Abstract
Background
The aim of this systematic review is to examine the epidemiological knowledge and gaps in understanding of the potential causes of chronic kidney disease of undetermined cause (CKDu) in Meso-America.
Methods
A systematic literature search of epidemiological studies of CKDu was conducted in PubMed, Embase and Web of Science from January 2000 to January 2017. Study quality was assessed by adapting the tool from Higgins et al. for observational studies. Where applicable, the summary prevalence odds ratio (POR) and 95% confidence interval (CI) were calculated using a random effects model.
Results
Twenty-five epidemiological studies were included in the analysis of risk factors for CKDu. The quality assessment of each occupational and community study was medium. The PORs for CKDu were males versus females 2.42 (95% CI 1.76–3.08), family history of CKD (versus none) 1.84 (95% CI 1.37–2.30), high water intake (versus low) 1.61 (95% CI 1.01–2.21) and low altitude (versus highland) 2.09 (95% CI 1.00–3.17). There were no significant associations between CKDu and pesticide exposure (versus no) 1.17 (95% CI 0.87–1.46), alcohol consumption (versus no) 1.34 (95% CI 0.84–1.84), non-steroidal anti-inflammatory drugs (versus no) 0.99 (95% CI 0.60–1.39) and heat stress (versus no) 1.52 (95% CI −0.91 – 3.95).
Conclusion
Our meta-analysis showed positive associations for males (versus females) and family history of CKD, water intake, lowland altitude and CKDu. There were no significant associations with pesticide exposure, non-steroidal anti-inflammatory drugs intake, heat stress and alcohol consumption.
Keywords: CKDu, Meso-America, Meso-American nephropathy, meta-analysis, Nicaragua, risk factors, systematic review
Introduction
Meso-American nephropathy (MeN), also known as chronic kidney disease of undetermined aetiology (CKDu), is a growing public health problem and young agricultural workers of the Pacific coast of Meso-America have been the most affected group [1–3]. There was an increased recognition of this problem by researchers, universities and policymakers after 2000 due to a marked increase in mortality and morbidity [4].
Three years later, the Program on Work and Health in Central America (SALTRA) organized the first regional workshop on chronic kidney disease (CKD); this reviewed available data, including several studies that showed increased risks for CKD among sugarcane workers and high mortality related to CKD in particular areas in Nicaragua and El Salvador [4]. In 2014, the Pan American Health Organization classified CKDu as a major public health problem in Central America that requires urgent, effective and concerted multisectoral action [5].
In the last 10 years, several narrative reviews about CKDu have been published [6–9]. None of these have conducted a formal meta-analysis and there was no systematic assessment of the quality of the available evidence considering inherent limitations within the design and analyses of available epidemiological studies. The purpose of this study is therefore to formally examine the epidemiological knowledge and gaps in understanding of the potential causes of CKD of undetermined cause in Meso-America.
Materials and methods
Research strategy
We searched on PubMed, MEDLINE, Embase and Web Science to identify all original research that had been published between January 2005 and January 2017 reporting prevalence and mortality of CKDu in Meso-America. Search terms included a combination of text words and headings for ‘Meso-American nephropathy’, ‘decreased kidney function’, ‘chronic kidney diseases of unknown cause’, ‘chronic kidney disease of non-traditional cause’, ‘agricultural’, ‘pesticide exposure’, ‘heat stress’, etc., were used. The full search strategy is outlined in the Supplementary Material (Supplementary data, Tables S1–S3).
Inclusion and exclusion criteria
The search was limited to ‘adolescent and adult human beings’ and only papers published in English and Spanish languages were considered. The search was restricted to studies conducted in Meso-America (Central America and Mexico). The exposures of interest included heat stress, dehydration, pesticide, non-steroidal anti-inflammatory drugs (NSAIDs), workplace conditions, environmental toxins and infectious diseases. The outcomes of interest included the reduced estimated glomerular filtration rate (eGFR), elevated serum creatinine (SCr) and CKD of undetermined cause. A wide range of study designs were assessed, including cross-sectional studies, case–control studies, retrospective or prospective cohort studies and ecological studies. We excluded animal studies, editorials, systematic reviews and case reports (Supplementary data, Table S4). However, systematic reviews were used to manually search for references.
Selection process
All titles and abstracts were examined by one reviewer (M.G.-Q.) according to the above inclusion criteria. Any disagreement of some marginal cases were discussed between M.G.-Q., B.C. and D.N. After review of the titles and abstract were independently reviewed by two authors (M.G.-Q. and D.N.). All full-text articles were assessed independently using the same criteria and included if both reviewers recommended inclusion. A second reviewer (D.N.) checked a sample of 45 titles and abstracts selected randomly after duplicated articles were removed. Agreement between authors was quantified by j-statistic calculation.
Data extraction and quality assessment
A standardized data extraction form was used by M.G.-Q. to extract study characteristics: authors, study design, year, country, sample size, altitude, exposure and outcome definitions, main findings, strengths and limitations and confounding factors. Any difficulty in data extraction was discussed by joint review of the original papers.
Quality was assessed for each selected study using standard quality assessment tools for trials [10] that we adapted for observational studies. Studies were assigned a high, low or uncertain risk according to the following criteria: selection bias, non-differential measurement error for exposure and outcome, information bias in exposure and outcome, confounding and reverse causation.
Data synthesis and analysis
We reviewed the exposure and outcome definitions and reported risk factors in each study. Where there were several studies with similar exposure definitions, data were included in a random effects meta-analysis for the respective exposure and CKDu and displayed in a forest plot. Funnel plot analysis and Egger’s test were performed to detect publication bias and P < 0.05 was considered significant.
The across-study heterogeneity was estimated by using Cochran’s Q-statistic and calculating the proportion of total variability explained by heterogeneity (I2) described by Higgins et al. [10]. All analyses were performed using Stata version 14 (StataCorp, College Station, TX, USA).
Results
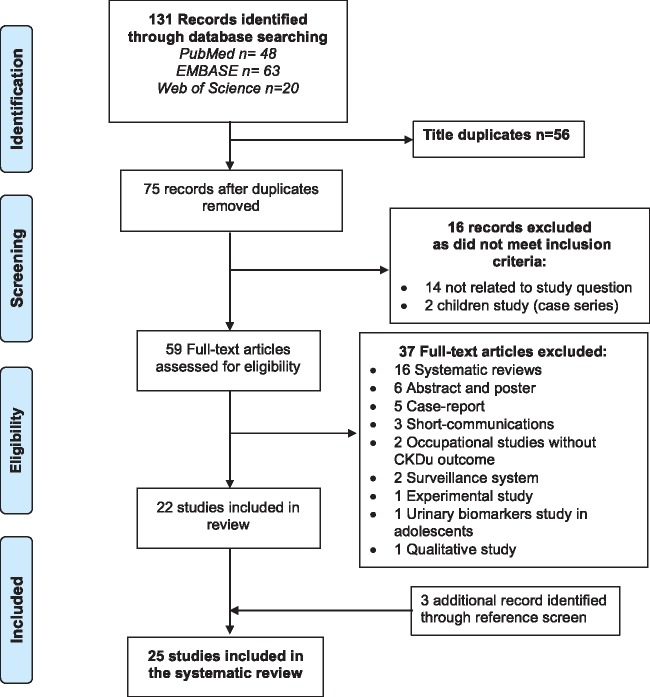
The two reviewers had excellent agreement (Cohen’ s κ = 1) on study inclusion after review of abstracts and titles. We identified 131 epidemiological studies on CKDu, of which 43% (56 papers) were duplicate studies using the same dataset. In addition, 53 studies did not meet the inclusion criteria and 3 were included by manual search, leaving 25 studies for the present systematic review (3 longitudinal occupational studies, 2 cross-sectional occupational studies, 14 community cross-sectional studies, 3 case–control studies and 3 ecological studies) (Fig. 1). The included studies were conducted from January 2000 to January 2017.
Fig. 1.
CKD of undetermined cause (eGFR <60 mL/min/1.73 m2), systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram.
Occupational studies mainly assessed how occupational risk factors in the workplace were associated with an eGFR cross-sectionally or a subsequent decline of eGFR across harvest or a single cross-shift in younger sugarcane workers (Table 1). For many risk factors there is only one estimate per risk factor in each study (Supplementary data, Table S5).
Table 1.
Characteristics of occupational studies included in the systematic review (n=5)
| Authors | Year | Design | Country | Region | Sample size/sex |
Age (years) | Exposures | Outcome definition | ||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Male | Female | ||||||||
| Wesseling et al. [11] | 2016 | Cohort | Nicaragua | León and Chinandega | 54 | 54 | – | 17–38 | Sugarcane cutters, anthropometrics measurements, fructose intake and urinary biomarkers | Decline in eGFR over time |
| Laws et al.a [12] | 2016 | Cohort | Nicaragua | Chinandega | 284 | 251 | 33 | 18–63 | Job category of sugarcane harvesters, years worked at sugar mill, water intake, isotonic solution intake, age and sex | Kidney urinary biomarkers and kidney function over harvest |
| Laws et al.a [13] | 2015 | Cohort | Nicaragua | Chinandega | 284 | 251 | 33 | 18–63 | Job category of sugarcane harvesters, years worked at sugar mill, water intake, isotonic solution intake, age and sex | Decline in eGFR over harvest |
| Wesseling et al. [14] | 2016 | Cross- sectional | Nicaragua | León and Chinandega | 194 | 194 | – | 17–39 | Differences between three occupations, socioeconomic status, hydration, lifestyle and health risk factors | eGFR <80 mL/min/1.73 m2 |
| Garcia-Trabanino et al. [15] | 2015 | Cross- sectional | El Salvador | Suchitoto, El Paisnal and San Luis Talpa | 189 | 168 | 21 | 18–49 | Sugarcane workers, workplace conditions, dehydration, heat stress, pesticide exposure and anthropometric measurements | Cross-shift changes in eGFR |
The baseline sample was 1104 sugarcane workers, but at the end of the harvest it was 284.
eGFR, estimated glomerular filtration rate.
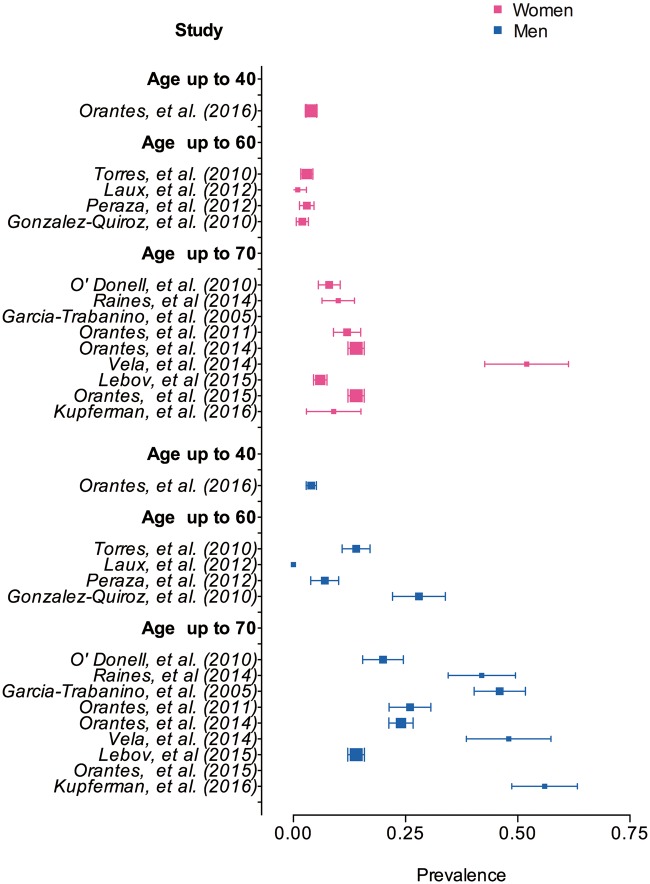
We identified only two longitudinal community studies. One involved follow-up of eGFR measurements in the subgroup that previously had abnormal SCr results, thus incident disease was not captured [16]. For the other study, only baseline data are available [17]. Most of the community-based studies were of cross-sectional design and recruited participants from different age groups and explored a variety of exposures. Two-thirds of these used a similar outcome definition for kidney function involving calculating an eGFR (<60 mL/min/1.73 m2) using the Modification of Diet in Renal Disease [18–25] or CKD Epidemiology Collaboration formula to quantify the prevalence of CKDu in the most affected regions [11–15, 26–28] (Table 2). Prevalence data among 14 cross-sectional community studies are confounded by age, as people with different ages were included, but overall more men than women were affected (Fig. 2). It was not possible to report an age-standardized CKDu prevalence because a breakdown of the findings by age was not available for most studies.
Table 2.
Characteristics of community-based studies included in the systematic review (n=20)
| Authors | Year | Design | Country | Region | Sample size/sex |
Age (years) | Exposures | Outcome definition | ||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Male | Female | ||||||||
| Nicaragua | ||||||||||
| González-Quiroz et al.a [17] | 2017 | Cohort | Nicaragua | León and Chinandega | 350 | 263 | 87 | 18–30 | Sociodemographic information, work history, lifestyle, work conditions, liquid intake and current diseases (hypertension or diabetes) | eGFR <90 mL/min/1.73 m2 |
| Minnings et al. [16] | 2016 | Cohort | Nicaragua | Rivas | 1242 | 537 | 705 | ≥18 | Demographic data, household membership, health symptoms, hydration practices, occupational and exposure history and personal medical history | SCr >1.5 mg/dL if male and >1.2 mg/dL if female or eGFR <60 mL/min/1.73 m2 |
| Kupferman et al. [27] | 2016 | Cross-sectional | Nicaragua | Chichigalpa | 226 | 178 | 88 | ≥18 | Clinical and demographic characteristics | SCr >1.5 mg/dL if male and >1.2 mg/dL if female or eGFR <60 mL/min/1.73 m2 |
| Lebov et al. [29] | 2015 | Cross-sectional | Nicaragua | León | 2275 | 1324 | 951 | 18–70 | Demographic indicators, source of drinking water, personal medical history, occupation and lifestyle | eGFR <60 mL/min/1.73 m2 |
| Laux et al.b [21] | 2012 | Cross-sectional | Nicaragua | Matagalpa | 267 | 120 | 147 | 20–60 | Demographic data, personal and family medical history, medications, occupation and lifestyle | eGFR <60 mL/min/1.73 m2 |
| Torres et al.c [18] | 2010 | Cross-sectional | Nicaragua | León and Chinandega | 1096 | 479 | 617 | 20–60 | Sociodemographic data, personal medical history (diabetes, hypertension, obesity and renal lithiasis), NSAIDs and occupation | SCr >1.2 mg/dL if male and >0.9 mg/dL if female or eGFR <60 mL/min/1.73 m2 |
| González-Quirozb [30] | 2010 | Cross-sectional | Nicaragua | Chichigalpa | 704 | 237 | 467 | 20–60 | Sociodemographic data, personal medical history (diabetes, hypertension, obesity and renal lithiasis), alcohol, NSAIDs, occupation and pesticide exposure | eGFR <60 mL/min/1.73 m2 |
| Raines et al.a [26] | 2014 | Case–control | Nicaragua | Chichigalpa | 424 | 166 | 258 | 15–69 | Demographic data, personal medical history, lifestyle, NSAIDs, cane chewing, inhaled pesticides, water intake, sugar beverage intake, occupation and personal protective equipment | eGFR <60 mL/min/1.73 m2 |
| O’Donnell et al.a [20] | 2010 | Case–control | Nicaragua | Quezalguaque | 771 | 298 | 473 | ≥18 | Age, sex, anthropometric measurements, education level, work history, exposure to pesticides, alcohol, cigarrete use and family and personal medical history | eGFR <60 mL/min/1.73 m2 |
| Sanoff et al.a [19] | 2010 | Case–control | Nicaragua | Chinandega | 997 | 848 | 149 | ≥18 | Demographic data, hypertension, diabetes, family history of kidney disease and occupational and non-occupational exposures | eGFR <60 mL/min/1.73 m2 |
| El Salvador | ||||||||||
| Orantes-Navarro et al.d [24] | 2016 | Cross-sectional | El Salvador | Bajo Lempa, Guayapa, Las Brisas | 2115 | 1058 | 1057 | <18 | Age, sex and region | eGFR <60 mL/min/1.73 m2, by a second measurement of SCr within 3 months’ difference |
| Orantes-Navarro et al.d [31] | 2015 | Cross-sectional | El Salvador | Bajo Lempa, Guayapa, Las Brisas | 1412 | – | 1412 | ≥18 | Age, sex, clinical history (hypertension and diabetes), family history (CKD, diabetes and hypertension), occupation, agrochemicals exposure and physical examination (weight, height and blood pressure) | eGFR <60 mL/min/1.73 m2, by a second measurement of SCr within 3 months’ difference |
| Vela et al.d [25] | 2014 | Cross-sectional | El Salvador | El Jicaro and Dimas Gutiérrez | 223 | 110 | 113 | ≥15 | Age, sex, physical measurements (weight, height, abdominal circumference and blood pressure), personal and family medical history, occupational and environmental exposures | eGFR <60 mL/min/1.73 m2, by a second measurement of SCr within 3 months’ difference |
| Orantes et al.a,d [23] | 2014 | Cross-sectional | El Salvador | Bajo Lempa, Guayapa abajo, Las Brisas | 2388 | 976 | 1412 | ≥18 | Age, sex, physical measurements (weight, height, waist circumference and blood pressure), personal and family medical history, occupational exposures and lifestyle | eGFR <60 mL/min/1.73 m2, by a second measurement of SCr within 3 months’ difference |
| Peraza et al.b [28] | 2012 | Cross-sectional | El Salvador | San Luis Talpa, Jiquilisco, Apastepeque, San Salvador and Ataco | 664 | 256 | 408 | 20–60 | Demographic data, occupational exposure, medical conditions and lifestyle | SCr >1.2 mg/dL if male and >0.9 mg/dL if female or eGFR <60 mL/min/1.73 m2 |
| Orantes et al.a,d [22] | 2011 | Cross-sectional | El Salvador | Bajo Lempa | 775 | 343 | 432 | ≥18 | Age, sex, physical measurements (weight, height, abdominal circumference and blood pressure), personal and family medical history, occupational, environmental exposures and lifestyle | eGFR <60 mL/min/1.73 m2, by a second measurement of SCr within 3 months’ difference |
| Garcia-Trabanino et al.a [32] | 2005 | Cross-sectional | El Salvador | Jaquilisco | 291 | 291 | – | 34–66 | Age, place of residence, occupation in agricultural work, history of pesticides exposure, alcohol consumption and basic medical history | SCr >1.5 mg/dL |
| Costa Rica, El Salvador and Guatemala | ||||||||||
| Wesseling et al. [33] | 2015 | Ecological | Costa Rica | Costa Rica | 6295 | 3843 | 2452 | ≥20 | Age, sex, region, altitude, climate and sugarcane production | CKD by death certificate |
| Laux et al. [34] | 2015 | Ecological | Guatemala | Guatemala | 3105 | 1591 | 1514 | Not reported | Sex, sugarcane cultivation, temperature, region, life expectancy, educational level and wealth | CKD by medical records from an RRT programme |
| VanDervort et al. [35] | 2014 | Ecological | El Salvador | El Salvador | 24 726 | – | – | No reported | Temperature, crops (sugarcane, sorghum, corn, beans, cotton and coffee) | CKD by medical records from National Surveillance Health System |
Altitude: at sea level.
More than 500 m above sea level.
At sea level and >500 m above sea level.
Five studies from El Salvador applied the CKD definition: persistence of renal damage markers for ≥3 months or GFR <60 mL/min/1.73 m2.
eGFR, estimated glomerular filtration rate; SCr, serum creatinine; CKD, chronic kidney disease; NSAIDs, non-steroidal antiinflammatory drugs; RRT, renal replacement therapy.
Fig. 2.
Forest plot of all prevalence of chronic kidney disease of undetermined cause (eGFR <60 mL/min/1.73 m2) by age group and sex from 14 cross-sectional community studies identified.
The quality of three longitudinal occupational studies was affected by severe loss of follow-up (up to 50%) during the study period, either due to changes in role or redundancy [11–13] (Table 3 and rationale in Supplementary data, Table S6). One cross-sectional occupational study [15] and cross-sectional community-based studies [23–25, 31, 32] had incomplete adjustment for confounding and reverse causation. One case–control study suffered from selection bias of participants, because researchers used improper procedures for selecting their cases and controls (volunteer participations at clinics) (Table 4). Many cross-sectional studies were limited to single measurements of creatinine, thus not fulfiling the chronicity criterion for CKD. Furthermore, the quality of three ecological studies was potentially affected by unmeasured confounding factors and within-regions variability in exposure and disease classification (outcome) (Table 4 and rationale in Supplementary data, Table S7).
Table 3.
Quality assessment of occupational studies (n=5)a
| Studies | Selection bias: participation | Selection bias: loss of follow-up | Non-differential misclassification exposure | Information bias of exposure | Non-differential misclassification of outcome | Information bias of outcome | Confounding | Reverse causation |
|---|---|---|---|---|---|---|---|---|
| Cohort studies | ||||||||
| Wesseling et al. [11] | ||||||||
| Laws et al. [12] | ||||||||
| Laws et al. [13] | ||||||||
| Cross-sectional studies | ||||||||
| Wesseling et al. [14] | N/A | |||||||
| Garcia-Trabanino et al. [15] | N/A |
Green bars: low risk of bias; yellow bars: medium risk of bias; red bars: high risk of bias.
N/A, not applicable.
Table 4.
Quality assessment of community-based studies (n=20)a
| Studies | Selection bias: participation | Selection bias: loss of follow-up | Non-differential misclassification exposure | Information bias of exposure | Non-differential misclassification of outcome | Information bias of outcome | Confounding | Reverse causation |
|---|---|---|---|---|---|---|---|---|
| Cohort studies | ||||||||
| González-Quiroz et al. [17] | N/A | |||||||
| Minnings et al. [16] | ||||||||
| Cross-sectional studies | ||||||||
| Orantes-Navarro et al. [24] | N/A | N/R | N/R | N/R | ||||
| Kupferman et al. [27] | N/A | |||||||
| Orantes-Navarro et al. [31] | N/A | |||||||
| Lebov et al. [29] | N/A | |||||||
| Vela et al. [25] | N/A | |||||||
| Orantes et al. [23] | N/A | |||||||
| Peraza et al. [28] | N/A | |||||||
| Laux et al. [21] | N/A | |||||||
| Orantes et al. [22] | N/A | |||||||
| Torres et al. [18] | N/A | |||||||
| Gonzalez-Quiroz [30] | N/A | |||||||
| Garcia-Trabanino et al. [32] | N/A | |||||||
| Case–control studies | ||||||||
| Raines et al. [26] | N/A | |||||||
| O’Donnell et al. [20] | N/A | |||||||
| Sanoff et al. [19] | N/A | |||||||
| Ecological studies | ||||||||
| Wesseling et al. [33] | N/A | |||||||
| Laux et al. [34] | N/A | |||||||
| VanDervort et al. [35] | N/A |
Green bars: low risk of bias; yellow bars: medium risk of bias; red bars: high risk of bias.
N/A, not applicable; N/R, not reported.
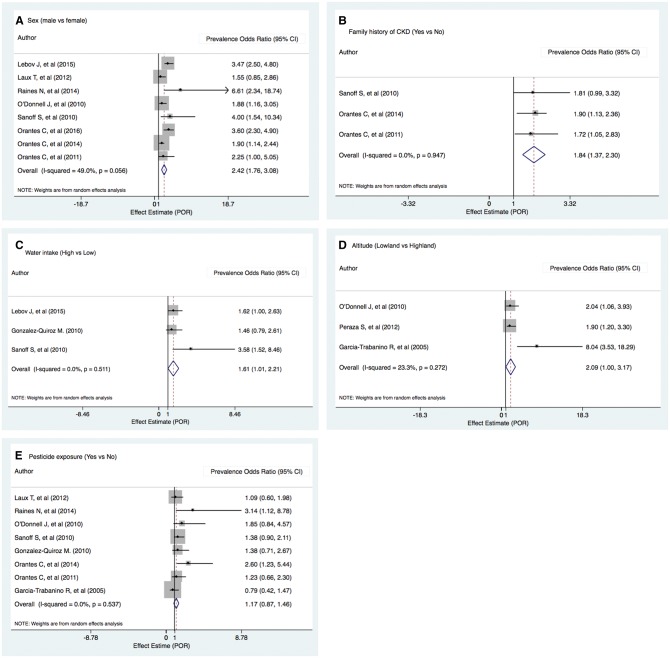
Sufficient data for meta-analysis were available for a subset of risk factors: male sex, family history of CKD, water intake, pesticide exposure, alcohol consumption, self-medication with NSAIDs, heat stress and altitude. These were from 10 cross-sectional community studies and 3 case–control community studies. In the meta-analysis, eight cross-sectional community studies showed positive associations between male and eGFR <60 mL/min/1.73 m2 {prevalence odds ratio [POR] 2.42 [95% confidence interval (CI) 1.76–3.08]; Cochran’s Q-statistic P = 0.056, I2 = 49.0%}. Three studies showed strong associations between family history of kidney disease and eGFR <60 mL/min/1.73 m2 [POR 1.84 (95% CI 1.37–2.30); Cochran’s Q-statistic P = 0.947, I2 = 0.0%]. For high water intake and eGFR <60 mL/min/1.73 m2 the POR was 1.61 (95% CI: 1.01–2.21; Cochran’s Q-statistic P = 0.511, I2 = 0.0%) and for lowland altitude (versus highland) it was 2.09 (95% CI 1.00–3.17; Cochran’s Q-statistic P = 0.272, I2 = 23.3%). The pooled POR for pesticide exposure was 1.17 (95% CI 0.87–1.46; Cochran’s Q-statistic P = 0.537, I2 = 0.0%) (Fig. 3). The summary estimate for alcohol consumption (yes versus no) was 1.34 (95% CI 0.84–1.84; Cochran’s Q-statistic P = 0.088, I2 = 45.5%), for NSAIDs intake (yes versus no) and eGFR <60 mL/min it was close to 1 [POR 0.99 (95% CI 0.60–1.39); Cochran’s Q-statistic P = 0.399, I2 = 0.0%] and for heat stress exposure (yes versus no) it was 1.52 (95% CI −0.91 to 3.95; Cochran’s Q-statistic P = 0.065, I2 = 70.6%) (Supplementary data, Fig. S1). A forest plot of occupation was not included because each study used different reference categories.
Fig. 3.
Forest plots of association with (A) sex, (B) family history of CKD, (C) water intake, (D) lowland altitude and (E) pesticide exposure estimates associated with CKD of undetermined caused (eGFR <60 mL/min/1.73 m2). Black diamond data markers express PORs; horizontal lines are the 95% CIs; grey square marker size indicating the statistical weight of the study using the random effects meta-analysis. A diamond data marker denotes the overall POR and 95% for the outcome of interest.
We tested for publication bias for sex, pesticide exposure and alcohol consumption risk factors. The funnel plot for studies that have assessed the above risk factors provides evidence for potential publication bias for pesticide exposure and alcohol consumption (P < 0.014 and P < 0.048, respectively) (Supplementary data, Fig. 2).
Discussion
We found 25 epidemiological studies that estimated the prevalence and assessed risk factors for CKDu in Meso-America. Our meta-analysis found a clear positive association between male sex, family history of CKD, high water intake, lowland altitude and reduced eGFR <60 mL/min/1.73 m2. There was no evidence for associations with pesticide exposure, NSAIDs intake, alcohol consumption and heat stress. The quality of cross-sectional studies was medium due to the potential for reverse causality, incomplete adjustment for confounding factors and the use of a single SCr measurement. Longitudinal occupational studies were affected by severe loss of follow-up.
A major issue impacting the quality of all the studies examined is that CKDu prevalence was estimated using a single SCr measurement rather than two measurements at least 3 months apart [36]. In affluent countries, a single measurement is frequently used to estimate the prevalence of CKD, as the intra-individual variability of creatinine under stable conditions is only a few percent. However, in a hot setting there is considerable seasonal variation and variation depending on work patterns and dehydration status; therefore, depending on when people are measured, they may have short-term fluctuations of creatinine that are far more pronounced than in cooler settings. Also, creatinine elevation can occur due to variations in factors such as exercise, muscle mass and diet. These factors may not only affect variability within individuals, but may also bias comparisons across populations studies, since each study may over- or underestimate kidney function depending on the season and setting of fieldwork or biological variation in the production of creatinine. In addition, creatinine levels may also be affected by ‘fixed’ factors such as ethnicity, which may also bias comparisons between populations.
The longitudinal occupational studies were affected by a loss of follow-up of up to 50% of their participants. This severely compromises study validity because those with CKDu are more likely to not be followed up [11–13]. Occupational studies are used to increase the power of a study when it is thought that a particular occupational exposure causes a problem. However, in the context of CKDu, it is not yet entirely clear whether occupation is the only risk factor or whether there are other risk factors that predispose young men to CKDu when they start working in sugarcane. Overall, considering the differential loss to follow-up of occupational studies, community cohorts have many advantages compared with occupational studies since they represent the entire risk population (workers from all occupations and both genders) and an assessment of environmental exposures at home [17].
Ecological studies may be affected by variability within regions in exposure and disease classification and by unmeasured confounding factors [33–35]. CKD mortality rate may vary across regions because of misclassification either of the cause reported by death certificate or by better case detection. Moreover, environmental temperature may be different within regions or areas due to variability in seasons and altitude. Finally, the lack of control for confounders have been an Achilles heel for ecological studies, even on the assumption that all variables have been accurately measured for all groups at a national level, basically due to the analysis strategy, which cannot completely remove bias due to the confounder.
Epidemiological studies have underlined many potential risk factors for CKDu, including male sex, occupation, high ambient temperature, self-medication with NSAIDs, altitude, exposure to heavy metals or pesticides and genetic susceptibility [11, 13–15, 18, 28]. While our systematic review could confirm the association with male sex, none of the other suggested risk factors were sufficiently well studied to conclusively prove or disprove their role. The most commonly cited working hypothesis for this disease has been heat stress causing repetitive episodes of dehydration in agricultural and non-agricultural workers due to working under heat stress and high humidity [15, 37], which may result in acute kidney injury (AKI) secondary to hypoperfusion or rhabdomyolysis [38]. However, although this hypothesis has been explored in an experimental study that suggested that dehydration and hyperosmolarity may induce tubular injury via activation of the polyolfructokinase pathway in the kidney [39], there have been no corresponding data in humans to support this hypothesis.
Our meta-analysis has identified positive associations of high water intake and CKDu in two cross-sectional community studies [29, 30] and one case–control study [19]. The study authors’ interpretation of these findings was that high water intake could be a proxy for exposure to heat stress and volume depletion during the workday secondary to high exertion and sweating [11, 14, 15, 40]. Some authors hypothesized that high water intake means that study participants drank more water trying to compensate for fluid deprivation, but that this is not enough to recover their hydration status [11, 14, 15]. Other authors have suggested that these associations are driven by intake from contaminated water sources (with pesticides or heavy metals) in the affected areas [41, 42]. An alternative interpretation could be reverse causation due to underlying kidney damage, in that those with kidney damage are unable to concentrate their urine and therefore need to drink more to not feel thirsty. To address the issue, it will be important to conduct more longitudinal studies to gain better insight into this association.
Pesticides are used extensively in Meso-America. Farmers in the cooler highland regions use pesticides similarly to farmers in coastal regions, yet CKDu prevalence is much lower at higher altitudes [15, 20–23, 26, 30]. Most of the studies that suggest a possible association between AKI and exposure to organochlorides, paraquat, 2,4-diclorophenoxyacetic and glyphosate have been conducted in animals [43, 44]. A single prospective cohort study among male licensed pesticide applicators in the USA reported an association between end-stage renal disease and exposure–response and increasing accumulated lifetime days in pesticide exposure and non-exposure for some herbicides such as alachlor, paraquat, pendimethalin, atrazine, permethrin and metolochlor [45]. The principal limitation of existing epidemiological studies is that exposure has been assessed using categorical questions (yes and no) and not by quantifying the pesticide residues in urine or blood [14, 19, 20, 26, 32]. Our findings suggest selective reporting of studies supporting an association with CKDu. Overall, the evidence about pesticide exposure and CKDu is still inconclusive.
Genetic predisposition may play a role in the CKDu epidemic, as some studies, and our meta-analysis, have suggested a positive association between family history of CKD and CKDu. Although CKD in general shows a high heritability of disease, suggesting familial clustering of risk factors, these have not been explained by genetic association studies [46, 47]. A positive association with family history of CKDu may simply be due to children who lost parents to CKDu or living in rural areas starting to work earlier in sugarcane or agriculture to support their household income.
Our systematic review has strengths and limitations. To our knowledge, this is the first systematic review that included a meta-analysis and evaluated the study quality of each epidemiological study by using a pre-specified tool adapted from Higgins et al. [10] for observational studies. Second, we included a broad definition of CKD of unknown aetiology and a variety of exposures. The main limitations of the review are that the available evidence on CKDu is overall patchy and inconclusive.
In summary, apart from male sex, positive family history, high water intake and lowland altitude, existing studies have been inconclusive with regards to potential risk factors for CKDu, such as pesticide use, NSAIDs, heavy metals, alcohol consumption, heat stress and dehydration. Longitudinal community-based studies are needed to address problems of reverse causality (as per existing cross-sectional studies) as well as differential loss to follow-up (as per existing occupational studies).
Authors' contributions
This study was conceived and designed by M.G.-Q., N.P., B.C. and D.N. Data collection was performed by M.G.-Q. and B.C. The analysis and interpretation of the results were done by M.G.-Q., B.C. and D.N. The draft was written by M.G.-Q. and D.N. All authors read and approved the final manuscript.
Supplementary data
Supplementary data are available at ckj online.
Funding
The study has been supported by a grant (CF/03/14) from the UK Colt Foundation.
Conflict of interest statement
None declared.
Supplementary Material
References
- 1. Weiner DE, McClean MD, Kaufman JS. et al. The Central American epidemic of CKD. Clin J Am Soc Nephrol 2013; 8: 504–511 [DOI] [PubMed] [Google Scholar]
- 2. Correa-Rotter R, Wesseling C, Johnson RJ.. CKD of unknown origin in Central America: the case for a Mesoamerican nephropathy. Am J Kidney Dis 2014; 63: 506–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wegman D, Crowe J, Hogstedt C. et al. (eds). Mesoamerican nephropathy: report from the second international research workshop on MeN. ISBN 978-9968-924-33-7 Heredia, Costa Rica: SALTRA/IRET-UNA, 2016 [Google Scholar]
- 4. Cuadra SN, Kristina J, Christer H. et al. Chronic Kidney Disease: Assessment of Current Knowledge and Feasibility for Regional Research Collaboration in Central America. Heredia, Costa Rica: SALTRA, 2006 [Google Scholar]
- 5. Pan American Health Organization–World Health Organization. Resolution CD52.R1: Chronic Kidney Disease in Agricultural Communities in Central America Washigton, DC: PAHO, 2014. http://www.paho.org/hq/index.php? option=com_content&view=article&id=8833&Itemid=40033&lang=en (last accessed 10 June 2017)
- 6. Lunyera J, Mohottige D, Isenburg MV. et al. CKD of uncertain etiology: a systematic review. Clin J Am Soc Nephrol 2016; 11: 379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gifford FJ, Gifford RM, Eddleston M. et al. Endemic nephropathy around the world. Kidney Int Rep 2017; 2: 282–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Madero M, García-Arroyo FE, Sánchez-Lozada LG.. Pathophysiologic insight into MesoAmerican nephropathy. Curr Opin Nephrol Hypertens 2017; 26: 296–302 [DOI] [PubMed] [Google Scholar]
- 9. Elinder C-G, Wijkström A, Wijkstrom J.. Mesoamerican nephropathy (MeN): a ‘new’ chronic kidney disease related to occupational heat exposure with repeated deprivation of salts and water. Int J Nephrol Kidney Failure 2015; 1: 1–9 [Google Scholar]
- 10. Higgins JP, Altman DG, Gøtzsche PC. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wesseling C, Aragón A, González M. et al. Kidney function in sugarcane cutters in Nicaragua—a longitudinal study of workers at risk of Mesoamerican nephropathy. Environ Res 2016; 147: 125–132 [DOI] [PubMed] [Google Scholar]
- 12. Laws RL, Brooks DR, Amador JJ. et al. Biomarkers of kidney injury among nicaraguan sugarcane workers. Am J Kidney Dis 2016; 67: 209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laws RL, Brooks DR, Amador JJ. et al. Changes in kidney function among Nicaraguan sugarcane workers. Int J Occup Environ Health 2015; 21: 241–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wesseling C, Aragón A, González M. et al. Heat stress, hydration and uric acid: a cross-sectional study in workers of three occupations in a hotspot of Mesoamerican nephropathy in Nicaragua. BMJ Open 2016; 6: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. García-Trabanino R, Jarquín E, Wesseling C. et al. Heat stress, dehydration, and kidney function in sugarcane cutters in El Salvador—a cross-shift study of workers at risk of Mesoamerican nephropathy. Environ Res 2015; 142: 746–755 [DOI] [PubMed] [Google Scholar]
- 16. Minnings K, Fiore M, Mosco M. et al. The Rivas Cohort Study: design and baseline characteristics of a Nicaraguan cohort. BMC Nephrol 2016; 17: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. González-Quiroz M, Camacho A, Faber D. et al. Rationale, description and baseline findings of a community-based prospective cohort study of kidney function amongst the young rural population of Northwest Nicaragua. BMC Nephrol 2017; 18: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Torres C, Aragón A, González M. et al. Decreased kidney function of unknown cause in Nicaragua: a community-based survey. Am J Kidney Dis 2010; 55: 485–496 [DOI] [PubMed] [Google Scholar]
- 19. Sanoff SL, Callejas L, Alonso CD. et al. Positive association of renal insufficiency with agriculture employment and unregulated alcohol consumption in Nicaragua. Ren Fail 2010; 32: 766–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O’Donnell JK, Tobey M, Weiner DE. et al. Prevalence of and risk factors for chronic kidney disease in rural Nicaragua. Nephrol Dial Transplant 2011; 26: 2798–2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laux TS, Bert PJ, Barreto Ruiz GM. et al. Nicaragua revisited: evidence of lower prevalence of chronic kidney disease in a high-altitude, coffee-growing village. J Nephrol 2012; 25: 533–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Orantes CM, Herrera R, Almaguer M. et al. Chronic kidney disease and associated risk factors in the Bajo Lempa region of El Salvador: Nefrolempa study, 2009. MEDICC Rev 2011; 13: 14–22 [DOI] [PubMed] [Google Scholar]
- 23. Orantes CM, Herrera R, Almaguer M. et al. Epidemiology of chronic kidney disease in adults of Salvadoran agricultural communities. MEDICC Rev 2014; 16: 23–30 [DOI] [PubMed] [Google Scholar]
- 24. Orantes-Navarro CM, Herrera-Valdes R, Almaguer-Lopez M. et al. Chronic kidney disease in children and adolescents in Salvadoran farming communities: NefroSalva Pediatric Study (2009–2011). MEDICC Rev 2016; 18; 15. [DOI] [PubMed] [Google Scholar]
- 25. Vela XF, Henriquez DO, Zelaya SM. et al. Chronic kidney disease and associated risk factors in two Salvadoran farming communities, 2012. MEDICC Rev 2014; 16: 55–60 [DOI] [PubMed] [Google Scholar]
- 26. Raines N, Gonzalez M, Wyatt C. et al. Risk factors for reduced glomerular filtration rate in a Nicaraguan community affected by Mesoamerican nephropathy. MEDICC Rev 2014; 16: 16–22 [DOI] [PubMed] [Google Scholar]
- 27. Kupferman J, Amador JJ, Lynch KE. et al. Characterization of Mesoamerican nephropathy in a kidney failure hotspot in Nicaragua. Am J Kidney Dis 2016; 68: 716–725 [DOI] [PubMed] [Google Scholar]
- 28. Peraza S, Wesseling C, Aragon A. et al. Decreased kidney function among agricultural workers in El Salvador. Am J Kidney Dis 2012; 59: 531–540 [DOI] [PubMed] [Google Scholar]
- 29. Lebov JF, Valladares E, Peña R. et al. A population-based study of prevalence and risk factors of chronic kidney disease in Leon, Nicaragua. Can J Kidney Health Dis 2015; 2: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gonzalez-Quiroz M. Enfermedad Renal Crónica: prevalencia y factores de riesgo ocupacionales en el municipio de Chichigalpa [Tesis de postgrado]. Universidad Nacional Autónoma de Nicaragua, León, 2010
- 31. Orantes Navarro CM, Herrera Valdés R, López MA. et al. Epidemiological characteristics of chronic kidney disease of non-traditional causes in women of agricultural communities of El Salvador. Clin Nephrol 2015; 83(Suppl 1): 24–31 [DOI] [PubMed] [Google Scholar]
- 32. Gracia-Trabanino R, Dominguez J, Jansa JM. et al. [Proteinuria and chronic renal failure in the coast of El Salvador: detection with low cost methods and associated factors]. Nefrologia 2005; 25: 31–38 [PubMed] [Google Scholar]
- 33. Wesseling C, van Wendel de Joode B, Crowe J. et al. Mesoamerican nephropathy: geographical distribution and time trends of chronic kidney disease mortality between 1970 and 2012 in Costa Rica. Occup Environ Med 2015; 72: 714–721 [DOI] [PubMed] [Google Scholar]
- 34. Laux TS, Barnoya J, Guerrero DR. et al. Dialysis enrollment patterns in Guatemala: evidence of the chronic kidney disease of non-traditional causes epidemic in Mesoamerica. BMC Nephrol 2015; 16: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. VanDervort DR, Lopez DL, Orantes CM. et al. Spatial distribution of unspecified chronic kidney disease in El Salvador by crop area cultivated and ambient temperature. MEDICC Rev 2014; 16: 31–38 [DOI] [PubMed] [Google Scholar]
- 36. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [DOI] [PubMed] [Google Scholar]
- 37. Crowe J, Wesseling C, Solano BR. et al. Heat exposure in sugarcane harvesters in Costa Rica. Am J Ind Med 2013; 56: 1157–1164 [DOI] [PubMed] [Google Scholar]
- 38. Paula Santos U, Zanetta DM, Terra-Filho M. et al. Burnt sugarcane harvesting is associated with acute renal dysfunction. Kidney Int 2015; 87: 792–799 [DOI] [PubMed] [Google Scholar]
- 39. Roncal Jimenez CA, Ishimoto T, Lanaspa MA. et al. Fructokinase activity mediates dehydration-induced renal injury. Kidney Int 2014; 86: 294–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lucas RA, Bodin T, García-Trabanino R. et al. Heat stress and workload associated with sugarcane cutting—an excessively strenuous occupation! Extrem Physiol Med 2015; 4(Suppl 1): A23 [Google Scholar]
- 41. Jayasumana C, Gunatilake S, Senanayake P.. Glyphosate, hard water and nephrotoxic metals: are they the culprits behind the epidemic of chronic kidney disease of unknown etiology in Sri Lanka? Int J Environ Res Public Health 2014; 11: 2125–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jayasumana C, Paranagama P, Agampodi S. et al. Drinking well water and occupational exposure to Herbicides is associated with chronic kidney disease, in Padavi-Sripura, Sri Lanka. Environ Health 2015; 14: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Uyanikgil Y, Ateş U, Baka M. et al. Immunohistochemical and histopathological evaluation of 2,4-dichlorophenoxyacetic acid-induced changes in rat kidney cortex. Bull Environ Contam Toxicol 2009; 82: 749–755 [DOI] [PubMed] [Google Scholar]
- 44. Poovala VS, Huang H, Salahudeen AK.. Role of reactive oxygen metabolites in organophosphate–bidrin-induced renal tubular cytotoxicity. J Am Soc Nephrol 1999; 10: 1746–1752 [DOI] [PubMed] [Google Scholar]
- 45. Lebov JF, Engel LS, Richardson D. et al. Pesticide use and risk of end-stage renal disease among licensed pesticide applicators in the Agricultural Health Study. Occup Environ Med 2016; 73: 3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wuttke M, Kottgen A.. Insights into kidney diseases from genome-wide association studies. Nat Rev Nephrol 2016; 12: 549–562 [DOI] [PubMed] [Google Scholar]
- 47. Gorski M, van der Most PJ, Teumer A. et al. 1000 Genomes-based meta-analysis identifies 10 novel loci for kidney function. Sci Rep 2017; 7: 45040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.