Abstract
Background
Several peritoneal dialysis catheter (PDC) placement techniques have been described. The objective of this study was to compare the fluoroscopy and ultrasound guidance technique with the laparoscopic technique.
Methods
We retrospectively reviewed the medical records of 260 patients who had their first PDC placed between January 2005 and June 2016. We compared the outcomes of the fluoroscopic and ultrasound-guided catheter placement technique (radiologic group, n = 50) with the laparoscopic catheter placement technique (laparoscopic group, n = 190). The primary endpoint was complication-free catheter survival at 365 days. Secondary endpoints were complication-free catheter survival at 90 days, overall catheter survival at 90 and 365 days, median days to first complication and median days to catheter removal.
Results
In the radiologic group, the complication-free catheter survival at 90 and 365 days was 64% and 48%, respectively, while in the laparoscopic group it was 71% (P = 0.374) and 53% (P = 0.494), respectively. Catheter malfunction was significantly higher in the laparoscopic group (30%) compared with the radiologic group (16%, P = 0.048). The overall catheter survival at 90 and 365 days was 76% and 52%, respectively, in the radiologic group, while in the laparoscopic group it was 88% (P = 0.0514) an 48% (P = 0.652), respectively. There was no significant difference in the median days to first complication and the median days to catheter removal between the two groups (P = 0.71).
Conclusion
The technique of fluoroscopic and ultrasound-guided PDC placement is a clinically effective and safe alternative to laparoscopic catheter placement with similar survival and complication rates.
Keywords: end-stage renal disease, fluoroscopic, hemodialysis, laparoscopic, peritoneal dialysis catheter, ultrasound
Introduction
Patients suffering from chronic kidney disease (CKD) and end-stage renal disease (ESRD) who are undergoing renal replacement therapy may elect to use peritoneal dialysis (PD) or hemodialysis (HD) or pursue preemptive renal transplantation. The overall cost for patients receiving PD has been shown to be an average of $20 000 per year lower than for patients receiving in-center HD. In addition to the favorable economic landscape for PD, the patient-centric factors that may make PD a favorable dialysis option are the ability to perform dialysis at home, largely during the nighttime to allow for more flexibility during the daytime, less interference with employment schedule, ability to travel and fewer dietary restrictions compared with in-center HD [1]. Recent comparisons of early and late survival between PD and HD suggest an early survival advantage to starting dialysis with PD and a similar longer-term survival at 5 years [2, 3].
These factors have led some clinicians to call for a ‘PD first’ position and to consider PD not just for the elective start to dialysis but for more urgent initiation of dialysis in patients presenting late in the course of their disease [1, 4, 5]. The recent interest in ‘urgent-start PD’ raised awareness of the need for more expeditious PD catheter (PDC) placement within 24–48 h to avoid unnecessary use of temporary vascular access catheters for HD [6]. This requirement faces obstacles in some institutions that use laparoscopic PDC insertion but there is suboptimal accessibility to surgical services for PDC placement, resulting in difficulties in clinic and operating room scheduling.
These operational inefficiencies drew attention to a different technique for PDC placement by interventional radiologists and nephrologists using fluoroscopy and ultrasound guidance, providing a minimally invasive approach to catheter placement that avoids general anesthesia or operating room logistical barriers [7–12].
The objective of this study was to compare the outcomes of PDC placement using fluoroscopic and ultrasound-guided technique with the laparoscopic technique. A recent study suggested PDC outcomes in prospective randomized trials may vary due to exclusion of obese patients and those who have had prior surgery, resulting in outcomes that represent what can be achieved under the most favorable circumstances [13]. Therefore, our study was designed to include obese patients and patients with prior surgery. We also included patients who underwent simultaneous adhesiolysis, omentopexy or hernia repairs, because exclusion of these patients might have adversely affected the results in favor of the radiologic technique.
Materials and methods
Study population
The study was approved by our institutional review board and patient’s informed consent was waived. The medical records of 260 patients who initiated PD between January 2005 and June 2016 as identified from the interventional radiology and dialysis registries were retrospectively reviewed. The patient population was divided into two groups: the radiologic group, which included patients who had a PDC placed by the fluoroscopic and ultrasound-guided technique by interventional radiologists or interventional nephrologists under conscious sedation, and the laparoscopic group, which included patients who received PDC insertion by the laparoscopic technique by surgeons and under general anesthesia. Inclusion criteria were patients with CKD Stage 5 or ESRD who were ≥ 19 years of age and had their first PDC placed during the study period. Exclusion criteria were patients who had no follow-up after catheter placement in our institution, patients who had a PDC placed but not used during the study period because they did not meet the criteria for dialysis (embedded catheters) and patients who had their catheters successfully placed after more than one attempt. Patients who required adhesiolysis, omentopexy or hernia repair during laparoscopic PDC placement and patients with prior abdominal surgery or severe obesity in both groups were not excluded. Figure 1 shows the algorithm for patients’ inclusion and exclusion.
Fig. 1.
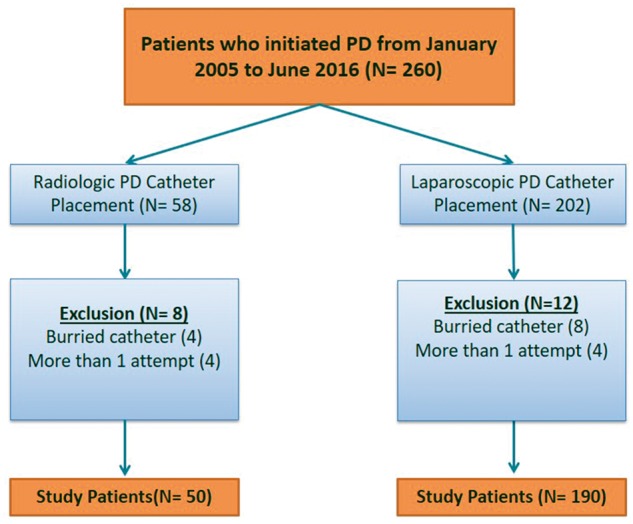
Algorithm for patients’ inclusion and exclusion.
Demographic data were obtained from the patients’ medical charts. Information regarding patients’ comorbidities was also recorded, including body mass index (BMI), coronary artery disease, hypertension, congestive heart failure, peripheral vascular disease, diabetes or cerebral vascular disease that would affect their eligibility to obtain general anesthesia for laparoscopic catheter placement. Obesity, defined as a BMI≥30, was included and the BMI was classified into three categories: Class 1 is BMI of 30–<35, Class 2 is BMI of 35–<40 and Class 3 is BMI≥40. We also recorded the failed attempts when placing a PDC with each technique as well as the prior surgical history of the patients. Advanced laparoscopic techniques such as adhesiolysis, omentopexy or hernia repairs were recorded if they were done simultaneously during laparoscopic PDC placement.
Study outcomes
The primary endpoint was the occurrence of PDC-related complications at 365 days (complication-free catheter survival at 365 days), which was a composite endpoint that includes mechanical, infectious, technical and miscellaneous complications. The mechanical complications included catheter malfunction related to inadequate drainage from the catheter, catheter leak through the exit site, and abdominal herniation. The infectious complications included peritonitis, tunnel infection and exit site infection. The technical complications included insertion failure, defined as an inability to insert the catheter or an inability to use the catheter after successful insertion, muscle hematoma, intraperitoneal bleeding and bowel perforation. Miscellaneous complications includes all other complications encountered during the follow-up period.
Secondary endpoints were the occurrence of PDC-related complications at 90 days (complication-free catheter survival at 90 days), catheter removal at 90 days (catheter survival at 90 days), catheter removal at 365 days (overall catheter survival at 365 days), median days to first complication and median days to catheter removal. Catheter complications from each group were also recorded. The data on catheter placement, complications and removal were obtained from the patients’ electronic medical records and from a prospective dialysis access database.
Technique of radiologic and laparoscopic catheter placement
Radiologic PDC placement using fluoroscopy and ultrasound guidance has been previously described and was performed by three interventional radiologists and one interventional nephrologist each with at least 5 years of experience [7, 9, 12]. A micropuncture set was used to access the peritoneum. The laparoscopic catheter insertion was performed by one surgeon with at least 5 years experience in placing PD catheters using this technique. The laparoscopic technique was previously described in the literature [13].
Statistical analysis
Medians and interquartile ranges (IQRs) were calculated for continuous variables and frequencies and percentages were calculated for categorical variables. Differences between the two groups were compared using Fisher’s exact test and Kruskal–Wallis one-way analysis of variance. Complication-free survival and overall catheter survival curves were estimated using the Kaplan–Meier approach. The log-rank tests were used to assess homogeneity across strata.
Results
There was a significant difference in the gender between both groups (P = 0.03), with more males in the radiologic group and more females in the laparoscopic group. Apart from this, there were no significant differences in the patient’s demographics or comorbidities (Table 1). The radiologic group comprised a total of 50 patients while the laparoscopic group comprised 190 patients. The radiologic group consisted of 21 females and 29 males with a median age of 56.3 years (IQR 47.17–66.41). The laparoscopic group included 113 females and 77 males with a median age of 54.3 years (IQR 40.26–63.41). Obesity in the radiologic and laparoscopic groups was seen in 15 patients (30%) and 68 patients (35.8%), respectively (P = 0.44) (Table 2). In the radiologic group, 11 patients (73.3%) and 4 patients (26.7%) were Class 1 and 2 obesity, respectively. There was no Class 3 obesity in the radiologic group. In the laparoscopic group, 28 patients (41.2%), 25 patients (36.8%) and 15 patients (22%) were Class 1, 2 and 3 obesity, respectively. Advanced techniques were simultaneously employed during laparoscopic PDC placement in 34 patients (17.9%), which included laparoscopic hernia repair in 15 patients (7.9%) and laparoscopic omentopexy in 19 patients (10%) (Table 3). Failed placement from the first attempt was seen in two (4%) and four (2%) patients in the radiologic and laparoscopic groups, respectively. These patients were excluded from the statistical analysis.
Table 1.
Demographics and baseline characteristics of the radiologic and laparoscopic groups
| Characteristics | Radiologic group (n = 50), n (%) | Laparoscopic group (n = 190), n (%) | P-value |
|---|---|---|---|
| Agea | 56 (47, 66) | 54 (40, 63) | 0.102 |
| Sex | 0.027 | ||
| Male | 29 (58) | 77 (41) | |
| Female | 21 (42) | 113 (59) | |
| BMIa | 27 (26, 32) | 28 (24, 35) | 0.916 |
| Diabetes | 25 (50) | 90 (47) | 0.740 |
| Hypertension | 44 (88) | 178 (94) | 0.223 |
| Coronary artery disease | 17 (34) | 42 (22) | 0.082 |
| Congestive heart failure | 14 (28) | 43 (23) | 0.427 |
| Peripheral vascular disease | 8 (16) | 21 (11) | 0.339 |
| Cerebrovascular disease | 3 (6) | 11 (6) | 0.999 |
Median (25th and 75th percentiles in parentheses).
Table 2.
BMI characteristics of the radiologic and laparoscopic groups
| BMI | Radiologic group (n = 50), n (%) | Laparoscopic group (n = 190), n (%) | P-value |
|---|---|---|---|
| Total (≥30) | 15 (30) | 68 (35.8) | 0.44 |
| Class 1 (30–< 35) | 11 (22) | 28 (14.7) | 0.22 |
| Class 2 (35–< 40) | 4 (8) | 25 (13.2) | 0.32 |
| Class 3 (≥40) | 0 (0) | 15 (7.9) | 0.04 |
Table 3.
Simultaneous advanced techniques in the laparoscopic groups
| Laparoscopic technique | n (%) |
|---|---|
| Total | 34 (17.9) |
| Omentopexy | 19 (10) |
| Hernia repair | 15 (7.9) |
Prior surgical procedures were performed in 19 patients (38%) in the radiologic group compared with 92 patients (48.4%) in the laparoscopic group (P = 0.19) (Table 4). The most common surgical procedure performed in both groups was hysterectomy, which accounted for 7 patients (36.8%) and 28 patients (30.4%) in the radiologic and laparoscopic groups, respectively. Other surgical procedures performed included abdominal exploration, appendectomy, cesarean section, cholecystectomy, colon surgery, fundoplication, gastric bypass, kidney transplantation, myomectomy, nephrectomy, salpingo-oophrectomy and tubal ligation.
Table 4.
Prior surgical procedures in the radiologic and laparoscopic groups
| Type of surgery | Radiologic group (n = 50), n (%) | Laparoscopic group (n = 190), n (%) | P-value |
|---|---|---|---|
| Total | 19 (38) | 92 (48.4) | 0.19 |
| Abdominal exploration | 2 (4) | 2 (1) | 0.15 |
| Appendectomy | 1 (2) | 3 (1.6) | 0.84 |
| Cesarean section | 2 (4) | 22 (11.6) | 0.11 |
| Cholecystectomy | 2 (4) | 6 (3.2) | 0.77 |
| Colon surgery | 0 (0) | 5 (2.6) | 0.25 |
| Fundoplication | 0 (0) | 1 (0.5) | 0.61 |
| Gastric bypass | 1 (2) | 1 (0.5) | 0.31 |
| Hysterectomy | 7 (14) | 28 (14.7) | 0.90 |
| Kidney transplantation | 3 (6) | 6 (3.2) | 0.35 |
| Myomectomy | 0 (0) | 1 (0.5) | 0.61 |
| Nephrectomy | 0 (0) | 8 (4.2) | 0.14 |
| Salpingo-oophorectomy | 0 (0) | 3 (1.6) | 0.37 |
| Tubal ligation | 1 (2) | 6 (3.2) | 0.67 |
The complication-free catheter survival at 90 days was 64% in the radiologic group and 71% in the laparoscopic group, which was not significant (P = 0.374). The complication-free catheter survival at 365 days was 48% in the radiologic group and 53% in the laparoscopic group, respectively, which was also not significant (P = 0.494). Catheter complication rates and complication-free survival times for the radiologic and laparoscopic groups are shown in Table 5.
Table 5.
Catheter complication rates and complication-free catheter survival times for radiologic and laparoscopic groups
| Radiologic (n = 50), n (%) | Laparoscopic (n = 190), n (%) | P-value | |
|---|---|---|---|
| Total complications | 31 (62) | 119 (63) | |
| Exit site infections | 2 (4) | 10 (5) | 0.999 |
| Peritonitis | 8 (16) | 32 (17) | 0.887 |
| Catheter malfunction | 8 (16) | 57 (30) | 0.048 |
| Catheter leak | 5 (10) | 6 (3) | 0.055 |
| Primary leak | 1 (2) | 2 (1) | 0.506 |
| Muscle hematoma or bleeding | 3 (6) | 3 (2) | 0.107 |
| Bowel perforation | 1 (2) | 0 (0) | 0.208 |
| Hernia | 3 (6) | 9 (5) | 0.718 |
| Complication-free survival at 90 days | 32 (64) | 134 (71) | 0.374 |
| Complication-free survival at 365 days | 24 (48) | 101 (53) | 0.494 |
| Complication-free daysa | 124 (17, 531) | 162 (64, 383) | 0.541 |
Median (25th and 75th percentiles in parentheses).
Catheter malfunction and peritonitis were the most frequent complications in both groups. Catheter malfunction occurred in 57 patients (30%) in the laparoscopic group, which was significantly higher compared with 8 patients (16%) in the radiologic group (P = 0.048). Peritonitis occurred in 8 patients (16%) in the radiologic group compared with 32 patients (17%) in the laparoscopic group, which was not statistically significant (P = 0.887). Bowel perforation occurred in only one case in the radiologic group and was treated conservatively by overnight observation and antibiotic administration. The remaining complications occurred at a higher rate in the laparoscopic group compared with the radiologic group, but without statistical significance. The median time to the first complication in the radiologic group was 124 days (IQR 17–531), which was not significantly different than 162 days (IQR 64–383) in the laparoscopic group (P = 0.54) (Table 2). Kaplan–Meier survival analysis showed no significant difference in the overall complication-free survival between both groups (P = 0.37) (Figure 2).
Fig. 2.
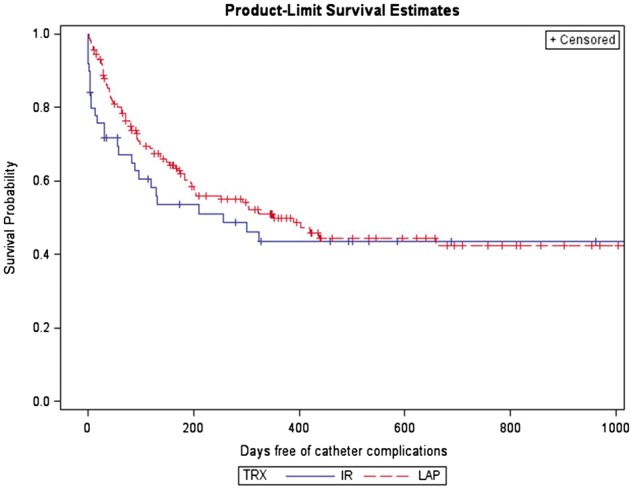
Kaplan–Meier survival curves for catheter complication in the radiologic and laparoscopic groups.
The overall catheter survival at 90 days was 70% in the radiologic group and 72% in the laparoscopic group, which was not significantly different (P = 0.0514). The overall catheter survival at 365 days was 48% in the radiologic group and 39% in the laparoscopic group, which was not significantly different (P = 0.652). The median time to catheter removal was 396 days (IQR 118–1335) in the radiologic group, which was not significantly different from 347 days (IQR 143–624) in the laparoscopic group (P = 0.71) (Table 6). Kaplan–Meier survival analysis showed no significant difference in the overall catheter survival between both groups (P = 0.50) (Figure 3).
Table 6.
Overall catheter survival for radiologic and laparoscopic groups
| Radiologic (n = 50), n (%) | Laparoscopic (n = 190), n (%) | P-value | |
|---|---|---|---|
| Total catheter removal | 12 (24) | 59 (31) | 0.321 |
| Overall survival at 90 days | 35 (70) | 136 (72) | 0.051 |
| Overall survival at 365 days | 24 (48) | 75 (39) | 0.652 |
| Days until removala | 396 (118, 1335) | 347 (143, 624) | 0.709 |
Median (25th and 75th percentiles in parentheses).
Fig. 3.
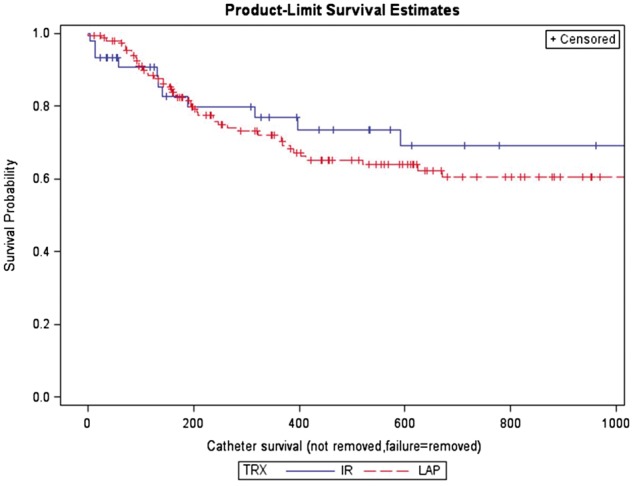
Kaplan–Meier survival curves for catheter removal in the radiologic and laparoscopic groups.
Discussion
PDC can be placed using either the laparoscopic technique by surgeons or the percutaneous image-guided approach by interventional radiologists. Several published studies comparing both techniques were retrospective and were limited mostly by inherent selection bias and institution-specific clinical practices, which makes the interpretation of catheter outcomes difficult. In clinical practice, patients with prior surgery as well as obese patients are usually referred for laparoscopic placement, while patients with advanced age and with multiple comorbidities are referred for percutaneous image-guided placement since these patients usually have one or more factors that make them ineligible to receive general anesthesia. In the current study there was no significant difference in the BMI, prior surgical procedures or other comorbidities between the radiologic and laparoscopic groups. On the other hand, patients who underwent adhesiolysis, omentopexy and hernia repair during laparoscopic catheter placement were not excluded from the study since these advanced laparoscopic techniques are now standard and have been shown to significantly improve laparoscopically placed catheters. Excluding these patients will adversely skew the outcomes in favor of the radiologic group.
The results of this study show that there were significantly more males in the radiologic group and significantly more females in the laparoscopic group. This bias may be due to the assumption by the referring nephrologists and other clinicians that males will tolerate the radiologic procedure, which was done under conscious sedation, while females are more likely to require general anesthesia.
The results of this study indicate that the complication-free catheter survival at 90 and 365 days as well as the overall catheter survival at 90 and 365 days were similar in both groups. Among the various complications encountered in our study in both groups, catheter malfunction appears to be the only complication that was significantly higher in the laparoscopic group when compared with the radiologic group. Bowel perforation was the only complication that was encountered exclusively in one case in the radiologic group and occurred during the authors’ early experience with the radiologic percutaneous image-guided technique. Therefore the radiologic percutaneous image-guided technique is not inferior to the laparoscopic catheter placement technique even in patient groups that in clinical practice are typically considered high risk or ineligible for the radiologic approach.
The results of the current study are slightly different than some of the studies published in the literature. In a prospective trial, Voss et al. [14] randomly assigned 113 patients to either fluoroscopic placement of a PDC under local anesthetic versus laparoscopic placement under general anesthesia. The primary outcome was complication-free catheter survival (complications secondary to mechanical or infectious causes) at 1 year. Secondary endpoints included catheter removal, procedure time, procedure pain, length of inpatient admissions, procedure room time utilization and direct hospital costs. The complication-free catheter survival was significantly higher in the radiologic group (42.5%) when compared to the laparoscopic group (18.1%; P = 0.03). Higher complication rates in the laparoscopic group included increased peritonitis and leak events. Hospital costs were significantly higher in the laparoscopic group. The results demonstrated the non-inferiority of fluoroscopically placed PDCs. However, the study was limited by several factors. First, the health care professionals who participated in this study were experts and trained to manage these study patients, which does not represent the natural circumstances elsewhere. Second, the patients included in the study were atypical, with exclusion of obese patients. Lastly, the patients included in the study were likely to receive better care than the usual circumstances regardless of the treatment allocation. The results of the study were therefore representative of what can be achieved in the most favorable circumstances.
Recently the same group published a retrospective cohort study comparing radiologically and laparoscopically placed PDCs [13]. Similar to the current study, there was no significant difference between the radiologic and laparoscopic groups regarding complication-free catheter survival or overall catheter survival at 365 days [13]. The overall patient survival, however, was statistically significantly higher for the laparoscopic group, due to the significant patient comorbidities and frail patients in the radiologic group, which was an inherent limitation of the study resulting from the selection bias when patients were referred to either technique.
Several published retrospective studies comparing the outcomes of traditional surgical placement of PDCs with the radiologic percutaneous image-guided technique concluded that the radiologic placement outcomes were comparable, yet allowed for more outpatient procedures, facilitated a more planned outpatient PD training and transition to home therapy and is a viable option compared to traditional surgical approaches [15–19].
Placement of PDCs by image-guided percutaneous techniques received renewed interest after reports of more rapid initiation of PD in patients presenting late in the course of their disease and needing more urgent PD therapy. To allow for the rapid initiation of PD, many centers have developed pathways for image-guided percutaneous placement of PDCs by interventional radiologists followed by assisted-PD treatments given by PD nurses in the outpatient setting until the patient has clinically improved and is able to be trained in self-care at home [20]. Development of the percutaneous image-guided technique for placement of PDC as well as advancement in the skill set among interventional radiologists in placing these catheters has helped tremendously in the establishment of urgent-start PD programs [21]. Fluoroscopic-guided techniques may also be used to attempt to restore function in those PDCs that have inflow or outflow disturbances, have migrated out of the pelvic location or have been occlude with fibrin or tissue. Various fluoroscopic stiff wire manipulations have been described in the literature and may offer a more cost-effective and expeditious pathway toward catheter revision compared with referring the patient to surgical PDC revision or repositioning [22–24].
The current study adds to the emerging literature suggesting that in centers with dedicated interventional radiology staff experienced in PDC placement, catheter outcomes can be comparable to laparoscopically placed catheters and therefore may offer a minimally invasive and cost-effective catheter placement option.
A limitation of this study includes its retrospective nature resulting in inherent selection bias. However, the existence of prospective dialysis registries at our center as well as the prospective collection of information on dialysis access procedures provide a high degree of confidence that the information on catheter placement, complications and removal is accurate and complete. The small sample size is another limitation. The study represents a single-center experience and the results cannot be generalized.
Conclusion
In conclusion, the fluoroscopy and ultrasound-guided minimally invasive technique for placement of PDCs can be performed safely and provides a clinically effective alternative to the laparoscopic technique, with similar survival and complication rates. This technique also allows for expeditious placement of PDCs in late-referred patients with ESRD and therefore facilitates urgent-start PD and avoids the need for placement of temporary vascular access catheters.
Conflict of interest statement
A.K.A.A. is a consultant for Abbott Medical, Bard Peripheral Vascular, Baxter Healthcare, Boston Scientific and W. L. Gore. S.S.G. is an employee of Baxter Healthcare. The other authors have nothing to disclose.
References
- 1. Chaudhary K, Sangha H, Khanna R.. Peritoneal dialysis first: rationale. Clin J Am Soc Nephrol 2011; 6: 447–456 [DOI] [PubMed] [Google Scholar]
- 2. Mehrotra R, Chiu Y-W, Kalantar-Zadeh K et al. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 2011; 171: 110–118 [DOI] [PubMed] [Google Scholar]
- 3. Kumar VA, Sidell MA, Jones JP. et al. Survival of propensity matched incident peritoneal and hemodialysis patients in a United States health care system. Kidney Int 2014; 86: 1016–1022 [DOI] [PubMed] [Google Scholar]
- 4. Ghaffari A. Urgent-start peritoneal dialysis: a quality improvement report. Am J Kidney Dis 2012; 59: 400–408 [DOI] [PubMed] [Google Scholar]
- 5. Arramreddy R, Zheng S, Saxena AB et al.. Urgent-start peritoneal dialysis: a chance for a new beginning. Am J Kidney Dis 2014; 63: 390–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ghaffari A, Kumar V, Guest S.. Infrastructure requirements for an urgent-start peritoneal dialysis program. Perit Dial Int 2013; 33: 611–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abdel-aal AK, Gaddikeri S, Saddekni S.. Technique of peritoneal catheter placement under fluoroscopic guidance. Radiol Res Pract 2011; 2011: 141707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reddy C, Dybbro PE, Guest S.. Fluoroscopically guided percutaneous peritoneal dialysis catheter placement: single center experience and review of the literature. Ren Fail 2010; 32: 294–299 [DOI] [PubMed] [Google Scholar]
- 9. Abdel-aal AK, Joshi AK, Saddekni S et al.. Fluoroscopic and sonographic guidance to place peritoneal catheters: how we do it. Am J Roentgenol 2009; 192: 1085–1089 [DOI] [PubMed] [Google Scholar]
- 10. Savader SJ. Percutaneous radiologic placement of peritoneal dialysis catheters. J Vasc Interv Radiol 1999; 10: 249–256 [DOI] [PubMed] [Google Scholar]
- 11. Savader SJ, Geschwind J, Lund GB et al.. Percutaneous radiological placement of peritoneal dialysis catheters: long-term results. J Vasc Interv Radiol 2000; 11: 965–970 [DOI] [PubMed] [Google Scholar]
- 12. Abdel-Aal AK, Dybbro P, Hathaway P et al.. Best practices consensus protocol for peritoneal dialysis catheter placement by interventional radiologists. Perit Dial Int 2014; 34: 481–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maher E, Wolley M, Abbas SA. et al. Fluoroscopic versus laparoscopic implantation of peritoneal dialysis catheters: a retrospective cohort study. J Vasc Interv Radiol 2014; 25: 895–903 [DOI] [PubMed] [Google Scholar]
- 14. Voss D, Hawkins S, Poole G. et al. Radiological versus surgical implantation of first catheter for peritoneal dialysis: a randomized non-inferiority trial. Nephrol Dial Transplant 2012; 27: 4196–4204 [DOI] [PubMed] [Google Scholar]
- 15. Brunier G, Hiller JA, Drayton S. et al. A change to radiologic peritoneal dialysis catheter insertion: three-month outcomes. Perit Dial Int 2010; 30: 528–533 [DOI] [PubMed] [Google Scholar]
- 16. Rosenthal MA, Yang PS, Liu I-LA et al.. Comparison of outcomes of peritoneal dialysis catheters placed by the fluoroscopically guided percutaneous method versus directly visualized surgical method. J Vasc Interv Radiol 2008; 19: 1202–1207 [DOI] [PubMed] [Google Scholar]
- 17. Moon J, Song S, Jung K. et al. Fluoroscopically guided peritoneal dialysis catheter placement: long-term results from a single center. Perit Dial Int 2008; 28: 163–169 [PubMed] [Google Scholar]
- 18. Medani S, Shantier M, Hussein W. et al. A comparative analysis of percutaneous and open surgical techniques for peritoneal catheter placement. Perit Dial Int 2012; 32: 628–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Georgiades CS, Geschwind JH.. Percutaneous peritoneal dialysis catheter placement for the management of end-stage renal disease: technique and comparison with the surgical approach. Tech Vasc Interv Radiol 2002; 5: 103–107 [DOI] [PubMed] [Google Scholar]
- 20. Liu FX, Ghaffari A, Dhatt H et al.. Economic evaluation of urgent-start peritoneal dialysis versus urgent-start hemodialysis in the United States. Medicine 2014; 28: e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Masseur A, Guest S, Kumar V.. Early technique success after initiation of treatment with urgent-start peritoneal dialysis. Adv Perit Dial 2014; 30: 36–39 [PubMed] [Google Scholar]
- 22. Miller M, McCormick B, Lavoie S. et al. Fluoroscopic manipulation of peritoneal dialysis catheters: outcomes and factors associated with successful manipulation. Clin J Am Soc Nephrol 2012; 7: 795–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Savader SJ, Lund G, Scheel PJ.. Guide wire directed manipulation of malfunctioning peritoneal dialysis catheters: a critical analysis. J Vasc Interv Radiol 1997; 8: 957–963 [DOI] [PubMed] [Google Scholar]
- 24. Ozyer U, Harman A, Aytekin C. et al. Correction of displaced peritoneal dialysis catheters with an angular stiff rod. Acta Radiol 2009; 50: 139–143 [DOI] [PubMed] [Google Scholar]


