects are associated with CD19-targeted CAR T-cell therapy. The 2 most common treatment-related toxic effects include cytokine release syndrome (CRS) and neurologic toxic effects. CRS, a potentially life-threatening condition mediated by the elevation of proinflammatory cytokines, including interleukin 6 (IL-6), coincides with CAR T-cell expansion and usually occurs during the first 3–4 weeks of CAR T-cell infusion (CTI) [3, 4].
Although CRS can be successfully managed with IL-6–directed therapy (eg, tocilizumab) or corticosteroids, the clinical presentations of CRS and sepsis are often indistinguishable. Thus, concomitant infection and CRS pose both diagnostic and management challenges and can be fatal [8]. However, little is known about the frequency and types of infectious complications after CD19 CAR T-cell therapy and whether current antimicrobial prophylactic practices are sufficient. Furthermore, patients are at increased risk of prolonged B-cell aplasia and hypogammaglobulinemia after CD19-targeted therapy, but the late infectious complications after CD19 CAR T-cell therapy remain unknown. To this end, we examined infections occurring within the first 180 days in 53 adult patients with relapsed or refractory B-ALL treated with CD19-targeted 19-28z CAR T cells in a phase I clinical trial at Memorial Sloan Kettering Cancer Center (MSKCC).
METHODS
Patients and Clinical Protocol
We reviewed 53 consecutive adults with relapsed or refractory B-ALL who received 19-28z CAR T cells at MSKCC in a phase I clinical trial (NCT01044069) from May 2010 until August 2016 when the study was completed. This historical cohort study was reviewed and approved by the institutional review board at MSKCC. Details regarding the study design were described elsewhere [3]. All patients underwent disease assessment with bone marrow evaluations immediately before CTI. The study included 3 stages designed to evaluate the safety and efficacy of 2 doses of CAR T cells (1 × 106/kg and 3 × 106/kg) and the addition of fludarabine to the conditioning chemotherapy regimen. Antimicrobial prophylaxis commenced with chemotherapy and was continued until count recovery or need to switch to therapy per institutional guidelines.
Definition of Infection
An episode of infection was defined as the presence of clinical symptoms along with corroborating laboratory, radiographic, microbiologic, and histopathologic results (Supplementary Material) [9–16]. Proven or probable invasive fungal infections (IFIs) were defined according to published criteria [17]. IFIs in which symptom onset occurred before CTI were excluded. A timeline of documented infections occurring between the day of CTI (day 0) and day 180 was constructed for each patient. Data were censored on receipt of allogeneic hematopoietic stem cell transplantation (HSCT), last MSKCC follow-up date, or death.
Cytokine Analysis
Serial serum samples were obtained before and after administration of conditioning chemotherapy and after CTI. Cytokine profiles were analyzed using the Luminex FlexMAP 3D system and commercially available 38-plex cytokine detection assays as described elsewhere [3, 18, 19].
Data Collection
Data extracted from the electronic medical record included demographics, prior treatment history, hospital and intensive care unit length of stay, conditioning chemotherapy before CTI, antimicrobial prophylaxis, subtype and duration of leukopenia, antimicrobial treatment received, occurrence and grade of CRS and/or neurotoxicity, serum immunoglobulin G (IgG) and cytokine levels, disease response, allogeneic HSCT after CTI, and survival. Neutropenia was defined as an absolute neutrophil count <500/μl and lymphopenia as an absolute lymphocyte count <300/μl. CRS was defined using the MSKCC grading criteria (Table 1). CRS grade 0–2 was considered mild, whereas grade ≥3 was considered severe, as described elsewhere [5, 7, 20, 21]. Neurotoxicity was assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03 [22].
Table 1.
Memorial Sloan Kettering Cancer Center Grading Criteria for Cytokine Release Syndrome
| Grade | Definitions |
|---|---|
| 1 | Mild symptoms, requiring observation or symptomatic management only (eg, antipyretics, antiemetics, pain medications) |
| 2 | Moderate symptoms: hypotension requiring vasopressors for <24 h or hypoxia or dyspnea requiring supplemental oxygen <40% (up to 6 L by nasal cannula) |
| 3 | Severe symptoms: hypotension requiring vasopressors for ≥24 h or hypoxia or dyspnea requiring supplemental oxygen ≥40% |
| 4 | Life-threatening symptoms: hypotension refractory to vasopressors or hypoxia or dyspnea requiring mechanical ventilation |
| 5 | Death |
Statistical Considerations
Clinical predictors of infection were assessed using survival analysis. Clinical variables analyzed included age, sex, prior lines of chemotherapy, prior allogeneic HSCT, conditioning chemotherapy, morphologic disease status (≥5% blasts or extramedullary involvement), CAR T-cell dose, quantitative IgG nadir, and maximum CRS grade. Cox proportional hazard modeling was used to examine these potential predictors; variables were first analyzed individually by means of univariate analysis. CRS was analyzed as a time-dependent predictor. Predictors with a univariate P value ≤.25 were analyzed in a multivariate model. Kaplan-Meier plots were used to further estimate the association between CRS grade and infection. All statistical analyses were performed using R software, version 3.2 (R Development Core Team).
RESULTS
Patients and Protocol Treatment
Fifty-three patients with relapsed or refractory B-ALL received CD19 CAR T cells during the study period. Detailed patient and laboratory characteristics are listed in Table 2. The median age was 45 years (interquartile range [IQR], 30–74 years); 39 (74%) were men, and the majority (43; 81%) were white. The study patients were pretreated with a median of 3 prior lines of chemotherapy (IQR, 2–7) before CTI, and 19 patients (36%) underwent a prior allogeneic HSCT. Per protocol, all received conditioning chemotherapy, either cyclophosphamide alone (n = 42; 79%), cyclophosphamide plus fludarabine (n = 10; 19%), or cyclophosphamide plus clofarabine (n = 1; 2%).
Table 2.
Demographic, Laboratory, and Clinical Characteristics
| Chararacteristic | Patients, No. (%)a (n = 53) |
|---|---|
| Demographics | |
| Age, median (IQR), y | 45 (30–74) |
| Male sex | 39 (74) |
| Race | |
| Asian | 2 (4) |
| Black | 1 (2) |
| Native American | 1 (2) |
| White | 43 (81) |
| Refused/unknown | 6 (11) |
| Disease status | |
| Prior lines of therapy, median (IQR) | 3 (2–7) |
| Relapse after HSCT | 19 (36) |
| Hematologic parameters before CTI | |
| ALC <300 cells/µL | 30 (57) |
| Lymphopenia duration, median (IQR), d | 3 (1–42) |
| ANC <500 cells/µL | 18 (34) |
| Neutropenia duration, median (IQR), d | 12 (3–83) |
| Hematologic parameters on day of CTI | |
| ALC <300 cells/µL | 31 (58) |
| ANC <500 cells/µL | 23 (43) |
| Antimicrobial prophylaxis before CTI | |
| Antibacterial prophylaxis | 0 (0) |
| Antifungal prophylaxis | |
| Micafungin | 32 (60) |
| Fluconazole | 1 (2) |
| Posaconazole | 3 (6) |
| Voriconazole | 6 (11) |
| None | 11 (21) |
| Anti-Pneumocystis prophylaxis | |
| Atovaquone | 2 (4) |
| Dapsone | 3 (6) |
| Pentamidine | 9 (17) |
| Trimethoprim-sulfamethoxazole | 32 (60) |
| None | 7 (13) |
| Antiviral prophylaxis | |
| Acyclovir | 38 (72) |
| Famciclovir | 1 (2) |
| Valacyclovir | 11 (21) |
| Valganciclovir | 2 (4) |
| None | 1 (2) |
| Cytokine release syndrome | |
| Grade 0 | 8 (15) |
| Grade 1–2 | 31 (59) |
| Grade 3–5 | 14 (26) |
Abbreviations: ALC, absolute lymphocyte count; ANC, absolute neutrophil count; CTI, chimeric antigen receptor T-cell infusion; HSCT, hematopoietic stem cell transplantation; IQR, interquartile range.
aData represent No. (%) of patients unless otherwise identified as median (IQR).
Before conditioning chemotherapy, 30 (57%) had a documented absolute lymphocyte count <300/µL with a median lymphopenia duration of 3 days (IQR, 1–42 days); 18 (34%) had an absolute neutrophil count <500/µL with a median neutropenia duration of 12 days (IQR, 3–83 days). On the day of CTI, 31 (58%) and 23 (43%) of the patients exhibited lymphopenia or neutropenia, respectively. After CTI, all patients (100%) exhibited lymphopenia, and 52 (98%) had neutropenia. The total median durations of lymphopenia and neutropenia among the 53 patients were 11 (IQR, 11–124) and 12 (IQR, 10–117) days, respectively.
Among the 53 treated patients, complete remission was achieved in 44 (83%). CRS of any grade occurred in 45 patients (85%), and severe CRS, defined as grade 3 and above, occurred in 14 (26%), including 1 patient in whom grade 5 CRS developed and who died of multiorgan failure on day 5 of treatment. CRS was managed with tocilizumab (n = 6), tocilizumab plus corticosteroid (n = 13), corticosteroid alone (n = 4), or supportive care in the other patients (n = 22). Grade 2, 3, or 4 neurotoxicity were observed in 1, 19, and 3 patients, respectively. There were no cases of cerebral edema and no fatal neurologic events. Twenty patients were transferred to the intensive care unit for closer monitoring of symptoms related to CRS and/or neurotoxicity.
Infections ≤30 Days After CTI
Details of antimicrobial prophylaxis are listed in Table 2. None received fluoroquinolone prophylaxis, 52 patients (98%) received antiviral prophylaxis, and 46 (87%) received anti-Pneumocystis prophylaxis. Forty-two patients (79%) were given antifungal prophylaxis, with micafungin (100 mg/d intravenously) the most frequently prescribed agent.
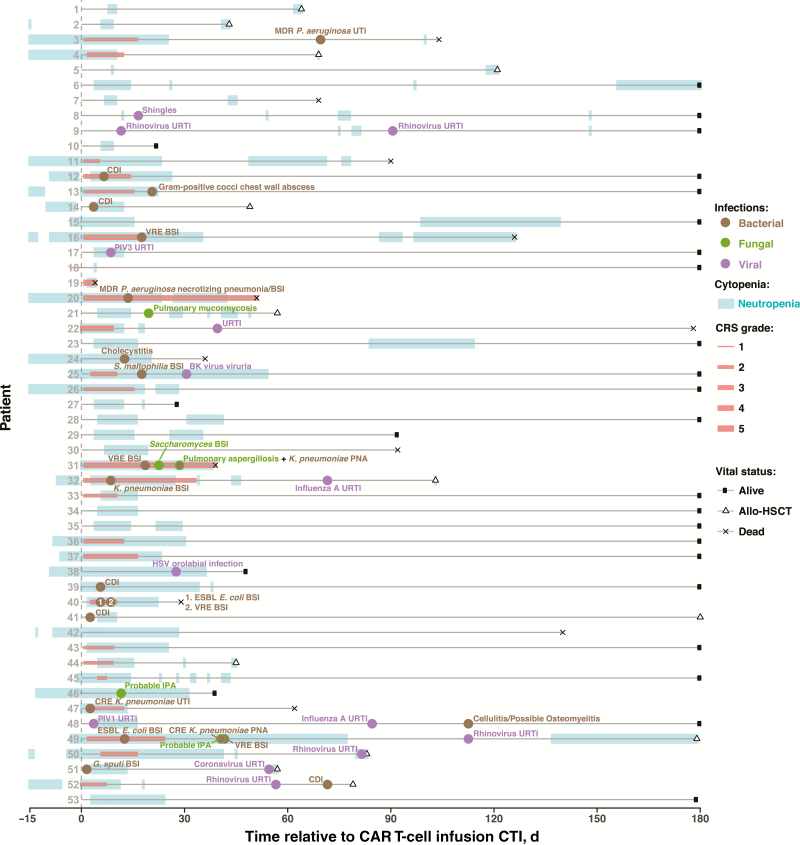
During the first 30 days after CTI, 26 infections developed in 22 patients (42%); all but 4 infections occurred while the patient was neutropenic (Table 3 and Figure 1). Bacterial infections predominated and included 8 bloodstream infections (BSIs), 1 intraabdominal infection, 4 cases of Clostridium difficile diarrhea, 2 pneumonias, 1 case of pyelonephritis, and 1 chest wall abscess. Four IFIs occurred in patients receiving micafungin prophylaxis. These included 1 case of Saccharomyces cerevisiae fungemia, 2 of probable invasive pulmonary aspergillosis, and 1 of proven pulmonary mucormycosis. There were 2 viral reactivations (1 herpes simplex and 1 varicella zoster virus); the case of shingles occurred in the absence of acyclovir prophylaxis. Three viral upper respiratory tract infections rounded out the remaining infections.
Table 3.
Comparison of Early Versus Late Infections After Chimeric antigen receptor T-cell infusion
| Earlya (Day 0–30) (n = 53) | Late (Day 31–180) (n = 32)b | |||
|---|---|---|---|---|
| Infections, No. | Patients, No. (%) | Infections, No. | Patients, No. (%) | |
| Any infection | 26 | 22 (42)c | 15 | 10 (31)d |
| Bacterial | ||||
| Bloodstreame | 8 | 7 (13) | 1 | 1 (3) |
| Bacterial sitef | 9 | 9 (17) | 4 | 4 (13) |
| Fungal | ||||
| Yeastg | 1 | 1 (2) | 0 | 0 (0) |
| Moldh | 3 | 3 (6) | 1 | 1 (3) |
| Viral | ||||
| Respiratory virusi | 3 | 3 (6) | 8 | 8 (25) |
| Other virusj | 2 | 2 (4) | 1 | 1 (3) |
aDay 0 was the day of Chimeric antigen receptor T-cell infusion (CTI).
bPatients with complete remission after CTI.
cTwo patients had >1 infection.
dThree patients had >1 infection.
eEarly bloodstream infections included 3 vancomycin-resistant Enterococcus faecium (VRE) and 2 extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli infections and 1 each of the following infections: Gordonia sputi, Klebsiella pneumoniae, and Stenotrophomonas maltophilia. The single late bloodstream infection was VRE.
fEarly bacterial infections included 4 cases of Clostridium difficile diarrhea, 2 cases of ventilator-associated pneumonia (1 K. pneumoniae, 1 multidrug-resistant Pseudomonas aeruginosa), and 1 case each of acute cholecystitis, pyelonephritis (carbapenemase-producing K. pneumoniae), and chest wall abscess. The late bacterial infections included 1 case each of C. difficile diarrhea, hospital-acquired pneumonia (carbapenemase-producing K. pneumoniae), cystitis (multidrug-resistant P. aeruginosa), and cellulitis.
gThe single yeast infection (early) was Saccharomyces cerevisiae fungemia.
hEarly mold infections included 2 cases of probable pulmonary aspergillosis (1 Aspergillus fumigatus and 1 patient with elevated serum and bronchoalveolar galactomannan levels but no recovered organism) and 1 case of proven pulmonary mucormycosis. The single late mold infection was probable pulmonary aspergillosis (patient had elevated serum galactomannan levels but no recovered organism).
iEarly respiratory virus infections included 2 parainfluenza virus and 1 rhinovirus/enterovirus infection. Late respiratory virus infections included 1 coronavirus, 2 influenza A, 4 rhinovirus/enterovirus, and 1 unknown infection.
jEarly infections with nonrespiratory viruses included 1 herpes simplex virus (orolabial) and 1 varicella zoster virus (shingles) infection. The single late infection was BK virus (hematuria).
Figure 1.
Overview of infections and clinical events in patients treated with chimeric antigen receptor (CAR) T cells. Timeline depicts the periods of neutropenia, cytokine release syndrome, and bacterial, fungal, and viral infections for 53 study patients after CAR T-cell infusion (CTI) until allogeneic transplantation, death, or last follow-up date through day 180. Abbreviations: allo-HSCT, allogeneic hematopoietic stem cell transplantation; BSI, bloodstream infection; CDI, Clostridium difficile infection; CRE, carbapenem-resistant Enterobacteriaceae; CTI, CAR T-cell infusion; E. coli, Escherichia coli; ESBL, extended-spectrum β-lactamase; G. sputi, Gordonia sputi; HSV, herpes simplex virus; IPA, invasive pulmonary aspergillosis; K. pneumoniae, Klebsiella pneumoniae; MDR, multidrug-resistant; P. aeruginosa, Pseudomonas aeruginosa; PIV, parainfluenza virus; PNA, pneumonia; S. maltophilia, Stenotrophomonas maltophilia; URTI, upper respiratory tract infection; UTI, urinary tract infection; VRE, vancomycin-resistant Enterococcus.
Infections >30 Days After CTI
Twenty-four of 32 surviving patients who attained complete remission had IgG levels checked between day 31 and receipt of HSCT, relapse, or last MSKCC follow-up, whichever date came first. Of the 24 patients, 20 (83%) had low IgG levels, with a median of 415 mg/dL (IQR, 338–635), and 1 patient received intravenous immunoglobulin. Ten of 32 patients (31%) had 15 infections between day 31 and day 180, which included 9 viral (8 respiratory viruses, 1 BK viruria), 5 bacterial, and 1 fungal infection (Table 3 and Figure 1). Of these 10 patients, 6 were hypogammaglobulinemic.
Infection-Related Deaths
Three patients died of infectious complications during the study period: 1 patient, who had a long-standing history of pneumonia and tracheostomy before CTI, died of necrotizing pneumonia and septicemia due to multidrug-resistant Pseudomonas aeruginosa 52 days after CTI; the other 2 patients who had undergone prior allogeneic HSCT died of polymicrobial sepsis at 30 and 41 days after CTI.
Clinical Factors Associated With Infections
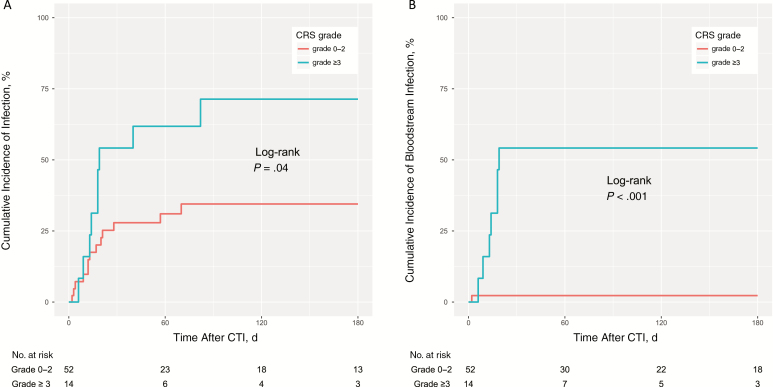
We then analyzed various pre- and posttreatment clinical factors to examine their association with increased infectious risk. Whereas age, sex, prior lines of chemotherapy, prior allogeneic HSCT, cyclophosphamide dose, conditioning regimen (cyclophosphamide alone vs cyclophosphamide plus fludarabine), disease burden, CAR T-cell dose, and hypogammaglobulinemia did not correlate with risk of infection in univariate analysis, CRS (grade ≥3) was associated with increased risk of subsequent infection, particularly BSI (Table 4). The association between grade ≥3 CRS and infection (adjusted hazard ratio [HR], 2.67; P = .05) or BSI (adjusted HR, 19.97; P < .001) remained significant in multivariate analysis. Cumulative incidence curves of time to infection and BSI within 180 days after CTI stratified by CRS grades are shown in Figure 2, demonstrating a significant association between high-grade CRS and the risk of infection.
Table 4.
Univariate and Multivariate Cox Models for Predictors of Infection and Bloodstream Infection
| Variable | Predictors of Infection | Predictors of Bloodstream Infection | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate HR (95% CI) | P Value | Multivariate HR (95% CI) | P Value | Univariate HR (95% CI) | P Value | Multivariate HR (95% CI) | P Value | |
| Age ≥50 y | 1.04 (.43–2.39) | .92 | … | … | 0.64 (.12–2.51) | .54 | … | … |
| Female sex | 1.29 (.51–3.00) | .57 | … | … | 2.76 (.71–10.69) | .14 | 2.63 (.67–10.37) | .16 |
| Prior chemotherapy (≥3 lines) | 0.86 (.38–2.03) | .72 | … | … | 1.66 (.42–9.04) | .48 | … | … |
| Prior allogeneic HSCT | 0.77 (.30–1.79) | .55 | … | … | 1.07 (.25–4.03) | .92 | … | … |
| Conditioning regimen | ||||||||
| Cy 1.5 g/m2 | 1.00 (—) | … | 1.00 (—) | … | 1.00 (—) | … | … | … |
| Cy 3.0 g/m2 | 0.47 (.18–1.36) | .16 | 0.35 (.12–1.05) | .06 | 1.12 (.21–11.16) | .9 | … | … |
| Cy/Flu or Cy/Clo | 1.41 (.49–4.21) | .52 | 0.83 (.23–3.02) | .77 | 2.58 (.42–26.63) | .31 | … | … |
| Morphologic disease (≥5% blasts or extramedullary disease) | 1.76 (.74–4.72) | .21 | 0.65 (.18–2.25) | .50 | 1.72 (.44–9.35) | .45 | … | … |
| CAR T-cell dose (3 × 106/kg vs 1 × 106/kg) | 0.44 (.19–1.01) | .05 | 0.47 (.16–1.35) | .16 | 0.72 (.19–2.78) | .62 | … | … |
| Hypogammaglobulinemia (IgG <600 mg/dL) | 1.10 (.32–5.66) | .89 | … | … | 2.21 (.24–292.76) | .56 | … | … |
| CRS grade ≥3a | 2.64 (1.11–6.03) | .03 | 2.67 (1.00–7.34) | .05 | 20.21 (4.40–192.13) | <.001 | 19.97 (4.32–190.31) | <.001 |
Abbreviations: CAR, chimeric antigen receptor; CI, confidence interval; Clo, clofarabine; CRS, cytokine release syndrome; Cy, cyclophosphamide; Flu, fludarabine; HR, hazard ratio; HSCT, hematopoietic stem cell transplantation; IgG, immunoglobulin G.
aAnalyzed as a time-dependent predictor. All the analysis time was CRS grade 3–4.
Figure 2.
Cumulative incidence of infection after chimeric antigen receptor T-cell infusion (CTI) and cytokine release syndrome (CRS). A, Cumulative incidence of any infection in patients by CRS grade. B, Cumulative incidence of bloodstream infection by CRS grade. Note that all the analysis time was CRS grade 3–4.
To examine whether serum cytokine profiles differ in patients with CRS and infection, we first identified serum cytokines correlated with grades of CRS: interferon γ, IL-6, interleukin 10 and 15, and tumor necrosis factor α (Supplementary Figure 1A). We then compared the serum cytokine panels in patients with CRS who had infections with those in patients who did not but found no difference in these serum levels (Supplementary Figure 1B). Considering the timing of infections by organism type, bacterial infections occurred a median of 18 days (IQR, 9–29) after CTI, followed by fungal infections (median 23 days; IQR, 20–29 days) and viral infections (median 48 days; IQR, 20–80 days).
DISCUSSION
Our study highlights the frequency, type, and severity of infectious complications in patients with relapsed or refractory B-ALL during the first 6 months after CD19 CAR T-cell therapy. Overall, 22 patients (42%) experienced ≥1 infection, predominantly bacterial, within the first 30 days of CTI; 10 (31%) of 32 patients with complete remission experienced ≥1 infection, many due to respiratory virus, between days 31 and 180. The infection-related mortality rate was low in these heavily treated patients, with deaths occurring in cases of either a multidrug-resistant organism (n = 1) or polymicrobial infection (n = 2).
In 1 recent publication, early (day 0–28) and late (day 29–90) infectious complications were seen in 30% (14 of 47) and 21% (9 of 43) of patients with ALL who underwent CAR T-cell therapy, respectively [23]. Although a direct comparison was limited by differences in methods and periods of observation, the types of infections occurring in the early and late periods after CTI were similar to those in our study, and life-threatening or fatal infections were also rare.
We found that the presence of grade ≥3 CRS was the only factor independently associated with any infection, and in particular BSI. It is unclear whether tocilizumab or corticosteroids used to treat high-grade CRS increases the risk of infection independent of CRS, but we were not able to find a significant association in univariate analysis (data not shown). Notably, a similar finding of an association between CRS and infection was recently reported in another trial of CD19-targeted CAR T cells, but with a different costimulatory domain (41BB vs CD28 in our study) and in patients with ALL, chronic lymphocytic leukemia, and non-Hodgkin lymphoma [23]. However, a potential mechanism underlying the association between high-grade CRS and infection is unclear. Although high-grade CRS is correlated with robust CAR T-cell expansion [3–7] and preclinical studies have demonstrated that tonic CAR signaling may induce early exhaustion of T cells [24], there is currently no evidence to suggest that these T cells lead to immune dysfunction.
Because sepsis due to infection can trigger a cytokine storm [25] and potentially lead to higher proinflammatory cytokines in patients with CRS due to CTI, we examined cytokine profiles in patients who experienced CRS with or without documented infection and found that although cytokine levels correspond to the severity of CRS, the pattern did not differ in patients with CRS with or without concomitant infection. This finding suggests that it is unlikely infection exacerbated CRS by altering cytokine levels, but further studies are required to investigate whether high levels of proinflammatory and anti-inflammatory cytokines and immunosuppressive therapies contribute to the increased risk of infections in patients with severe CRS. Nonetheless, infections can occur together during times of CRS, and clinicians should be aware of infectious complications, because signs and symptoms of CRS and sepsis are often difficult to distinguish, with differences in management (eg, use of IL-6-directed therapies and corticosteroids in CRS) [26–28].
To date, there are no standardized antimicrobial prophylactic practices in patients undergoing CTI for B-ALL. However, we have made several observations. First, our study found that there were no cases of Pneumocystis jiroveci pneumonitis and rare herpetic viral reactivations. Nearly all viral infections were due to respiratory viruses, occurring late after CTI, at a median of 48 days.
We also observed that bacterial infections occurred a median of 18 days after CTI, followed by fungal infections at a median of 23 days. The early occurrence of bacterial infections, followed by fungal infections, mirrors that in patients undergoing allogeneic HSCT or high-risk patients with acute leukemia or myelodysplastic syndrome receiving cytotoxic chemotherapy [29, 30]. Although no study patient received fluoroquinolone prophylaxis before CAR T-cell therapy, it is debatable whether fluoroquinolone prophylaxis would have had significant utility in this particular cohort. Once C. difficile infections were excluded, we found that 7 of the 13 remaining documented bacterial infections diagnosed within the first 30 days after CTI were due to resistant organisms. These included 3 VRE, 2 ESBL-producing Escherichia coli, 1 multidrug-resistant P. aeruginosa, and 1 carbapenemase-producing Klebsiella pneumoniae infection. All study patients had previously experienced ≥2 lines of chemotherapy for B-ALL, and 19 patients (36%) had also undergone prior allogeneic HSCT. We can thus infer that these patients had significant antimicrobial exposure before CAR T-cell therapy. Although prior antimicrobial exposure is a well-known risk factor for colonization by resistant organisms, it is difficult to know to what degree it contributed in this study cohort [31].
The decision regarding choice of antifungal prophylaxis depends on several factors, including local epidemiology, drug interactions, and adverse effect profile. At MSKCC, there were 4 breakthrough IFIs (9.5%) among 42 patients receiving antifungal prophylaxis. The majority of patients (32 of 42; 76%) received micafungin prophylaxis (100 mg intravenously daily). Compared with posaconazole suspension, micafungin was associated with a lower rate of premature discontinuation from gastrointestinal intolerance or adverse effects in patients with acute leukemia or myelodysplastic syndrome in a single-center, randomized, open-label study [32]. Although all 4 breakthrough IFIs occurred in patients receiving micafungin (4 of 32; 12.5%), this finding is comparable to the 8.6% rate of proven or probable IFI in the micafungin prophylactic arm [32]. It remains to be seen whether patients undergoing CAR T-cell therapy can be better stratified to identify those at higher risk for invasive mold infections and better target antifungal prophylaxis.
Because prolonged B-cell aplasia is a known complication after targeted CD19 therapy, we looked for infections consistent with a humoral immune deficiency (eg, recurrent sinopulmonary infections, diarrhea) and evaluated hypogammaglobulinemia in our survival model. However, in the patients for whom IgG levels were available, hypogammaglobulinemia was mild, obviating the need for routine immunoglobulin replacement. Although we did not see clear examples of humoral immune deficiency, the number of examined patients (n = 24) was small, and the rate, degree, and duration of B-cell aplasia and hypogammaglobulinemia may vary, depending on the patients’ age (children vs adults) and the duration of CAR T-cell persistence and therefore may require further prospective evaluations.
Although CAR T-cell therapy may impart some additional infectious risk not explained by the patient’s backdrop of prior cancer treatment, it was not possible to quantify the attributable risk, in part owing to the difficulty of selecting an appropriate comparison group for this highly customized patient cohort participating in a phase I clinical trial. Because this was a single-center study, the epidemiology of infections along with susceptibility patterns may differ from that in other centers and thus may affect how one’s institution will approach antimicrobial prophylaxis.
In summary, infections are common in adults with relapsed B-ALL after CD19 CAR T-cell therapy and can co-occur during times of CRS, although infection-related deaths were low. Based on our observations of the frequency and timing of infections, antimicrobial prophylaxis probably will parallel that for high-risk neutropenic patients although local bacterial and fungal epidemiology should be considered. As we gain experience with the different CAR constructs in various hematologic cancers, further studies should be conducted so we can better understand acute and delayed infectious complications in order to develop optimal prophylactic and management strategies and improve clinical outcomes in patients with various hematologic cancers receiving CD19 CAR T cells.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by William H. Goodwin and Alice Goodwin, the Commonwealth Foundation for Cancer Research and the Center for Experimental Therapeutics at Memorial Sloan Kettering Cancer Center, the Carson Family Charitable Trust, the Emerald Foundation, the Annual Terry Fox Run for Cancer Research organized by the Canada Club of New York, Kate’s Team, the William Lawrence and Blanche Hughes Foundation, the Lake Road Foundation, Juno Therapeutics, National Institutes of Health/National Cancer Institute Cancer Center Support Grant (P30 CA008748), the American Society of Clinical Oncology (J. H. P.), the Leukemia and Lymphoma Society (J. H. P.), and the National Comprehensive Cancer Network (J. H. P.).
Potential conflicts of interest. J. H. P. reports connections with Amgen (consulting/advisory role), Juno Therapeutics (research funding), Genentech/Roche (research funding), and Pfizer (consulting/advisory role). M. S. and R. J. B. report connections with Juno Therapeutics (consulting/advisory role, royalty sharing agreement, and stock ownership for both; research funding for R. J. B.). All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: IDWeek 2016, New Orleans, Louisiana, 25–30 October 2016.
References
- 1. Fielding AK, Richards SM, Chopra R, et al. ; Medical Research Council of the United Kingdom Adult ALL Working Party; Eastern Cooperative Oncology Group Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood 2007; 109:944–50. [DOI] [PubMed] [Google Scholar]
- 2. Davila ML, Bouhassira DC, Park JH, et al. Chimeric antigen receptors for the adoptive T cell therapy of hematologic malignancies. Int J Hematol 2014; 99:361–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014; 6:224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014; 371:1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015; 385:517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park JH, Geyer MB, Brentjens RJ. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood 2016; 127:3312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 2016; 126:2123–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frey NV, Levine BL, Lacey SF, et al. Refractory cytokine release syndrome in recipients of chimeric antigen receptor (CAR) T cells. Blood 2014; 24:2296. [Google Scholar]
- 9. Centers for Disease Control and Prevention. Bloodstream infection event (central line-associated bloodstream infection and non-central line-associated bloodstream infection Available at: https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf CfDCaP. Accessed 27 June 2017.
- 10. Mandell LA, Wunderink RG, Anzueto A, et al. ; Infectious Diseases Society of America; American Thoracic Society Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(suppl 2):S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohen SH, Gerding DN, Johnson S, et al. ; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–55. [DOI] [PubMed] [Google Scholar]
- 12. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis 2010; 50:133–64. [DOI] [PubMed] [Google Scholar]
- 13. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52:e103–20. [DOI] [PubMed] [Google Scholar]
- 14. Hirsch HH, Martino R, Ward KN, Boeckh M, Einsele H, Ljungman P. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis 2013; 56:258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stevens DL, Bisno AL, Chambers HF, et al. ; Infectious Diseases Society of America Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:e10–52. [DOI] [PubMed] [Google Scholar]
- 16. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63:e61–e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Pauw B, Walsh TJ, Donnelly JP, et al. ; European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013; 5:177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brentjens RJ, Rivière I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011; 118:4817–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kochenderfer JN, Somerville RPT, Lu T, et al. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J Clin Oncol 2017; 35:1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 2017; 129:3322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v4.03 Available at: http://www.hrc.govt.nz/sites/default/files/CTCAE%20manual%20-%20DMCC.pdf. Accessed 20 March 2018.
- 23. Hill JA, Li D, Hay KA, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood 2018; 131:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Long AH, Haso WM, Shern JF, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med 2015; 21:581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chaudhry H, Zhou J, Zhong Y, et al. Role of cytokines as a double-edged sword in sepsis. In Vivo 2013; 27:669–84. [PMC free article] [PubMed] [Google Scholar]
- 26. Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics 2016; 3:16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 2016; 127:3321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol 2018; 15:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tomblyn M, Chiller T, Einsele H, et al. ; Center for International Blood and Marrow Research; National Marrow Donor program; European Blood and Marrow Transplant Group; American Society of Blood and Marrow Transplantation; Canadian Blood and Marrow Transplant Group; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America; Association of Medical Microbiology and Infectious Disease Canada; Centers for Disease Control and Prevention Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 2009; 15:1143–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2011; 52:e56–93. [DOI] [PubMed] [Google Scholar]
- 31. MacDougall C, Polk RE. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev 2005; 18:638–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Epstein DJ, Seo SK, Huang Y-T, et al. Micafungin versus posaconazole prophylaxis in patients with acute leukemia or myelodysplastic syndrome: a randomized, open-label study. Open Forum Infect Dis 2017; 4:S538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.